Energy-Density Improvement in Li-Ion Rechargeable Batteries Based on LiCoO2 + LiV3O8 and Graphite + Li-Metal Hybrid Electrodes
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dunn, B.; Kamath, H.; Tarascon, J.-M. Electrical energy storage for the grid: A battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.C.; Østergaard, J. Battery energy storage technology for power systems—An overview. Electr. Power Syst. Res. 2009, 79, 511–520. [Google Scholar] [CrossRef]
- Reddy, M.V.; Jie, T.W.; Jafta, C.J.; Ozoemena, K.I.; Mathe, M.K.; Nair, A.S.; Peng, S.S.; Idris, M.S.; Geetha, B.G.; Ezema, F.I.; et al. Studies on bare and Mg-doped LiCoO2 as a cathode material for lithium ion batteries. Electrochim. Acta 2014, 128, 192–197. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Yang, J.; Mu, Y.; Wang, Y. Fabrication of Fe-doped LiCoO2 sandwich-like nanocomposites as excellent performance cathode materials for lithium-ion batteries. Chem. Eur. J. 2015, 21, 19104–19111. [Google Scholar] [CrossRef] [PubMed]
- Ning, F.; Xu, B.; Shi, J.; Wu, M.; Hu, Y.; Ouyang, C. Structural, electronic, and Li migration properties of RE-doped (RE = Ce, La) LiCoO2 for Li-ion batteries: A first-principles investigation. J. Phys. Chem. C 2016, 120, 18428–18434. [Google Scholar] [CrossRef]
- Jung, Y.S.; Lu, P.; Cavanagh, A.S.; Ban, C.; Kim, G.-H.; Lee, S.-H.; George, S.M.; Harris, S.J.; Dillon, A.C. Unexpected improved performance of ALD coated LiCoO2/graphite Li-ion batteries. Adv. Energy Mater. 2013, 3, 213–219. [Google Scholar] [CrossRef]
- Teranishi, T.; Yoshikawa, Y.; Sakuma, R.; Hashimoto, R.; Hayashi, H.; Kishimoto, A.; Fujii, T. High-rate performance of ferroelectric BaTiO3-coated LiCoO2 for Li-ion batteries. Appl. Phys. Lett. 2014, 105, 143904. [Google Scholar] [CrossRef]
- Shim, J.-H.; Lee, S.; Park, S.S. Effects of MgO coating on the structural and electrochemical characteristics of LiCoO2 as cathode materials for lithium ion battery. Chem. Mater. 2014, 26, 2537–2543. [Google Scholar] [CrossRef]
- Shim, J.-H.; Lee, K.-S.; Missyul, A.; Lee, J.; Linn, B.; Lee, E.C.; Lee, S. Characterization of Spinel LixCo2O4 coated LiCoO2 prepared with post-thermal treatment as a cathode material for lithium ion batteries. Chem. Mater. 2015, 27, 3273–3279. [Google Scholar] [CrossRef]
- Kim, M.H.; Kang, Y.C.; Jeong, S.M.; Choi, Y.J.; Kim, Y.S. Morphologies and electrochemical properties of 0.6Li2MnO3·0.4LiCoO2 composite cathode powders prepared by spray pyrolysis. Mater. Chem. Phys. 2013, 142, 438–444. [Google Scholar] [CrossRef]
- Wang, F.X.; Xiao, S.Y.; Chang, Z.; Li, M.X.; Wu, Y.P.; Holze, R. Coaxial LiCoO2@Li2MnO3 nanoribbon as a high capacity cathode for lithium ion batteries. Int. J. Electrochem. Sci. 2014, 9, 6182–6190. [Google Scholar]
- Long, B.R.; Croy, J.R.; Dogan, F.; Suchomel, R.; Key, B.; Wen, J.; Miller, D.J.; Thackeray, M.M.; Balasubramanian, M. Effect of cooling rates on phase separation in 0.5Li2MnO3·0.5LiCoO2 electrode materials for Li-ion batteries. Chem. Mater. 2014, 26, 3565–3572. [Google Scholar] [CrossRef]
- Teranishi, T.; Yoshikawa, Y.; Sakuma, R.; Okamura, H.; Hashimoto, H.; Hayashi, H.; Fujii, T.; Kishimoto, A.; Takeda, Y. High-rate capabilities of ferroelectric BaTiO3–LiCoO2 composites with optimized BaTiO3 loading for Li-ion batteries. ECS Electrochem. Lett. 2015, 4, A137–A140. [Google Scholar] [CrossRef]
- Jiao, L.; Liu, L.; Sun, J.; Yang, L.; Zhang, Y.; Yuan, H.; Wang, Y.; Zhou, X. Effect of AlPO4 nanowire coating on the electrochemical properties of LiV3O8 cathode material. J. Phys. Chem. C 2008, 112, 18249–18254. [Google Scholar] [CrossRef]
- Ren, W.; Zheng, Z.; Luo, Z.; Chen, W.; Niu, C.; Zhao, K.; Yan, M.; Zhang, L.; Meng, J.; Mai, L. An electrospun hierarchical LiV3O8 nanowire-in-network for high-rate and long-life lithium batteries. J. Mater. Chem. A 2015, 3, 19850–19856. [Google Scholar] [CrossRef]
- Huang, S.; Tu, J.P.; Jian, X.M.; Lu, Y.; Shi, S.J.; Zhao, X.Y.; Wang, T.X.; Wang, X.L.; Gu, C.D. Enhanced electrochemical properties of Al2O3-coated LiV3O8 cathode materials for high-power lithium-ion batteries. J. Power Sources 2014, 245, 698–705. [Google Scholar] [CrossRef]
- Gao, X.-W.; Wang, J.Z.; Chou, S.L.; Liu, H.K. Synthesis and electrochemical performance of LiV3O8/polyaniline as cathode material for the lithium battery. J. Power Sources 2012, 220, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Pan, A.; Zhang, J.-G.; Cao, G.; Liang, S.; Wang, C.; Nie, Z.; Arey, B.W.; Xu, W.; Liu, D.; Xiao, J.; et al. Nanosheet-structured LiV3O8 with high capacity and excellent stability for high energy lithium batteries. J. Mater. Chem. 2011, 21, 10077–10084. [Google Scholar] [CrossRef]
- Mori, M.; Naruok, Y.; Naoi, K. Modification of the lithium metal surface by nonionic polyether surfactants: Quartz crystal microbalance studies. J. Electrochem. Soc. 1998, 145, 2340–2348. [Google Scholar] [CrossRef]
- Aurbach, D.; Zinigrad, E.; Cohen, Y.; Teller, H. A short review of failure mechanisms of lithium metal and lithiated graphite anodes in liquid electrolyte solutions. Solid State Ion. 2002, 148, 405–416. [Google Scholar] [CrossRef]
- Kim, J.-S.; Yoon, W.-Y.; Kim, B.-K. Morphological differences between lithium powder and lithium foil electrode during discharge/charge. J. Power Sources 2006, 163, 258–263. [Google Scholar] [CrossRef]
- Kim, J.S.; Yoon, W.Y.; Yi, K.Y.; Kim, B.K.; Cho, B.W. The dissolution and deposition behavior in lithium powder electrode. J. Power Sources 2007, 165, 620–624. [Google Scholar] [CrossRef]
- Seong, I.W.; Hong, C.H.; Kim, B.K.; Yoon, W.Y. The effects of current density and amount of discharge on dendrite formation in the lithium powder anode electrode. J. Power Sources 2008, 178, 769–773. [Google Scholar] [CrossRef]
- Kim, J.S.; Baek, S.H.; Yoon, W.Y. Electrochemical behavior of compacted lithium powder electrode in LiOV2O5 rechargeable battery. J. Electrochem. Soc. 2010, 157, A984–A987. [Google Scholar] [CrossRef]
- Kong, S.-K.; Kim, B.-K.; Yoon, W.-Y. Electrochemical behavior of Li-powder anode in high Li capacity used. J. Electrochem. Soc. 2012, 159, A1551–A1553. [Google Scholar] [CrossRef]
- Seong, I.W.; Kim, K.T.; Yoon, W.Y. Electrochemical behavior of a lithium-pre-doped carbon-coated silicon monoxide anode cell. J. Power Sources 2009, 189, 511–514. [Google Scholar] [CrossRef]
- Seong, I.W.; Yoon, W.Y. Electrochemical behavior of a silicon monoxide and Li-powder double layer anode cell. J. Power Sources 2010, 195, 6143–6147. [Google Scholar] [CrossRef]
- Park, J.S.; Mane, A.U.; Elam, J.W.; Croy, J.R. Amorphous metal fluoride passivation coatings prepared by atomic layer deposition on LiCoO2 for Li-ion batteries. Chem. Mater. 2015, 27, 1917–1920. [Google Scholar] [CrossRef]
- Kwak, W.J.; Shin, H.J.; Reiter, J.; Tsiouvaras, N.; Hassoun, J.; Passerini, S.; Scrosati, B.; Sun, Y.K. Understanding problems of lithiated anodes in lithium oxygen full-cells. J. Mater. Chem. A 2016, 4, 10467–10471. [Google Scholar] [CrossRef]
- Ma, J.M.; Liu, Z.; Chen, B.; Wang, L.; Yue, L.; Liu, H.; Zhang, J.; Liu, Z.; Cui, G. A strategy to make high voltage LiCoO2 compatible with polyethylene oxide electrolyte in all-solid-state lithium ion batteries. J. Electrochem. Soc. 2017, 164, A3454–A3461. [Google Scholar] [CrossRef]
- Liu, H.; Chem, Z.; Zhou, L.; Li, X.; Pei, K.; Zhang, J.; Song, Y.; Fang, F.; Che, R.; Sun, D. Rooting bismuth oxide nanosheets into porous carbon nanoboxes as a sulfur immobilizer for lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 7074–7081. [Google Scholar] [CrossRef]
- Wu, N.; Zhang, Y.; Wei, Y.; Liu, H.; Wu, H. Template-engaged synthesis of 1D hierarchical chainlike LiCoO2 cathode materials with enhanced high-voltage lithium storage capabilities. ACS Appl. Mater. Interfaces 2016, 8, 25361–25368. [Google Scholar] [CrossRef]
- Zhao, M.; Zuo, X.; Ma, X.; Xiao, X.; Yu, L.; Nan, J. Diphenyl disulfide as a new bifunctional film-forming additive for high-voltage LiCoO2/graphite battery charged to 4.4 V. J. Power Sources 2016, 323, 29–36. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, J.; Liu, Y.; Wang, H.; Liu, C.; Wu, T.; Hsu, P.-C.; Lin, D.; Jin, Y.; Cui, Y. Engineering the surface of LiCoO2 electrodes using atomic layer deposition for stable high-voltage lithium ion batteries. Nano Res. 2017, 10, 3754–3764. [Google Scholar] [CrossRef]
- Cho, S.H.; Hwang, S.W.; Kim, B.H.; Bae, K.Y.; Yoon, H.; Yoon, S.S.; Yoon, W.Y. Electrochemical properties of Li metal batteries with P(PEGMA)-coated lithium trivanadate cathode and Li powder anode. J. Nanosci. Nanotechnol. 2016, 16, 10607–10612. [Google Scholar] [CrossRef]
- Xie, L.-L.; You, L.-Q.; Cao, Z.-Y.; Zhang, C.-F.; Song, D.-W.; Qu, L.-B. Co3(PO4)2-coated LiV3O8 as positive materials for rechargeable lithium batteries. Electron. Mater. Lett. 2012, 8, 411–415. [Google Scholar] [CrossRef]
- Jouanneau, S.; Le Gal La Salle, A.; Verbaere, A.; Guyomard, D. The Origin of capacity fading upon lithium cycling in Li1.1V3O8. J. Electrochem. Soc. 2005, 152, A1660–A1667. [Google Scholar] [CrossRef]
- Ren, X.; Hu, S.; Shi, C.; Zhang, P.; Yuan, Q.; Liu, J. Preparation and electrochemical properties of Zr-doped LiV3O8 cathode materials for lithium-ion batteries. J. Solid State Electrochem. 2012, 16, 2135–2141. [Google Scholar] [CrossRef]
- Feng, Y.; Li, Y.; Hou, F. Preparation and electrochemical properties of Cr doped LiV3O8 cathode for lithium ion batteries. Mater. Lett. 2009, 63, 1338–1340. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Zhang, C.; Liu, C.; Cao, G. Mo-doped LiV3O8 nanorod-assembled nanosheets as a high performance cathode material for lithium ion batteries. J. Mater. Chem. A 2015, 3, 3547–3558. [Google Scholar] [CrossRef]
- Zhang, T.; Imanishi, N.; Shimonishi, Y.; Hirano, A.; Takeda, Y.; Yamamoto, O.; Sammes, N. A novel high energy density rechargeable lithium/air battery. Chem. Commun. 2010, 46, 1661–1663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-K.; Oh, S.-M.; Park, E.; Scrosati, B.; Hassoun, J.; Park, M.-S.; Kim, Y.-J.; Kim, H.; Belharouak, I.; Sun, Y.-K. Highly cyclable lithium−sulfur batteries with a dual-type sulfur cathode and a lithiated Si/SiOx nanosphere anode. Nano. Lett. 2015, 15, 2863–2868. [Google Scholar] [CrossRef] [PubMed]
- Nie, P.; Shen, L.; Luo, H.; Li, H.; Xu, G.; Zhang, X. Synthesis of nanostructured materials by using metalcyanide coordination polymers and their lithium storage properties. Nanoscale 2013, 5, 11087–11093. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, S.; Kanamura, K.; Takehara, Z.-I. Surface condition changes in lithium metal deposited in nonaqueous electrolyte containing HF by dissolution-deposition cycles. J. Electrochem. Soc. 1999, 146, 1633–1639. [Google Scholar] [CrossRef]
- Sinha, N.N.; Munichandraiah, N. Synthesis and characterization of carbon-coated LiNi1/3Co1/3Mn1/3O2 in a single step by an inverse microemulsion route. ACS Appl. Mater. Interfaces 2009, 1, 1241–1249. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, J.K.; Yoon, W.Y. Electrochemical analysis of the effect of Cr coating the LiV3O8 cathode in a lithium ion battery with a lithium powder anode. ACS Appl. Mater. Interfaces 2013, 5, 7058–7064. [Google Scholar] [CrossRef] [PubMed]
- Meer, S.; Yong, H.; Amir, C.; Ebenezer, A.; Meha, C.; Chunlei, W.; Bilal, E. Capacity fading mechanism in lithium-sulfur battery using poly(ionicliquid) gel electrolyte. Electrochim. Acta 2017, 258, 1284–1292. [Google Scholar]
- Wang, Z.K.; Shu, J.; Zhu, Q.-C.; Cao, B.-Y.; Chen, H.; Wu, X.Y.; Bartlett, B.M.; Wang, K.X.; Chen, J.S. Graphene-nanosheet-wrapped LiV3O8 nanocomposites as high performance cathode materials for rechargeable lithium-ion batteries. J. Power Sources 2016, 307, 426–434. [Google Scholar] [CrossRef]
- Qiao, Y.Q.; Tu, J.O.; Wang, X.L.; Zhang, J.; Yu, Y.X.; Gu, C.D. Self-assembled synthesis of hierarchical wafer-like porous Li-V-O composites as cathode materials for lithium ion batteries. J. Phys. Chem. C 2011, 115, 25508–25518. [Google Scholar] [CrossRef]

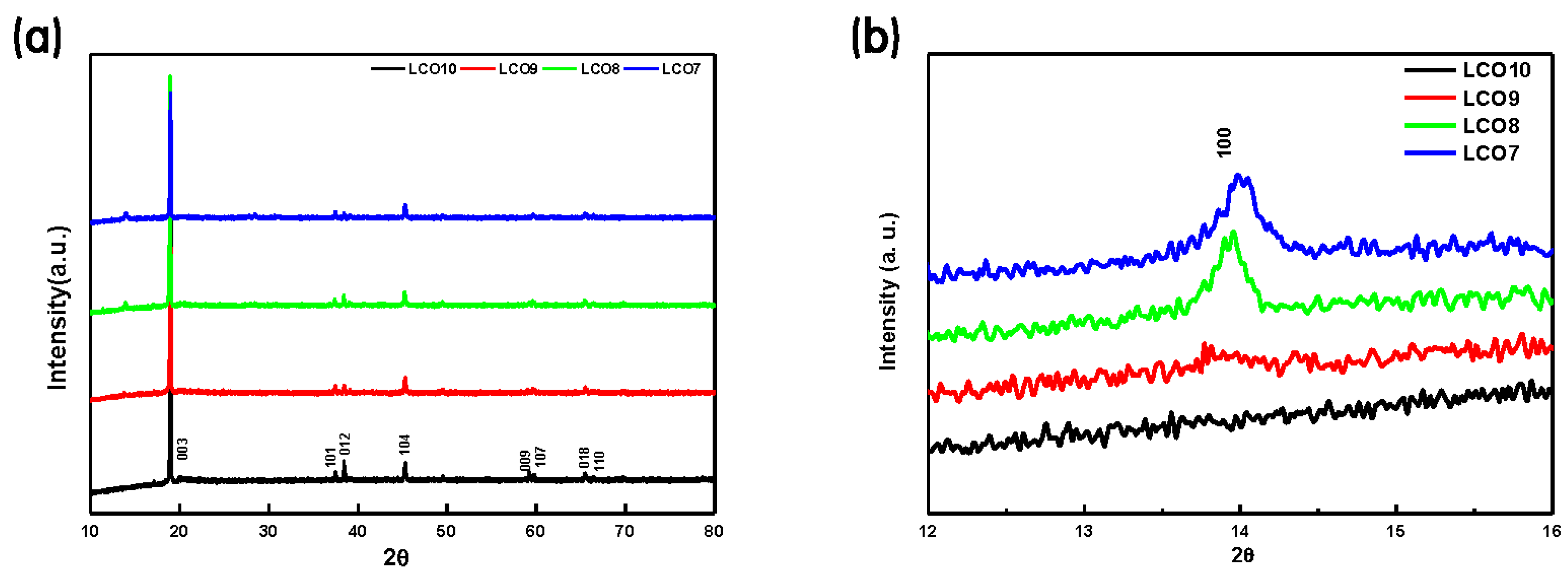
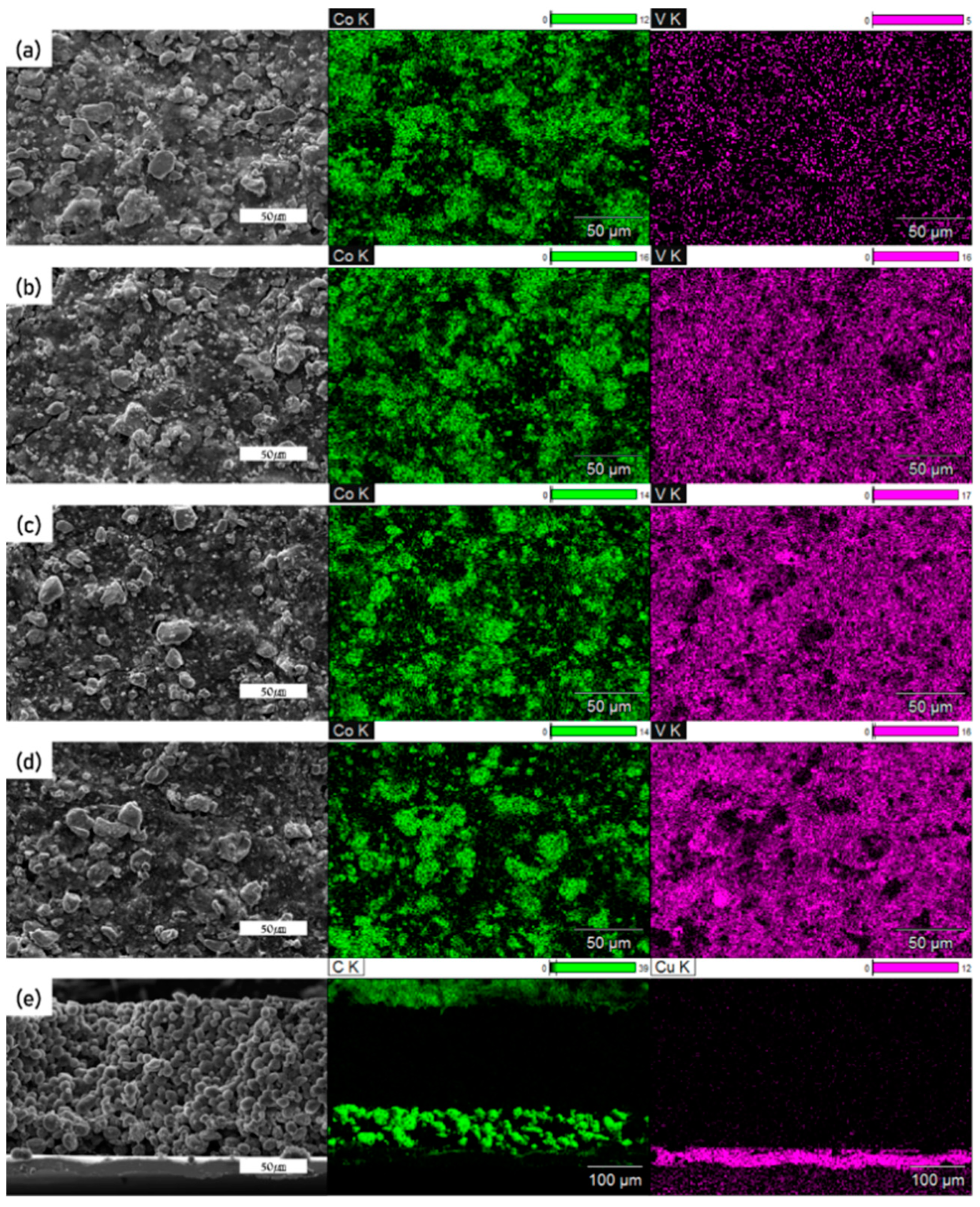
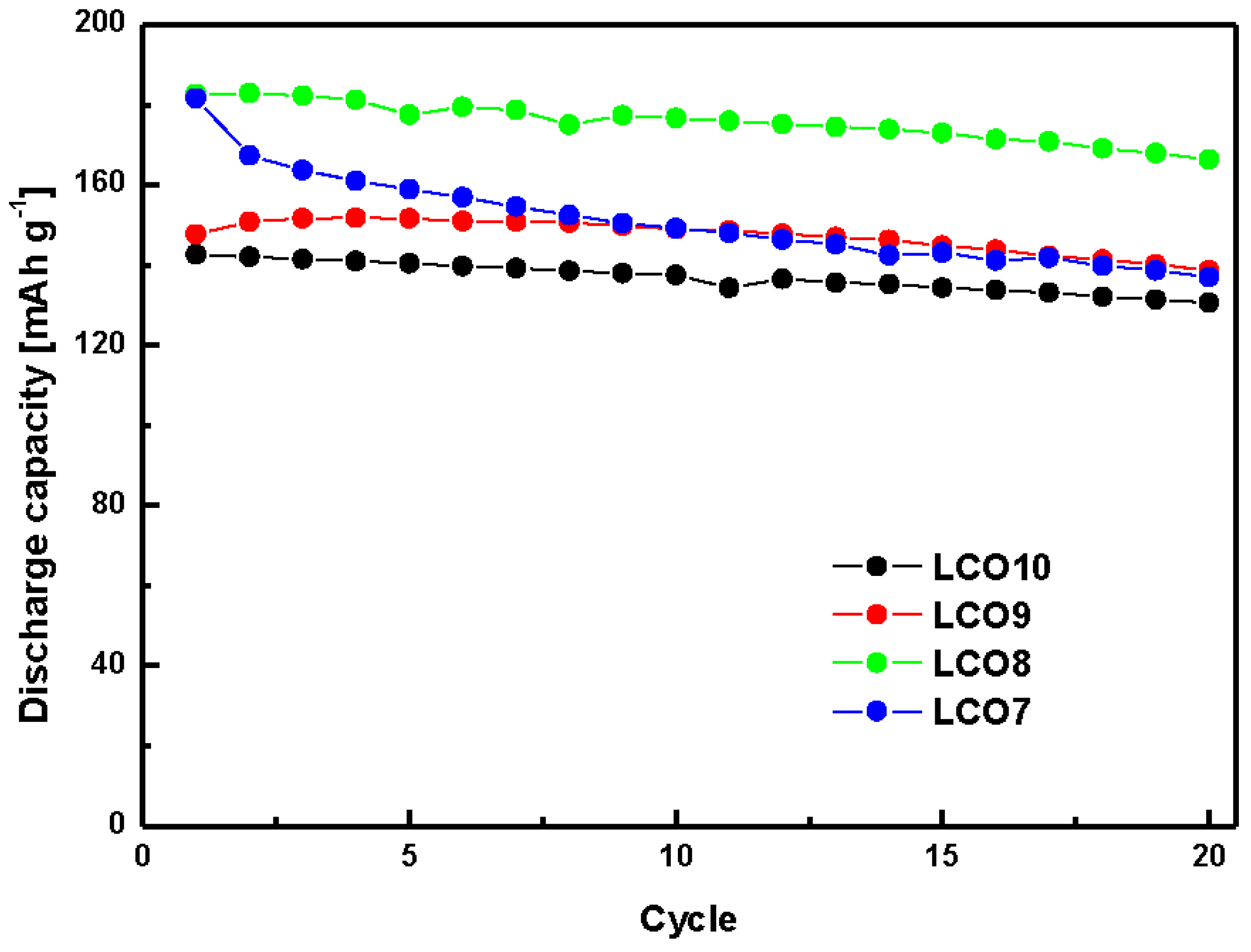

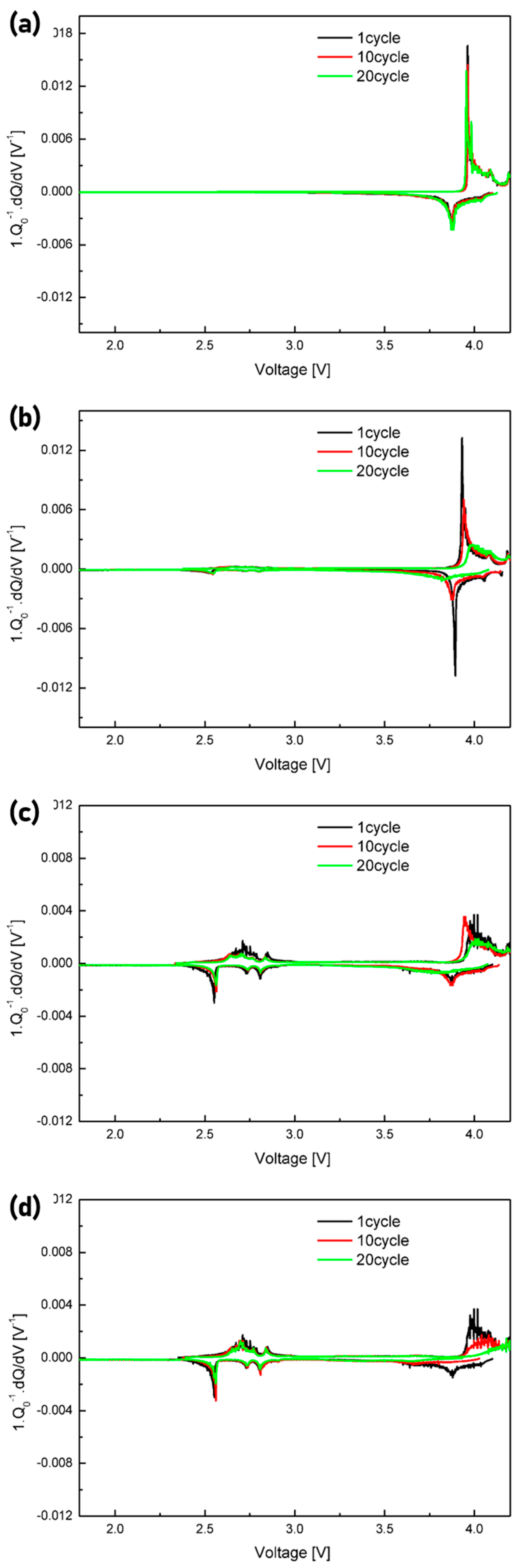
| Hybrid Cathode (wt%) | Hybrid Anode (wt%) | ||||
|---|---|---|---|---|---|
| LCO | LVO | Graphite (g) | LP (g) | Graphite + LP (g) | |
| LCO10 | 100 | 0 | 100 (0.737) | 0 (0) | 0.737 |
| LCO9 | 90 | 10 | 88 (0.663) | 12 (0.007) | 0.670 |
| LCO8 | 80 | 20 | 78 (0.589) | 22 (0.015) | 0.604 |
| LCO7 | 70 | 30 | 70 (0.516) | 30 (0.022) | 0.537 |
| LCO10 | LCO9 | LCO8 | LCO7 | |
|---|---|---|---|---|
| Energy density [W h kg−1] | 545.96 | 543.84 | 629.24 | 551.49 |
| Energy density [W h L−1] | 2686.12 | 2578.89 | 2871.85 | 2418.84 |
| Discharge capacity [mA h g−1] | 142.03 | 150.84 | 182.88 | 167.35 |
| Nominal voltage [V] | 3.844 | 3.605 | 3.441 | 3.295 |
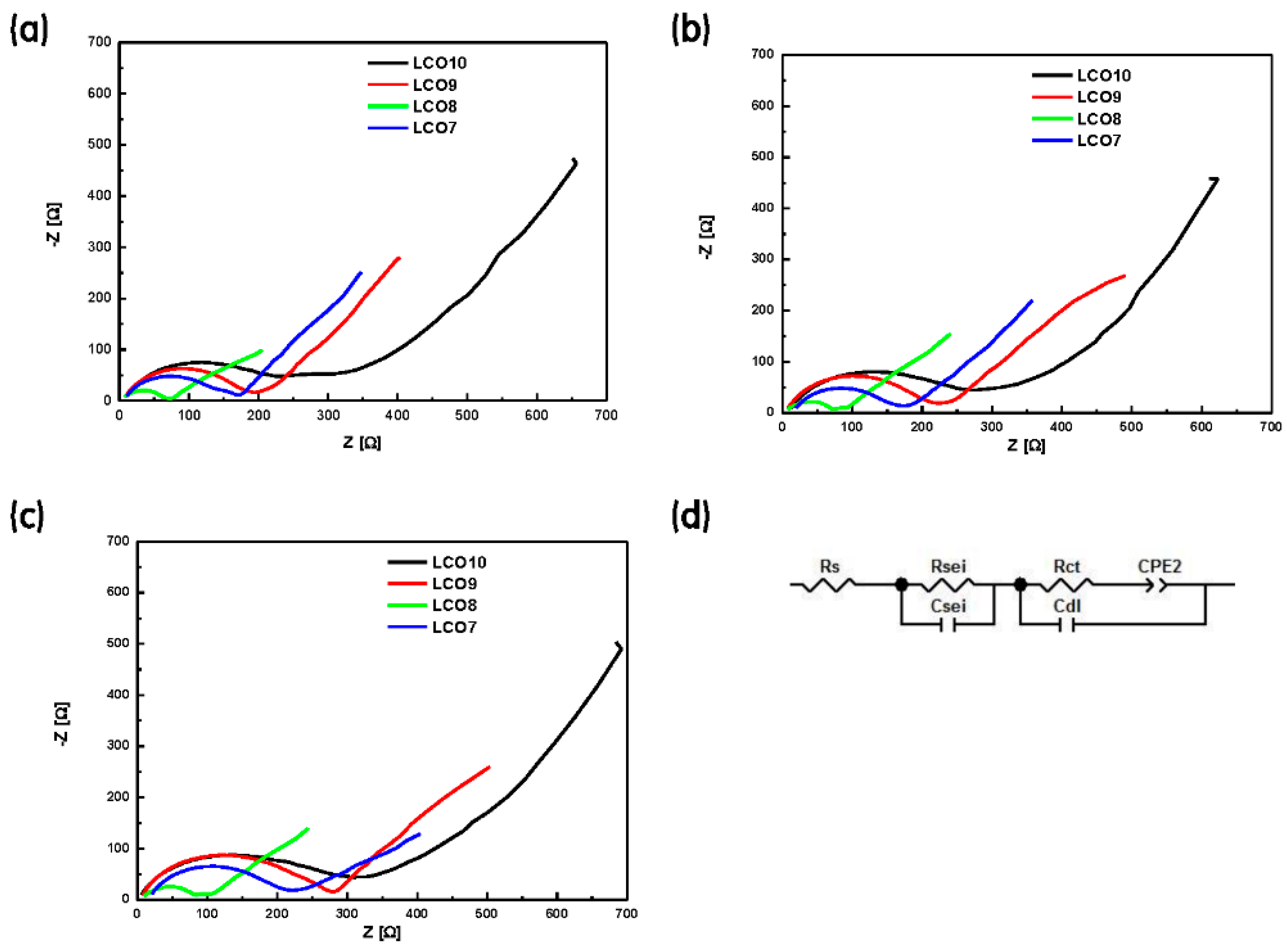
| LCO10 | Cycle | LCO9 | Cycle | ||||
| 1st | 10th | 20th | 1st | 10th | 20th | ||
| Rs [Ω] | 11.99 | 13.56 | 6.04 | Rs [Ω] | 9.58 | 8.42 | 8.19 |
| Rsei [Ω] | 62.48 | 73.45 | 74.01 | Rsei [Ω] | 49.37 | 49.34 | 70.05 |
| Rct [Ω] | 138.50 | 154.90 | 179.60 | Rct [Ω] | 120.40 | 142.90 | 171.60 |
| LCO8 | Cycle | LCO7 | Cycle | ||||
| 1st | 10th | 20th | 1st | 10th | 20th | ||
| Rs [Ω] | 6.23 | 6.51 | 8.38 | Rs [Ω] | 11.46 | 20.01 | 20.09 |
| Rsei [Ω] | 21.41 | 21.83 | 20.76 | Rsei [Ω] | 74.54 | 61.02 | 44.56 |
| Rct [Ω] | 35.90 | 42.66 | 51.16 | Rct [Ω] | 70.26 | 76.85 | 115.90 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bae, K.Y.; Cho, S.H.; Kim, B.H.; Son, B.D.; Yoon, W.Y. Energy-Density Improvement in Li-Ion Rechargeable Batteries Based on LiCoO2 + LiV3O8 and Graphite + Li-Metal Hybrid Electrodes. Materials 2019, 12, 2025. https://doi.org/10.3390/ma12122025
Bae KY, Cho SH, Kim BH, Son BD, Yoon WY. Energy-Density Improvement in Li-Ion Rechargeable Batteries Based on LiCoO2 + LiV3O8 and Graphite + Li-Metal Hybrid Electrodes. Materials. 2019; 12(12):2025. https://doi.org/10.3390/ma12122025
Chicago/Turabian StyleBae, Ki Yoon, Sung Ho Cho, Byung Hyuk Kim, Byung Dae Son, and Woo Young Yoon. 2019. "Energy-Density Improvement in Li-Ion Rechargeable Batteries Based on LiCoO2 + LiV3O8 and Graphite + Li-Metal Hybrid Electrodes" Materials 12, no. 12: 2025. https://doi.org/10.3390/ma12122025
APA StyleBae, K. Y., Cho, S. H., Kim, B. H., Son, B. D., & Yoon, W. Y. (2019). Energy-Density Improvement in Li-Ion Rechargeable Batteries Based on LiCoO2 + LiV3O8 and Graphite + Li-Metal Hybrid Electrodes. Materials, 12(12), 2025. https://doi.org/10.3390/ma12122025




