Behavior of Colloidal Nanosilica in an Ultrahigh Performance Concrete Environment Using Dynamic Light Scattering
Abstract
1. Introduction
1.1. Experimental Theory
1.1.1. Colloidal NS
1.1.2. Dynamic Light Scattering
1.1.3. Zeta Potential
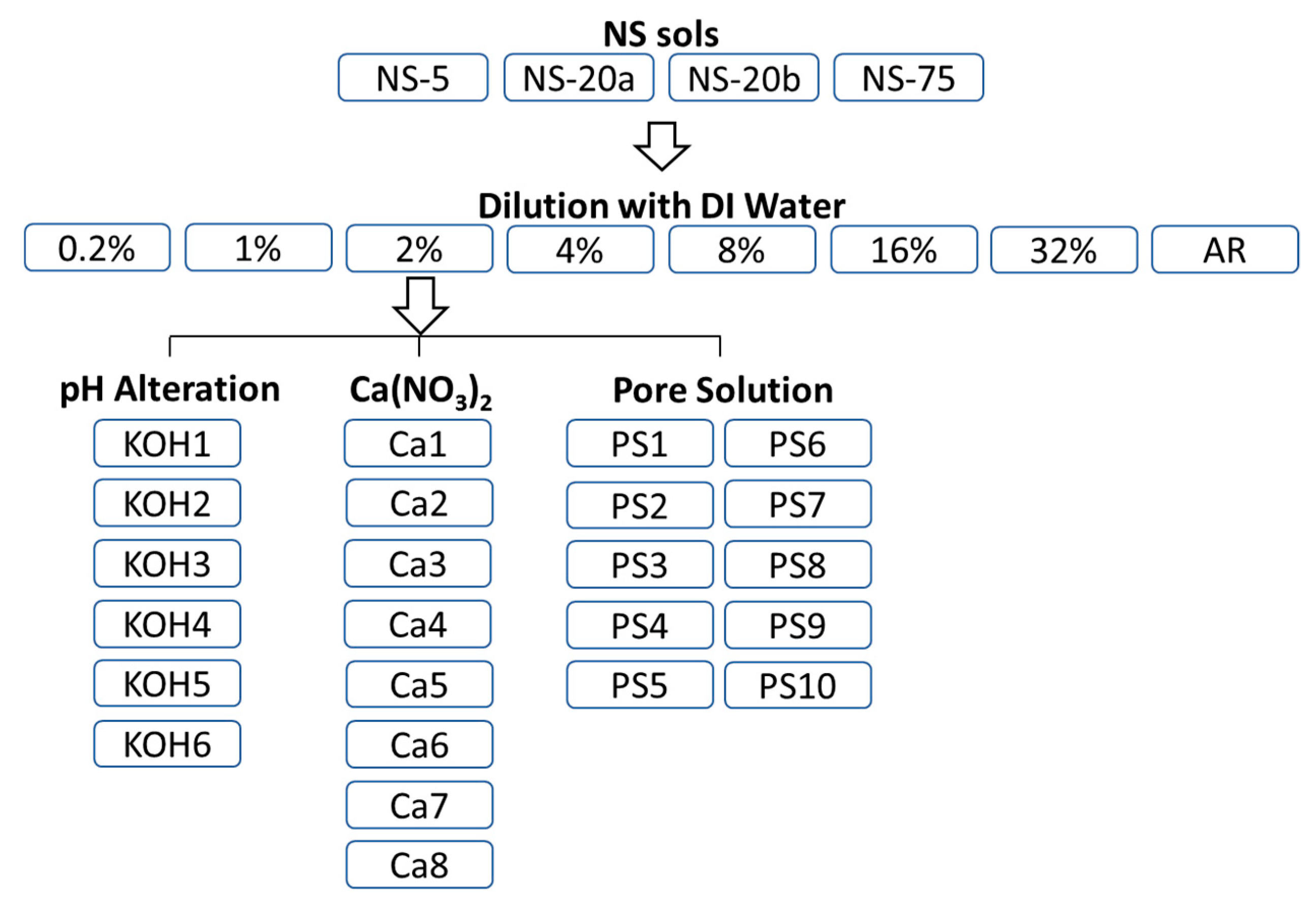
2. Materials and Methods
2.1. Experimental Parameters
2.1.1. Colloidal NS
2.1.2. Dynamic Light Scattering
2.1.3. Zeta Potential
2.1.4. Sample Preparation
2.1.5. CryoSEM
3. Results
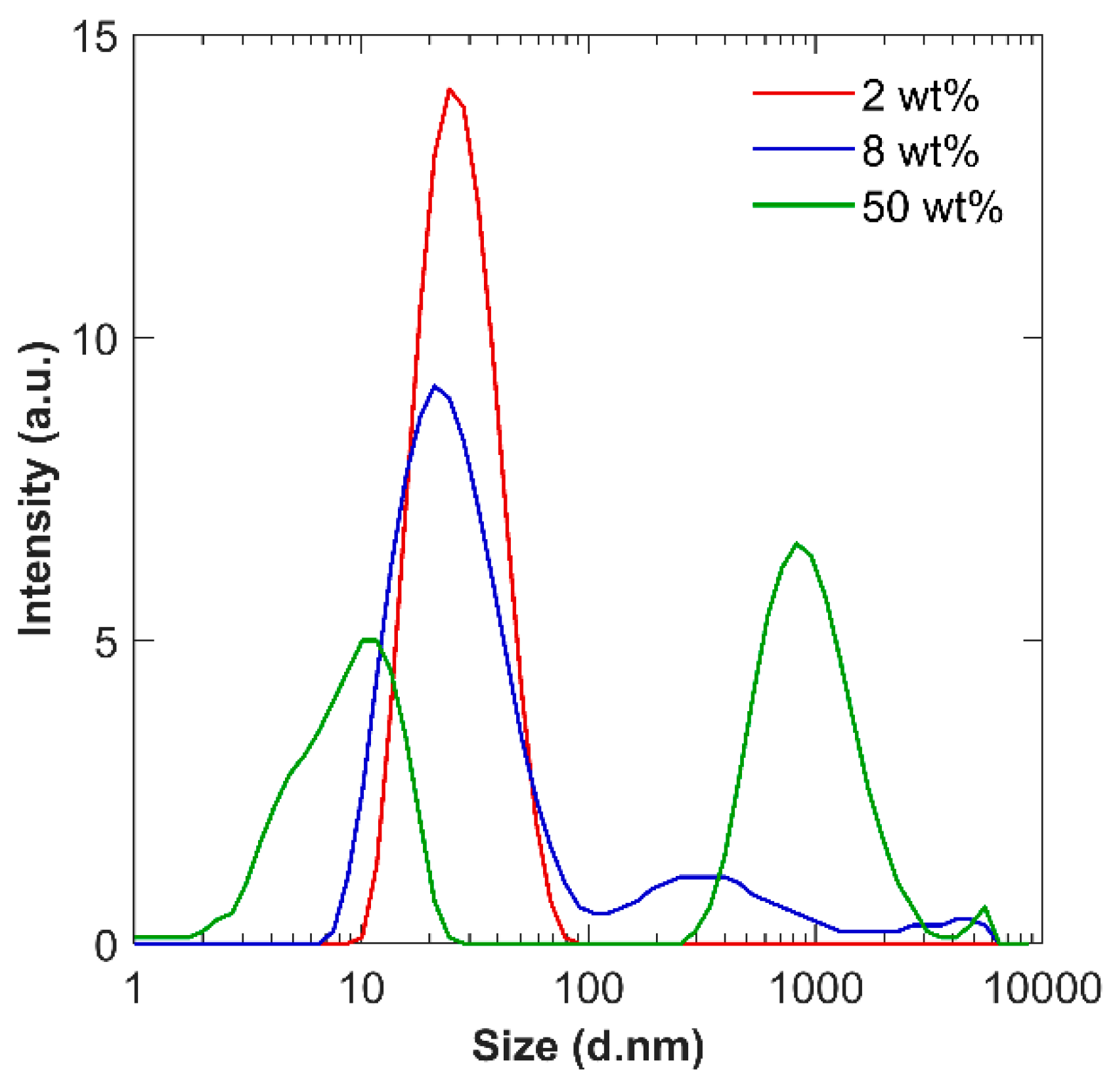
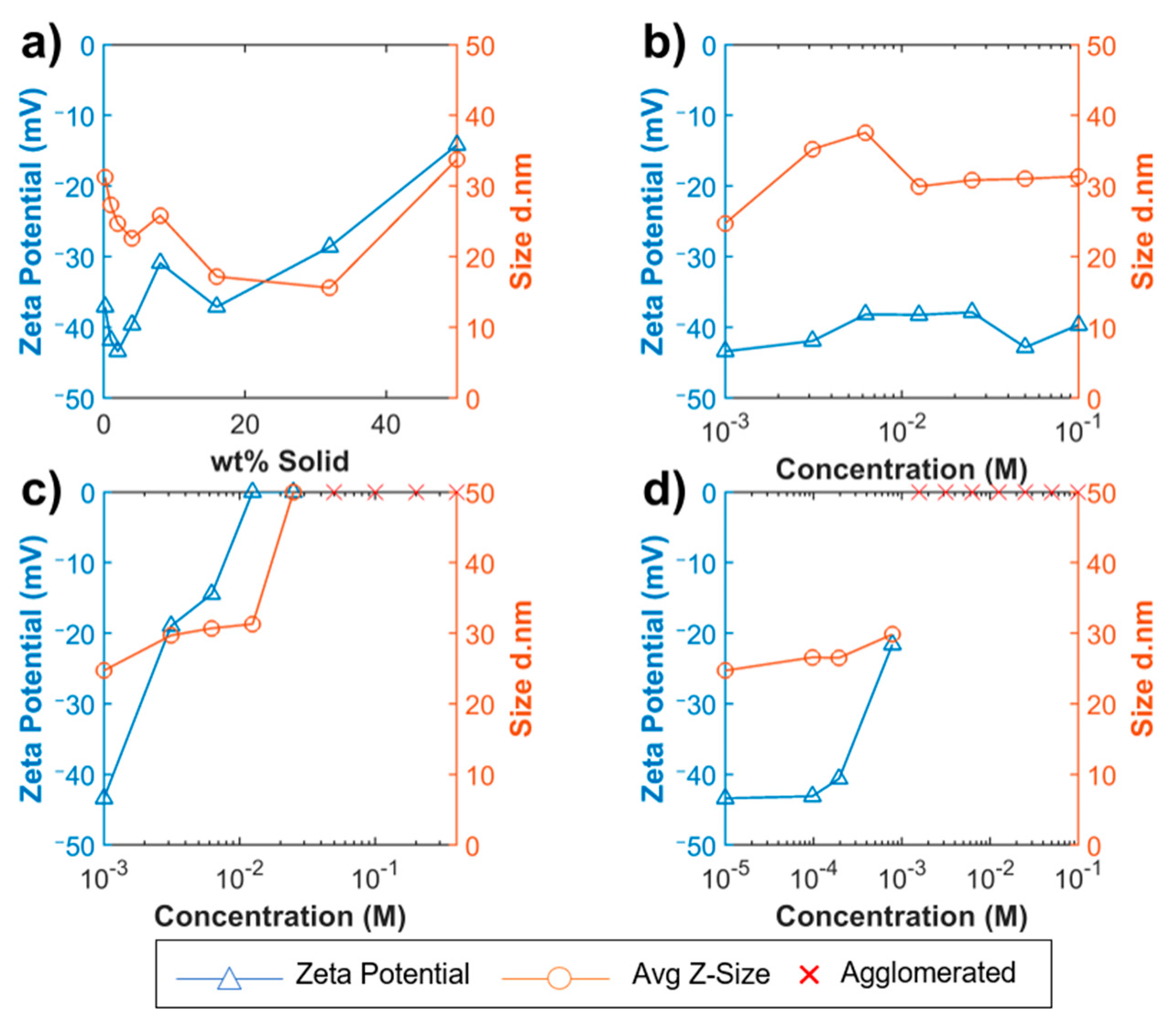
3.1. Diluting NS
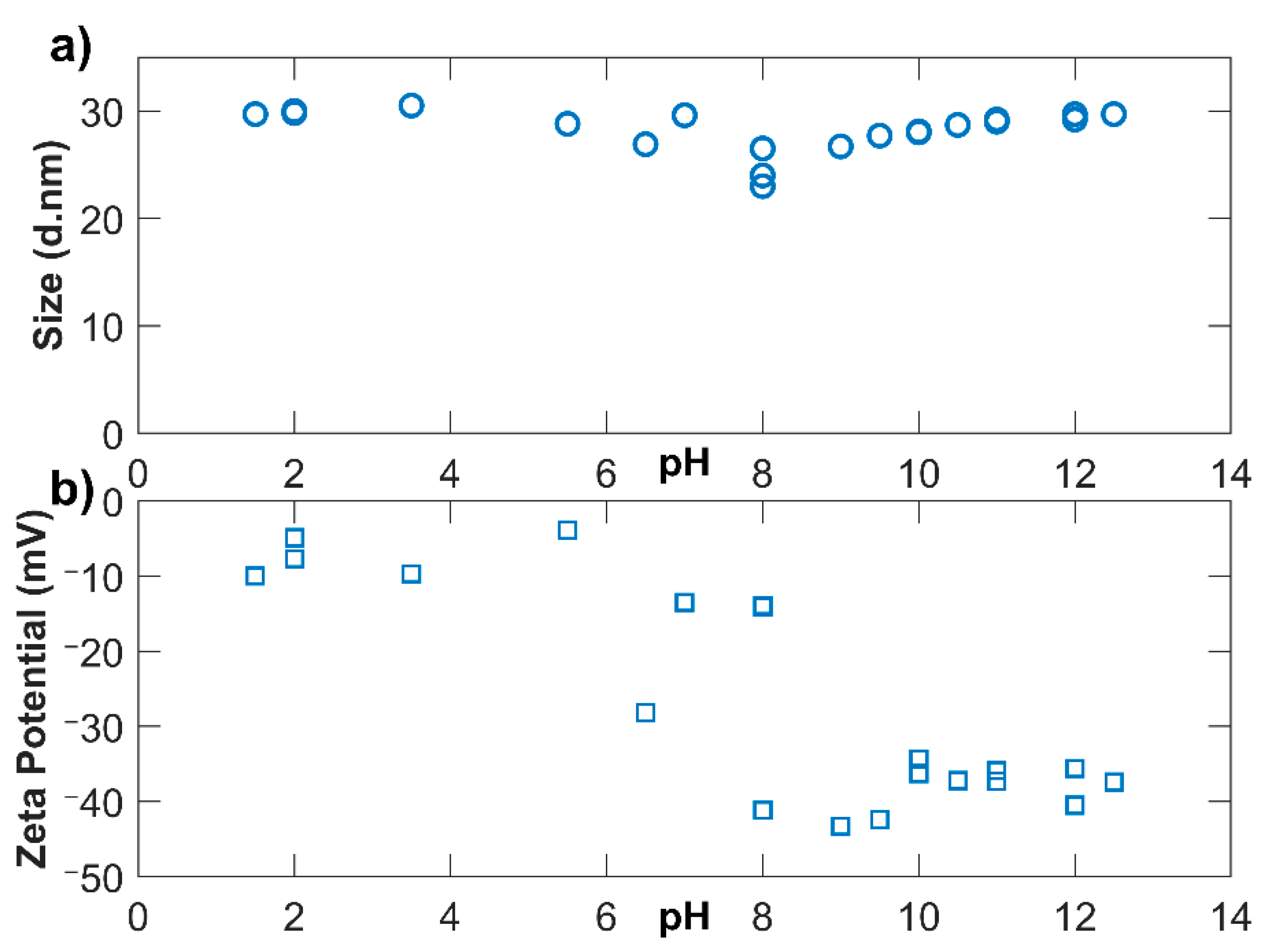
3.2. pH Adjustment
3.3. Calcium Nitrate
3.4. Synthetic Pore Solution
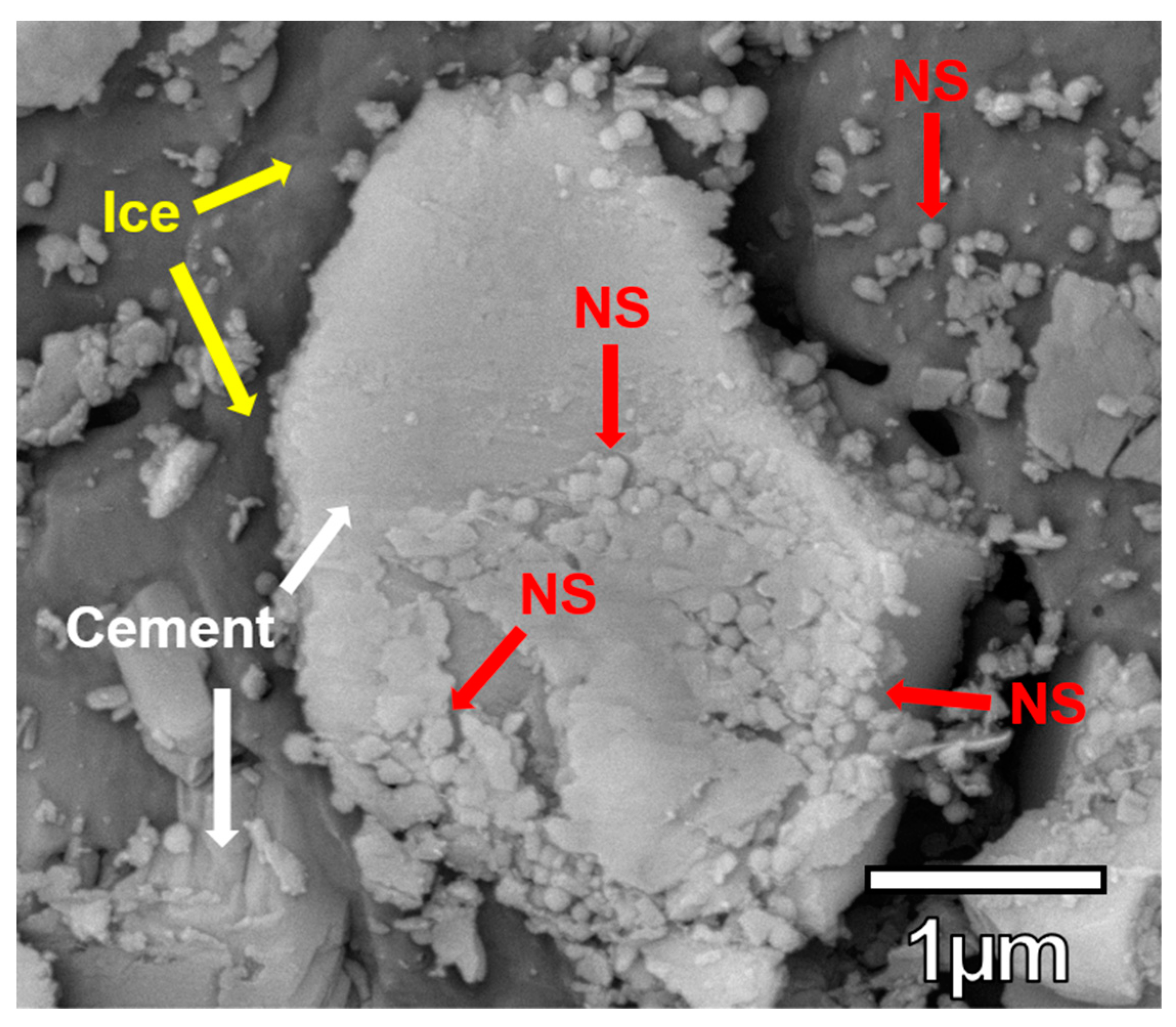
3.5. NS Visualization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Sobolev, K.; Flores, I.; Hermosillo, R.; Torres-Martinez, L. Nanomaterials and Nanotechnology for High-Performance Cement Composites. Proc. ACI Sess. Nanotechnol. Concr. Recent Dev. Futur. Prospect. 2006, 254, 91–118. [Google Scholar] [CrossRef]
- Silvestre, J.; Silvestre, N.; de Brito, J. Review on Concrete Nanotechnology. Eur. J. Environ. Civ. Eng. 2016, 20, 455–485. [Google Scholar] [CrossRef]
- Aggarwal, P.; Singh, R.P.; Aggarwal, Y. Use of Nano-Silica in Cement Based Materials—A Review. Cogent Eng. 2015, 2, 1078018. [Google Scholar] [CrossRef]
- Chen, J.; Kou, S.; Poon, C. Hydration and Properties of Nano-TiO2 Blended Cement Composites. Cem. Concr. Compos. 2012, 34, 642–649. [Google Scholar] [CrossRef]
- Metaxa, Z.S.; Konsta-Gdoutos, M.S.; Shah, S.P. Carbon Nanotubes Reinforced Concrete. In Proceedings of the Nanotechnology of Concrete: The Next Big Thing is Small, Session at the ACI Fall 2009 Convention, New Orleans, LA, USA, 8–12 November 2009; pp. 11–20. [Google Scholar] [CrossRef]
- Chuah, S.; Pan, Z.; Sanjayan, J.G.; Wang, C.M.; Duan, W.H. Nano Reinforced Cement and Concrete Composites and New Perspective from Graphene Oxide. Constr. Build. Mater. 2014, 73, 113–124. [Google Scholar] [CrossRef]
- Janković, K.; Stankovic, S.; Dragan, B.; Stojanovic, M.; Antić, L. The Influence of Nano-Silica and Barite Aggregate on Properties of Ultra High Performance Concrete. Constr. Build. Mater. 2016, 126, 147–156. [Google Scholar] [CrossRef]
- Kontoleontos, F.; Tsakiridis, P.E.; Marinos, A.; Kaloidas, V.; Katsioti, M. Influence of Colloidal Nanosilica on Ultrafine Cement Hydration: Physicochemical and Microstructural Characterization. Constr. Build. Mater. 2012, 35, 347–360. [Google Scholar] [CrossRef]
- Land, G.; Stephan, D. The Influence of Nano-Silica on the Hydration of Ordinary Portland Cement. J. Mater. Sci. 2012, 47, 1011–1017. [Google Scholar] [CrossRef]
- Lim, S.; Mondal, P. Effects of Incorporating Nanosilica on Carbonation of Cement Paste. J. Mater. Sci. 2015, 50, 3531–3540. [Google Scholar] [CrossRef]
- Hou, P.; Cheng, X.; Qian, J.; Zhang, R.; Cao, W.; Shah, S.P. Characteristics of Surface-Treatment of Nano-SiO2 on the Transport Properties of Hardened Cement Pastes with Different Water-to-Cement Ratios. Cem. Concr. Compos. 2015, 55, 26–33. [Google Scholar] [CrossRef]
- Torabian Isfahani, F.; Redaelli, E.; Lollini, F.; Li, W.; Bertolini, L. Effects of Nanosilica on Compressive Strength and Durability Properties of Concrete with Different Water to Binder Ratios. Adv. Mater. Sci. Eng. 2016, 2016. [Google Scholar] [CrossRef]
- Ji, T. Preliminary Study on the Water Permeability and Microstructure of Concrete Incorporating Nano-SiO2. Cem. Concr. Res. 2005, 35, 1943–1947. [Google Scholar] [CrossRef]
- Jo, B.-W.; Kim, C.-H.; Tae, G.; Park, J.-B. Characteristics of Cement Mortar with Nano-SiO2 Particles. Constr. Build. Mater. 2007, 21, 1351–1355. [Google Scholar] [CrossRef]
- Berra, M.; Carassiti, F.; Mangialardi, T.; Paolini, A.E.; Sebastiani, M. Effects of Nanosilica Addition on Workability and Compressive Strength of Portland Cement Pastes. Constr. Build. Mater. 2012, 35, 666–675. [Google Scholar] [CrossRef]
- Khaloo, A.; Mobini, M.H.; Hosseini, P. Influence of Different Types of Nano-SiO2 Particles on Properties of High-Performance Concrete. Constr. Build. Mater. 2016, 113, 188–201. [Google Scholar] [CrossRef]
- Madani, H.; Bagheri, A.; Parhizkar, T. The Pozzolanic Reactivity of Monodispersed Nanosilica Hydrosols and Their Influence on the Hydration Characteristics of Portland Cement. Cem. Concr. Res. 2012, 42, 1563–1570. [Google Scholar] [CrossRef]
- Richard, P.; Cheyrezy, M. Composition of Reactive Powder Concretes. Cem. Concr. Res. 1995, 25, 1501–1511. [Google Scholar] [CrossRef]
- Sonebi, M.; García-Taengua, E.; Hossain, K.M.A.; Khatib, J.; Lachemi, M. Effect of Nanosilica Addition on the Fresh Properties and Shrinkage of Mortars with Fly Ash and Superplasticizer. Constr. Build. Mater. 2015, 84, 269–276. [Google Scholar] [CrossRef]
- Yu, R.; Spiesz, P.; Brouwers, H.J.H. Effect of Nano-Silica on the Hydration and Microstructure Development of Ultra-High Performance Concrete (UHPC) with a Low Binder Amount. Constr. Build. Mater. 2014, 65, 140–150. [Google Scholar] [CrossRef]
- Hou, P.; Kawashima, S.; Wang, K.; Corr, D.J.; Qian, J.; Shah, S.P. Effects of Colloidal Nanosilica on Rheological and Mechanical Properties of Fly Ash–cement Mortar. Cem. Concr. Compos. 2013, 35, 12–22. [Google Scholar] [CrossRef]
- Bi, J.; Pane, I.; Hariandja, B.; Imran, I. The Use of Nanosilica for Improving of Concrete Compressive Strength and Durability. Appl. Mech. Mater. 2012, 204, 4059–4062. [Google Scholar] [CrossRef]
- Qing, Y.; Zenan, Z.; Deyu, K.; Rongshen, C. Influence of Nano-SiO2 Addition on Properties of Hardened Cement Paste as Compared with Silica Fume. Constr. Build. Mater. 2007, 21, 539–545. [Google Scholar] [CrossRef]
- Rong, Z.; Sun, W.; Xiao, H.; Jiang, G. Effects of Nano-SiO2 Particles on the Mechanical and Microstructural Properties of Ultra-High Performance Cementitious Composites. Cem. Concr. Compos. 2015, 56, 25–31. [Google Scholar] [CrossRef]
- Sanjuán, M.Á.; Argiz, C.; Gálvez, J.C.; Moragues, A. Effect of Silica Fume Fineness on the Improvement of Portland Cement Strength Performance. Constr. Build. Mater. 2015, 96, 55–64. [Google Scholar] [CrossRef]
- Wille, K.; Naaman, A.E.; Parra-Montesinos, G.J. Ultra-High Performance Concrete with Compressive Strength Exceeding 150 MPa (22 Ksi): A Simpler Way. ACI Mater. J. 2011, 108, 34–46. [Google Scholar]
- De Larrard, F.; Sedran, T. Optimization of Ultra-High-Performance Concrete by the Use of a Packing Model. Cem. Concr. Res. 1994, 24, 997–1009. [Google Scholar] [CrossRef]
- Bagheri, A.; Parhizkar, T.; Madani, H.; Raisghasemi, A.M. The Influence of Different Preparation Methods on the Aggregation Status of Pyrogenic Nanosilicas Used in Concrete. Mater. Struct. 2013, 46, 135–143. [Google Scholar] [CrossRef]
- Zabihi, N.; Ozkul, M.H. The Effect of Colloidal Nano-Silica as a Cementitious Material, on Durability and Mechanical Properties of Mortar. In Proceedings of the 11th International Congress on Advances in Civil Engineering (ACE 2014), Istanbul, Turkey, 21–25 October 2014. [Google Scholar]
- Böschel, D.; Janich, M.; Roggendorf, H. Size Distribution of Colloidal Silica in Sodium Silicate Solutions Investigated by Dynamic Light Scattering and Viscosity Measurements. J. Coll. Interface Sci. 2003, 267, 360–368. [Google Scholar] [CrossRef]
- Hayrapetyan, S.S.; Khachatryan, H.G. Control of the Growth Processes of the Silica Sols Colloidal Particles. Microporous Mater. 2005, 78, 151–157. [Google Scholar] [CrossRef]
- Quercia, G.; Lazaro, A.; Geus, J.W.; Brouwers, H.J.H. Characterization of Morphology and Texture of Several Amorphous Nano-Silica Particles Used in Concrete. Cem. Concr. Compos. 2013, 44, 77–92. [Google Scholar] [CrossRef]
- Oertel, T.; Hutter, F.; Helbig, U.; Sextl, G. Amorphous Silica in Ultra-High Performance Concrete: First Hour of Hydration. Cem. Concr. Res. 2014, 58, 131–142. [Google Scholar] [CrossRef]
- Quercia, G.; Hüsken, G.; Brouwers, H.J.H. Water Demand of Amorphous Nano Silica and Its Impact on the Workability of Cement Paste. Cem. Concr. Res. 2012, 42, 344–357. [Google Scholar] [CrossRef]
- Sanchez, F.; Sobolev, K. Nanotechnology in Concrete–a Review. Constr. Build. Mater. 2010, 24, 2060–2071. [Google Scholar] [CrossRef]
- Sobolev, K.; Gutiérrez, M.F. How Nanotechnology Can Change the Concrete World. Am. Ceram. Soc. Bull. 2005, 84, 14. [Google Scholar]
- Rashad, A.M. A Comprehensive Overview about the Effect of Nano-SiO2 on Some Properties of Traditional Cementitious Materials and Alkali-Activated Fly Ash. Constr. Build. Mater. 2014, 52, 437–464. [Google Scholar] [CrossRef]
- Rashad, A.M. A Synopsis about the Effect of Nano-Al2O3, Nano-Fe2O3, Nano-Fe3O4 and Nano-Clay on Some Properties of Cementitious Materials–A Short Guide for Civil Engineer. Mater. Des. 2013, 52, 143–157. [Google Scholar] [CrossRef]
- Iler, R.K. The Colloid Chemistry of Silica and Silicates; Cornell University Press: New York, NY, USA, 1955. [Google Scholar]
- Bergna, H.E.; Roberts, W.O. Colloidal Silica: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2005; Volume 131. [Google Scholar]
- Iler, K.R. The Chemistry of Silica: Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1979. [Google Scholar]
- Reches, Y. Nanoparticles as Concrete Additives: Review and Perspectives. Constr. Build. Mater. 2018, 175, 483–495. [Google Scholar] [CrossRef]
- Vrij, A.; Sonntag, H.; Tezak, B.; Kitchener, J.A.; Matijevic, E.; Mysels, K.J.; Overbeek, J.T.G.; Corkill, J.M.; Goodman, J.F.; Fowkes, F.M.; et al. General Discussion. Discuss. Faraday Soc. 1966, 42, 60–68. [Google Scholar] [CrossRef]
- Iler, R.K. Coagulation of Colloidal Silica by Calcium Ions, Mechanism, and Effect of Particle Size. J. Coll. Interface Sci. 1975, 53, 476–488. [Google Scholar] [CrossRef]
- Baxter, S.; Bryant, K.C. 578. Silica Sols. Part III. Accelerated Gelation, and Particle Size. J. Chem. Soc. 1952, 3024–3027. [Google Scholar] [CrossRef]
- Tadros, T.F.; Lyklema, J. The Electrical Double Layer on Silica in the Presence of Bivalent Counter-Ions. J. Electroanal. Chem. Interfacial Electrochem. 1969, 22, 1–7. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential–What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Ball, S. Colloidal Gold: The Gold Standard for Drug Delivery? Drug Dev. Deliv. 2015, 15, 32–35. [Google Scholar]
- Carpenter, D.K. Dynamic Light Scattering with Applications to Chemistry, Biology, and Physics. J. Chem. Educ. 1977, 54, A430. [Google Scholar] [CrossRef]
- Koppel, D.E. Analysis of Macromolecular Polydispersity in Intensity Correlation Spectroscopy: The Method of Cumulants. J. Chem. Phys. 1972, 57, 4814–4820. [Google Scholar] [CrossRef]
- Nägele, E. The Zeta-Potential of Cement. Cem. Concr. Res. 1985, 15, 453–462. [Google Scholar] [CrossRef]
- Malvern Instruments Ltd. Zetasizer Nano Series User Manual; MAN0317; Malvern Instruments Ltd.: Malvern, UK, 2004. [Google Scholar]
- Kitamura, R.; Pilon, L.; Jonasz, M. Optical Constants of Silica Glass from Extreme Ultraviolet to Far Infrared at near Room Temperature. Appl. Opt. 2007, 46, 8118–8133. [Google Scholar] [CrossRef]
- Schröfl, C.; Gruber, M.; Plank, J. Preferential Adsorption of Polycarboxylate Superplasticizers on Cement and Silica Fume in Ultra-High Performance Concrete (UHPC). Cem. Concr. Res. 2012, 42, 1401–1408. [Google Scholar] [CrossRef]
- Plank, J.; Schroefl, C.; Gruber, M.; Lesti, M.; Sieber, R. Effectiveness of Polycarboxylate Superplasticizers in Ultra-High Strength Concrete: The Importance of PCE Compatibility with Silica Fume. J. Adv. Concr. Technol. 2009, 7, 5–12. [Google Scholar] [CrossRef]



| Property | NS-5 | NS-20a | NS-20b | NS-75 |
|---|---|---|---|---|
| Particle Size (nm) | 5 | 20 | 20 | 75 |
| Surface Area (m2/g) | 600 | 150 | 150 | 40 |
| % SiO2 | 15 | 34 | 50 | 40 |
| pH | 9.0 | 2.8 | 9.0 | 8.4 |
| Specific Gravity | 1.09 | 1.23 | 1.39 | 1.29 |
| Viscosity (cP) | <10 | <10 | 55 | 10 |
| Stabilizing Ion | Ammonium | — | Sodium | Sodium |
| % Na2O | 0.02 | 0.04 | 0.40 | 0.30 |
| 2% Solid NS | |||||
|---|---|---|---|---|---|
| pH Alteration | Molarity (mol/L) | Ca(NO3)2 | Molarity (mol/L) | Pore Solution | Molarity of KOH (mol/L) |
| KOH0 | 0 | Ca0 | 0 | PS0 | 0 |
| PS1 | 9.77 × 10−5 | ||||
| PS2 | 1.95 × 10−4 | ||||
| Ca1 | 3.13 × 10−3 | PS3 | 7.81 × 10−4 | ||
| Ca2 | 6.25 × 10−3 | PS4 | 1.56 × 10−3 | ||
| KOH1 | 3.13 × 10−3 | Ca3 | 1.25 × 10−2 | PS5 | 3.13 × 10−3 |
| KOH2 | 6.25 × 10−3 | Ca4 | 2.50 × 10−2 | PS6 | 6.25 × 10−3 |
| KOH3 | 1.25 × 10−3 | Ca5 | 5.00 × 10−2 | PS7 | 1.25 × 10−2 |
| KOH4 | 2.50 × 10−3 | Ca6 | 1.00 × 10−1 | PS8 | 2.50 × 10−2 |
| KOH5 | 5.00 × 10−3 | Ca7 | 2.00 × 10−1 | PS9 | 5.00 × 10−2 |
| KOH6 | 1.00 × 10−3 | Ca8 | 4.00 × 10−1 | PS10 | 1.00 × 10−1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hendrix, D.; McKeon, J.; Wille, K. Behavior of Colloidal Nanosilica in an Ultrahigh Performance Concrete Environment Using Dynamic Light Scattering. Materials 2019, 12, 1976. https://doi.org/10.3390/ma12121976
Hendrix D, McKeon J, Wille K. Behavior of Colloidal Nanosilica in an Ultrahigh Performance Concrete Environment Using Dynamic Light Scattering. Materials. 2019; 12(12):1976. https://doi.org/10.3390/ma12121976
Chicago/Turabian StyleHendrix, Douglas, Jessica McKeon, and Kay Wille. 2019. "Behavior of Colloidal Nanosilica in an Ultrahigh Performance Concrete Environment Using Dynamic Light Scattering" Materials 12, no. 12: 1976. https://doi.org/10.3390/ma12121976
APA StyleHendrix, D., McKeon, J., & Wille, K. (2019). Behavior of Colloidal Nanosilica in an Ultrahigh Performance Concrete Environment Using Dynamic Light Scattering. Materials, 12(12), 1976. https://doi.org/10.3390/ma12121976





