Hydrogen Storage for Mobility: A Review
Abstract
1. Introduction
2. Hydrogen for Mobility
2.1. Overall Efficiency
2.2. Costs of Battery vs. Fuel Cell
2.3. Practical Advantages of Fuel Cells
3. Ideal Storage Method
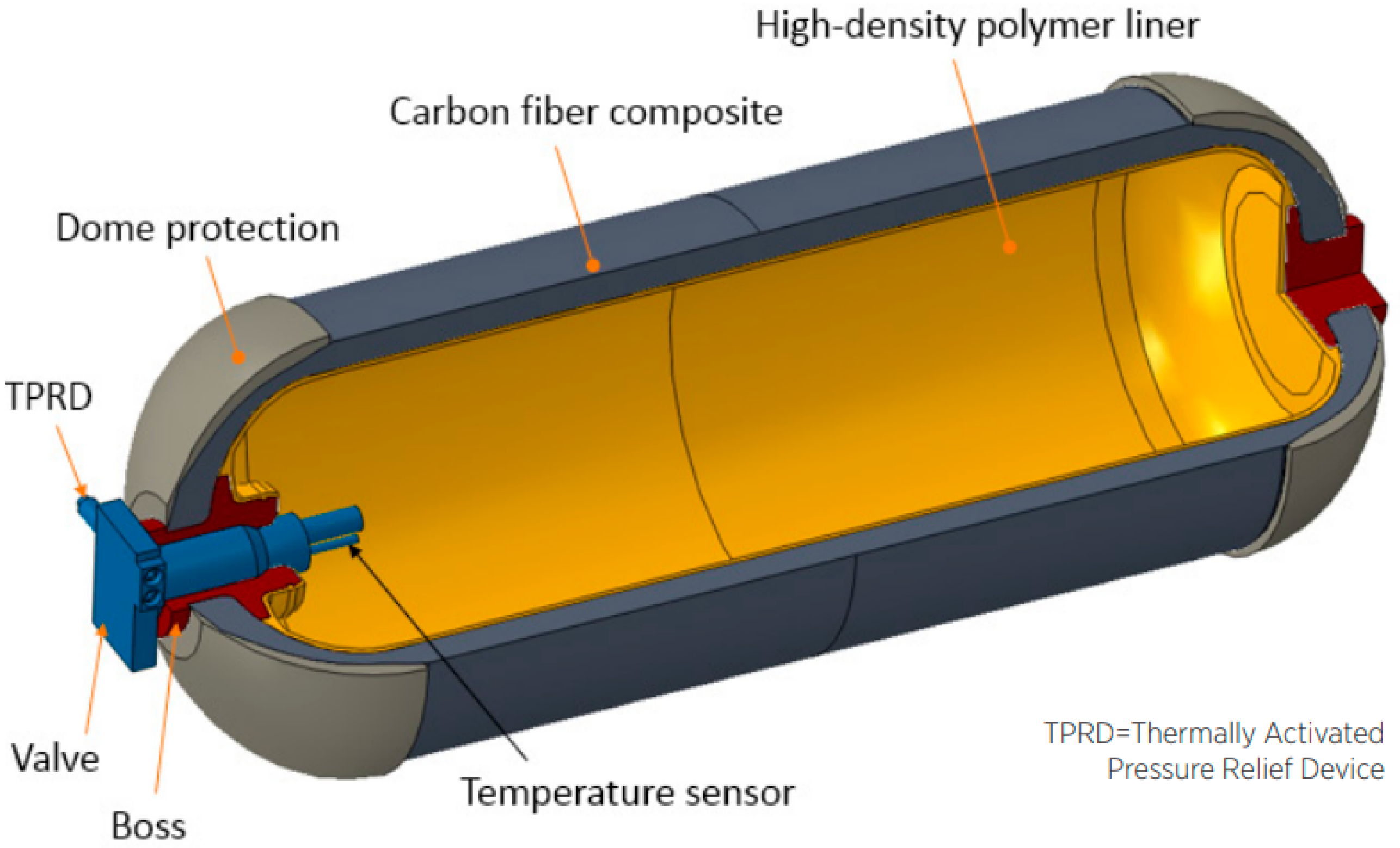
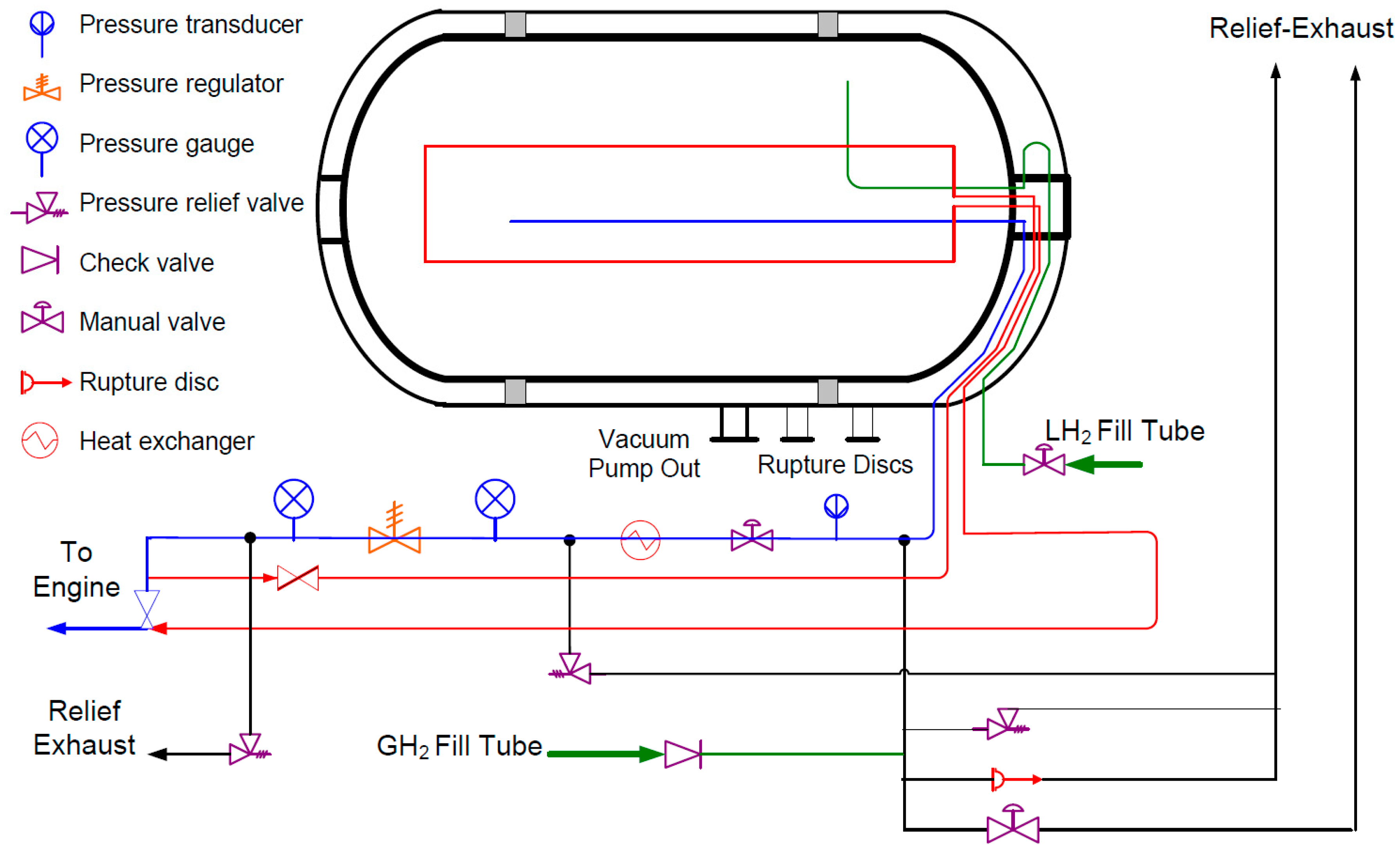
4. Present Industry Choice: Compressed Gas
5. Other Storage Methods
5.1. Liquid Hydrogen
5.2. Cold/Cryo Compression
5.3. Metal–Organic Framework
5.4. Carbon Nanostructures
5.5. Metal Hydrides
5.6. Metal Borohydrides
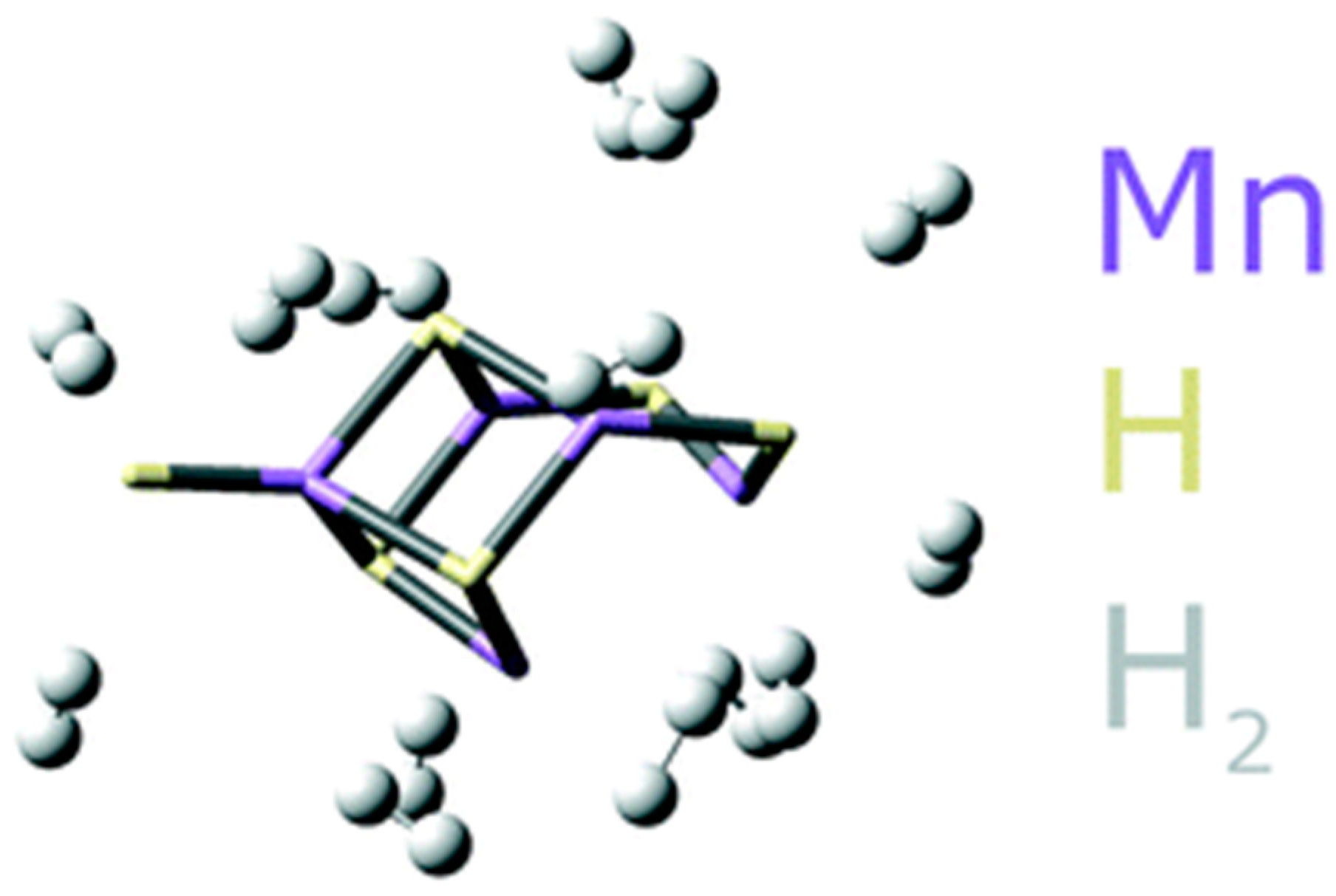
5.7. Kubas-Type Hydrogen
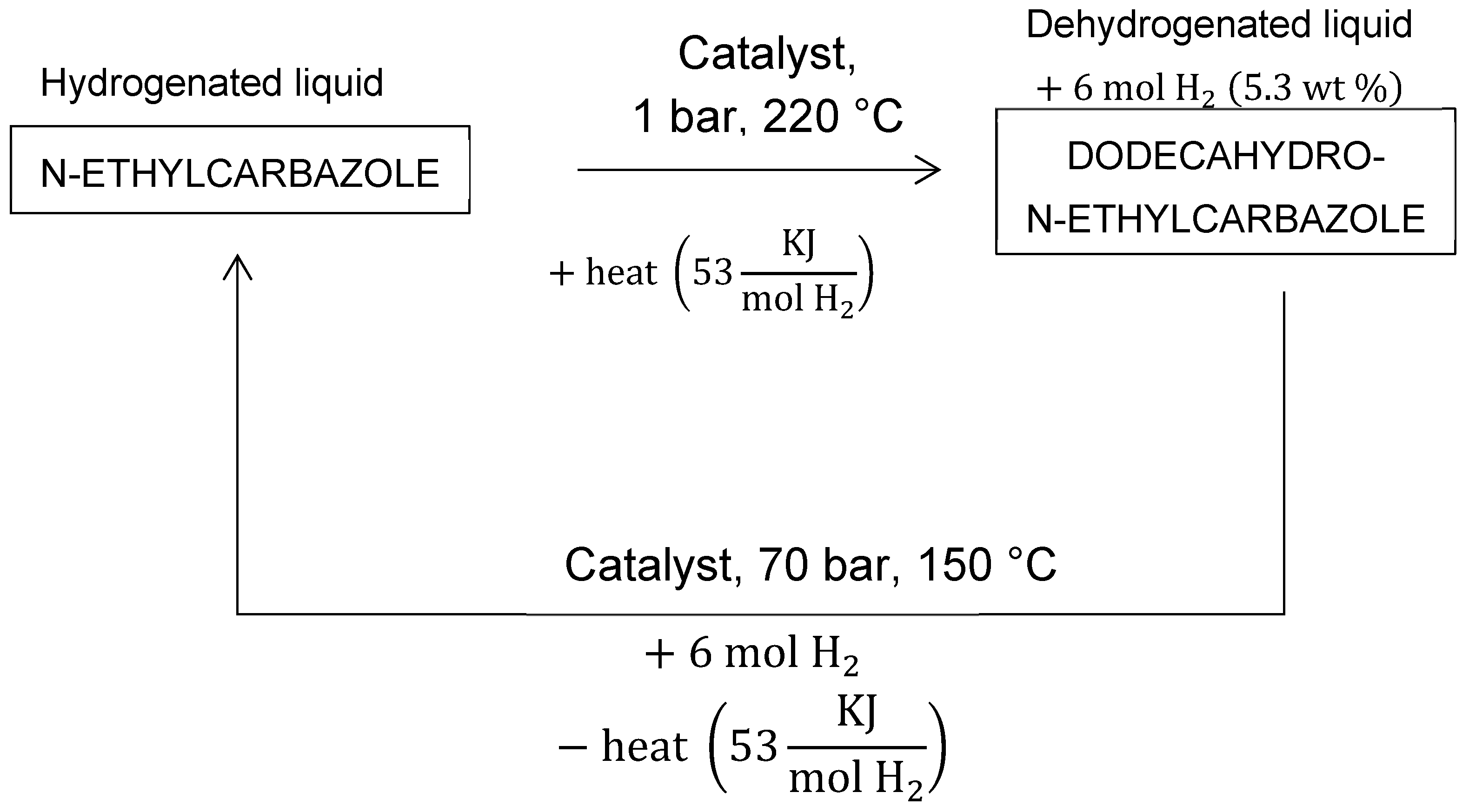
5.8. Liquid Organic Hydrogen Carriers
5.9. Chemical Hydrogen
6. Overview
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Stocker, T.F.; Qin, D.; Plattner, G.-K.; Tignor, M.; Allen, S.K.; Boschung, J.; Nauels, A.; Xia, Y.; Bex, V.; Midgley, P.M. IPCC, 2013: Summary for Policymakers. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2013. [Google Scholar]
- Walsh, B.S.; Parratt, S.R.; Hoffmann, A.A.; Atkinson, D.; Snook, R.R.; Bretman, A.; Price, T.A.R. The Impact of Climate Change on Fertility. Trends Ecol. Evol. 2019, 34, 249–259. [Google Scholar] [CrossRef] [PubMed]
- CaraDonna, P.J.; Cunningham, J.L.; Iler, A.M. Experimental warming in the field delays phenology and reduces body mass, fat content and survival: Implications for the persistence of a pollinator under climate change. Funct. Ecol. 2018, 32, 2345–2356. [Google Scholar] [CrossRef]
- Hernandez-Ochoa, I.M.; Asseng, S.; Kassie, B.T.; Xiong, W.; Robertson, R.; Pequeno, D.N.L.; Sonder, K.; Reynolds, M.; Babar, M.A.; Milan, A.M.; et al. Climate change impact on Mexico wheat production. Agric. For. Meteorol. 2018, 263, 373–387. [Google Scholar] [CrossRef]
- Kontgis, C.; Schneider, A.; Ozdogan, M.; Kucharik, C.; Tri, V.P.D.; Duc, N.H.; Schatz, J. Climate change impacts on rice productivity in the Mekong River Delta. Appl. Geogr. 2019, 102, 71–83. [Google Scholar] [CrossRef]
- Raymundo, R.; Asseng, S.; Robertson, R.; Petsakos, A.; Hoogenboom, G.; Quiroz, R.; Hareau, G.; Wolf, J. Climate change impact on global potato production. Eur. J. Agron. 2018, 100, 87–98. [Google Scholar] [CrossRef]
- Mazdiyasni, O.; AghaKouchak, A. Substantial increase in concurrent droughts and heatwaves in the United States. Proc. Natl. Acad. Sci. USA 2015, 112, 11484–11489. [Google Scholar] [CrossRef]
- Ogburn, S.P. Indian Monsoons Are Becoming More Extreme. Scientific American. 2014. Available online: https://www.scientificamerican.com/article/indian-monsoons-are-becoming-more-extreme/ (accessed on 13 February 2019).
- Warner, K.; Ehrhart, C.; de Sherbinin, A.; Adamo, S.; Chai-Onn, T. In Search of Shelter: Mapping the Effects of Climate Change on Human Migration and Displacement; Climate Change CARE International: London, UK, 2009; 26p. [Google Scholar]
- Black, R.; Bennett, S.R.; Thomas, S.M.; Beddington, J.R. Climate change: Migration as adaptation. Nature 2011, 478, 447–449. [Google Scholar] [CrossRef] [PubMed]
- McLeman, R.; Smit, B. Migration as an Adaptation to Climate Change. Clim. Chang. 2006, 76, 31–53. [Google Scholar] [CrossRef]
- Reuveny, R. Climate change-induced migration and violent conflict. Political Geogr. 2007, 26, 656–673. [Google Scholar] [CrossRef]
- Nordås, R.; Gleditsch, N.P. Climate change and conflict. Political Geogr. 2007, 26, 627–638. [Google Scholar] [CrossRef]
- Barnett, J. Security and climate change. Glob. Environ. Chang. 2003, 13, 7–17. [Google Scholar] [CrossRef]
- AFHYPAC and FNCCR. Déployer les stations hydrogène dans votre territoire. 2018. Available online: http://www.afhypac.org/documents/divers/GUIDE-STATION-HYDROGENE-WEB.pdf (accessed on 13 February 2019).
- United States Environmental Protection Agency. Inventory of U.S. Greenhouse Gas Emissions and Sinks: 1990–2015. 2017. Available online: https://www.epa.gov/sites/production/files/2017-02/documents/2017_complete_report.pdf (accessed on 13 February 2019).
- BP Statistical Review of World Energy. 2018. Available online: https://www.bp.com/content/dam/bp/en/corporate/pdf/energy-economics/statistical-review/bp-stats-review-2018-full-report.pdf (accessed on 12 October 2018).
- Saito, S. Role of nuclear energy to a future society of shortage of energy resources and global warming. J. Nucl. Mater. 2010, 398, 1–9. [Google Scholar] [CrossRef]
- Salameh, M.G. Can renewable and unconventional energy sources bridge the global energy gap in the 21st century? Appl. Energy 2003, 75, 33–42. [Google Scholar] [CrossRef]
- Friedrichs, J. Global energy crunch: How different parts of the world would react to a peak oil scenario. Energy Policy 2010, 38, 4562–4569. [Google Scholar] [CrossRef]
- Lund, H.; Mathiesen, B.V. Energy system analysis of 100% renewable energy systems—The case of Denmark in years 2030 and 2050. Energy 2009, 34, 524–531. [Google Scholar] [CrossRef]
- Singer, S.; Denruyter, J.-P.; Yener, D. The Energy Report: 100% Renewable Energy by 2050. In Towards 100% Renewable Energy; Springer: Cham, Switzerland, 2017. [Google Scholar]
- Züttel, A.; Borgschulte, A.; Schlapbach, L. Hydrogen as a Future Energy Carrier; John Wiley & Sons: Hoboken, NJ, USA, 2011; p. 441. [Google Scholar]
- Winter, C.-J.; Nitsch, J. Hydrogen as an Energy Carrier; Springer: Berlin/Heidelberg, Germany, 1988; p. 380. [Google Scholar]
- Mazloomi, K.; Gomes, C. Hydrogen as an energy carrier: Prospects and challenges. Renew. Sustain. Energy Rev. 2012, 16, 3024–3033. [Google Scholar] [CrossRef]
- Cipriani, G.; Di Dio, V.; Genduso, F.; La Cascia, D.; Liga, R.; Miceli, R.; Galluzzo, G.R. Perspective on hydrogen energy carrier and its automotive applications. Int. J. Hydrogen Energy 2014, 39, 8482–8494. [Google Scholar] [CrossRef]
- Züttel, A.; Remhof, A.; Borgschulte, A.; Friedrichs, O. Hydrogen: The future energy carrier. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2010, 368, 3329–3342. [Google Scholar] [CrossRef]
- Nakicenovic, N.; Grübler, A.; McDonald, A. Global Energy Perspectives; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Arnold, R. Are Renewable Energy Cost Reductions Coming to an End? What Next? 2018. Available online: http://energypost.eu/are-renewable-energy-cost-reductions-coming-to-an-end-what-next/ (accessed on 13 February 2019).
- Kikuchi, Y.; Ichikawa, T.; Sugiyama, M.; Koyama, M. Battery-assisted low-cost hydrogen production from solar energy: Rational target setting for future technology systems. Int. J. Hydrogen Energy 2019, 44, 1451–1465. [Google Scholar] [CrossRef]
- Dincer, I.; Ratlamwala, T. Development of novel renewable energy based hydrogen production systems: A comparative study. Energy Convers. Manag. 2013, 72, 77–87. [Google Scholar] [CrossRef]
- Nafchi, F.M.; Baniasadi, E.; Afshari, E.; Javani, N. Performance assessment of a solar hydrogen and electricity production plant using high temperature PEM electrolyzer and energy storage. Int. J. Hydrogen Energy 2018, 43, 5820–5831. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Durbin, D.; Malardier-Jugroot, C. Review of hydrogen storage techniques for on board vehicle applications. Int. J. Hydrogen Energy 2013, 38, 14595–14617. [Google Scholar] [CrossRef]
- Wang, L.; Yang, R.T. New sorbents for hydrogen storage by hydrogen spillover–A review. Energy Environ. Sci. 2008, 1, 268–279. [Google Scholar] [CrossRef]
- Jain, I.; Jain, P.; Jain, A. Novel hydrogen storage materials: A review of lightweight complex hydrides. J. Alloy Compd. 2010, 503, 303–339. [Google Scholar] [CrossRef]
- Fakioğlu, E.; Yürüm, Y.; Nejat Veziroğlu, T. A review of hydrogen storage systems based on boron and its compounds. Int. J. Hydrogen Energy 2004, 29, 1371–1376. [Google Scholar] [CrossRef]
- Marbán, G.; Valdés-Solís, T. Towards the hydrogen economy? Int. J. Hydrogen Energy 2007, 32, 1625–1637. [Google Scholar] [CrossRef]
- Muradov, N.; Vezirolu, T. From hydrocarbon to hydrogen?carbon to hydrogen economy. Int. J. Hydrogen Energy 2005, 30, 225–237. [Google Scholar] [CrossRef]
- Barreto, L.; Makihira, A.; Riahi, K. The hydrogen economy in the 21st century: A sustainable development scenario. Int. J. Hydrogen Energy 2003, 28, 267–284. [Google Scholar] [CrossRef]
- Penner, S.S. Steps toward the hydrogen economy. Energy 2006, 31, 33–43. [Google Scholar] [CrossRef]
- Bossel, U. Does a Hydrogen Economy Make Sense? Proc. IEEE 2006, 94, 1826–1837. [Google Scholar] [CrossRef]
- ITM Power. Hydrogen Refuelling Infrastructure. 2017. Available online: http://www.level-network.com/wp-content/uploads/2017/02/ITM-Power.pdf (accessed on 18 March 2019).
- Gardiner, M.; Satyapal, S. Energy Requirements for Hydrogen Gas Compression and Liquefaction as Related to Vehicle Storage Needs. DOE Hydrogen and Fuel Cells Program Record; 2009. Available online: https://www.hydrogen.energy.gov/pdfs/9013_energy_requirements_for_hydrogen_gas_compression.pdf (accessed on 13 June 2019).
- Fuel Cell Technologies Office. Fuel Cells. 2015. Available online: https://www.energy.gov/sites/prod/files/2015/11/f27/fcto_fuel_cells_fact_sheet.pdf (accessed on 18 March 2019).
- Office of Energy Efficiency and Renewable Energy. All-Electric Vehicles. Available online: https://www.fueleconomy.gov/feg/evtech.shtml (accessed on 18 March 2019).
- Curry, C. Lithium-ion Costs and Market. 2017. Available online: https://data.bloomberglp.com/bnef/sites/14/2017/07/BNEF-Lithium-ion-battery-costs-and-market.pdf (accessed on 13 June 2019).
- Tajitsu, N.; Shiraki, M. Toyota Plans to Expand Production, Shrink Cost of Hydrogen Fuel Cell Vehicles, in Business News; Reuters: London, UK, 2018. [Google Scholar]
- Brown, E.G. Joint Agency Staff Report on Assembly Bill 8: Assessment of Time and Cost Needed to Attain 100 Hydrogen Refueling Stations in California; California Energy Commission: Sacramento, CA, USA, 2015.
- Thomas, C. Fuel cell and battery electric vehicles compared. Int. J. Hydrogen Energy 2009, 34, 6005–6020. [Google Scholar] [CrossRef]
- Heraeus, Heraeus Precious Appraisal. 2018. Available online: https://www.heraeus.com/media/media/hpm/doc_hpm/precious_metal_update/en_6/Appraisal_20190429.pdf (accessed on 13 June 2019).
- ToolBox, T.E. Fossil and Alternative Fuels-Energy Content. Available online: https://www.engineeringtoolbox.com/fossil-fuels-energy-content-d_1298.html (accessed on 13 February 2019).
- AFHYPAC. Les données de base physico-chimiques sur l’hydrogène. Mémento de l’Hydrogène. 2013. Available online: http://www.afhypac.org/documents/tout-savoir/fiche_1.2_donnees_physicochimiques_rev.mars_2013.pdf (accessed on 13 February 2019).
- International Group of Liquefied Natural Gas Importers. Basic Properties of LNG. LNG Information Papers. Available online: http://www.kosancrisplant.com/media/5648/1-lng_basics_82809_final_hq.pdf (accessed on 13 February 2019).
- Mori, D.; Hirose, K. Recent challenges of hydrogen storage technologies for fuel cell vehicles. Int. J. Hydrogen Energy 2009, 34, 4569–4574. [Google Scholar] [CrossRef]
- Crabtree, R.H. Hydrogen storage in liquid organic heterocycles. Energy Environ. Sci. 2008, 1, 134. [Google Scholar] [CrossRef]
- Eberle, U.; Arnold, G.; Von Helmolt, R. Hydrogen storage in metal–hydrogen systems and their derivatives. J. Power Sources 2006, 154, 456–460. [Google Scholar] [CrossRef]
- Aardahl, C.; Rassat, S. Overview of systems considerations for on-board chemical hydrogen storage. Int. J. Hydrogen Energy 2009, 34, 6676–6683. [Google Scholar] [CrossRef]
- Zhang, J.; Fisher, T.S.; Ramachandran, P.V.; Gore, J.P.; Mudawar, I. A Review of Heat Transfer Issues in Hydrogen Storage Technologies. J. Heat Transf. 2005, 127, 1391–1399. [Google Scholar] [CrossRef]
- U.S. Department of Energy. Fuel Cell Technologies Office Multi-Year Research, Development, and Demonstration Plan. 2012. Available online: https://www.energy.gov/eere/fuelcells/downloads/fuel-cell-technologies-office-multi-year-research-development-and-22 (accessed on 6 February 2019).
- SAE International. SAE J2600 Compressed Hydrogen Surface Vehicle Fueling Connection Devices. 2015. Available online: https://www.sae.org/standards/content/j2600_201211/ (accessed on 13 February 2019).
- Yamashita, A.; Kondo, M.; Goto, S.; Ogami, N. Development of High-Pressure Hydrogen Storage System for the Toyota “Mirai”. SAE Tech. Pap. Ser. 2015. [Google Scholar] [CrossRef]
- Legault, M. Pressure Vessel Tank Types. 2012. Available online: https://www.compositesworld.com/articles/pressure-vessel-tank-types (accessed on 13 February 2019).
- Law, K.; Rosenfeld, J. Cost Analyses of Hydrogen Storage Materials and On-Board Systems. 2011. Available online: https://www.hydrogen.energy.gov/pdfs/review11/st002_law_2011_o.pdf (accessed on 18 March 2019).
- Hua, T.; Ahluwalia, R.; Peng, J.-K.; Kromer, M.; Lasher, S.; McKenney, K.; Law, K.; Sinha, J. Technical Assessment of Compressed Hydrogen Storage Tank Systems for Automotive Applications; Office of Scientific and Technical Information (OSTI): Oak Ridge, TN, USA, 2010.
- Fuel Cell Technologies Office, Hydrogen Storage. 2017. Available online: https://www.energy.gov/sites/prod/files/2017/03/f34/fcto-h2-storage-fact-sheet.pdf (accessed on 13 June 2019).
- Toyota. 2017 Mirai Product Information. 2017. Available online: https://ssl.toyota.com/mirai/assets/core/Docs/Mirai%20Specs.pdf (accessed on 13 February 2019).
- Gye, H.-R.; Seo, S.-K.; Bach, Q.-V.; Ha, D.; Lee, C.-J. Quantitative risk assessment of an urban hydrogen refueling station. Int. J. Hydrogen Energy 2019, 44, 1288–1298. [Google Scholar] [CrossRef]
- Alperowicz, N. Ube Industries’ New Nylon Resin to be Used in Toyota Fuel-Cell Vehicles. 2014. Available online: chemweek.com (accessed on 13 June 2019).
- Wyckaert, P.; Nadeau, S.; Bouzid, H.A. Analysis of risks of pressure vessels. In Proceedings of the Kongress der Gesellschaft für Arbeitswissenschaft, Zurich, Switzerland, 15–17 February 2017. [Google Scholar]
- Rossini, F.D. Report on International Practical Temperature Scale of 1968. J. Chem. Thermodyn. 1970, 2, 447–459. [Google Scholar] [CrossRef]
- Xu, W.; Li, Q.; Huang, M. Design and analysis of liquid hydrogen storage tank for high-altitude long-endurance remotely-operated aircraft. Int. J. Hydrogen Energy 2015, 40, 16578–16586. [Google Scholar] [CrossRef]
- Babac, G.; Şişman, A.; Çimen, T. Two-dimensional thermal analysis of liquid hydrogen tank insulation. Int. J. Hydrogen Energy 2009, 34, 6357–6363. [Google Scholar] [CrossRef]
- Petitpas, G. Simulation of boil-off losses during transfer at a LH2 based hydrogen refueling station. Int. J. Hydrogen Energy 2018, 43, 21451–21463. [Google Scholar] [CrossRef]
- Gürsu, S.; Lordgooei, M.; Sherif, S.; Veziroglu, T. An optimization study of liquid hydrogen boil-off losses. Int. J. Hydrogen Energy 1992, 17, 227–236. [Google Scholar] [CrossRef]
- Zhou, L. Progress and problems in hydrogen storage methods. Renew. Sustain. Energy Rev. 2005, 9, 395–408. [Google Scholar] [CrossRef]
- Amos, W.A. Costs of Storing and Transporting Hydrogen; National Technical Information Service (NTIS): Springfield, VA, USA, 1998. [Google Scholar]
- Colozza, A.J. Hydrogen Storage for Aircraft Applications Overview; NASA: Washington, DC, USA, 2002.
- Sirosh, N. Hydrogen Composite Tank Program. In Proceedings of the 2002 U.S. DOE Hydrogen Program Review, Golden, CO, USA, 6–10 May 2002. [Google Scholar]
- Hydrogen Delivery Technical Team Roadmap. 2017. Available online: energy.gov (accessed on 13 June 2019).
- Krasae-In, S.; Stang, J.H.; Neksa, P. Development of large-scale hydrogen liquefaction processes from 1898 to 2009. Int. J. Hydrogen Energy 2010, 35, 4524–4533. [Google Scholar] [CrossRef]
- Petitpas, G.; Simon, A.J. Liquid Hydrogen Infrastructure Analysis; Lawrence Livermore National Laboratory: Livermore, CA, USA, 2017.
- Meneghelli, B.; Tamburello, D.; Fesmire, J.; Swanger, A. Integrated Insulation System for Automotive Cryogenic; U.S. DOE Hydrogen and Fuel Cells Program, 2017. Available online: https://www.hydrogen.energy.gov/pdfs/progress17/iv_d_4_meneghelli_2017.pdf (accessed on 13 June 2019).
- D1655-18a; ASTM International: West Conshohocken, PA, USA, 2018.
- Crabtree, G.W.; Dresselhaus, M.S.; Buchanan, M.V. The Hydrogen Economy. Phys. Today 2004, 57, 39–44. [Google Scholar] [CrossRef]
- Moreno-Blanco, J.; Petitpas, G.; Espinosa-Loza, F.; Elizalde-Blancas, F.; Martinez-Frias, J.; Aceves, S.M. The fill density of automotive cryo-compressed hydrogen vessels. Int. J. Hydrogen Energy 2019, 44, 1010–1020. [Google Scholar] [CrossRef]
- Kunze, K.; Oliver, K. Cryo-Compressed Hydrogen Storage; BMW Group: Munich, Germany, 2012. [Google Scholar]
- Ahluwalia, R.K.; Peng, J.-K.; Hua, T.Q. Cryo-compressed hydrogen storage. In Compendium of Hydrogen Energy; Woodhead Publishing: Cambridge, UK, 2016; pp. 119–145. [Google Scholar]
- Argonne National Laboratory. Technical Assessment of Cryo-Compressed Hydrogen Storage Tank Systems for Automotive Applications; U.S. Department of Energy: Oak Ridge, TN, USA, 2009.
- Ahluwalia, R.; Peng, J.; Roh, H.; Hua, T.; Houchins, C.; James, B. Supercritical cryo-compressed hydrogen storage for fuel cell electric buses. Int. J. Hydrogen Energy 2018, 43, 10215–10231. [Google Scholar] [CrossRef]
- Reed, R.; Golda, M. Cryogenic properties of unidirectional composites. Cryogenics 1994, 34, 909–928. [Google Scholar] [CrossRef]
- Ahluwalia, R.K.; Hua, T.Q.; Peng, J.-K.; Lasher, S.; McKenney, K.; Sinha, J.; Llc, T. Nuclear Engineering Division Technical assessment of cryo-compressed hydrogen storage tank systems for automotive applications. Int. J. Hydrogen Energy 2010, 35, 4171–4184. [Google Scholar] [CrossRef]
- Aceves, S.M.; Petitpas, G.; Espinosa-Loza, F.; Matthews, M.J.; Ledesma-Orozco, E. Safe, long range, inexpensive and rapidly refuelable hydrogen vehicles with cryogenic pressure vessels. Int. J. Hydrogen Energy 2013, 38, 2480–2489. [Google Scholar] [CrossRef]
- He, Y.; Chen, F.; Li, B.; Qian, G.; Zhou, W.; Chen, B. Porous metal–organic frameworks for fuel storage. Coord. Chem. Rev. 2018, 373, 167–198. [Google Scholar] [CrossRef]
- Zhao, R.; Liang, Z.; Zou, R.; Xu, Q. Metal-Organic Frameworks for Batteries. Joule 2018, 2, 2235–2259. [Google Scholar] [CrossRef]
- Mehtab, T.; Yasin, G.; Arif, M.; Shakeel, M.; Korai, R.M.; Nadeem, M.; Muhammad, N.; Lu, X. Metal-organic frameworks for energy storage devices: Batteries and supercapacitors. J. Energy Storage 2019, 21, 632–646. [Google Scholar] [CrossRef]
- Qiu, J.; Zhang, X.; Feng, Y.; Zhang, X.; Wang, H.; Yao, J. Modified metal-organic frameworks as photocatalysts. Appl. Catal. B Environ. 2018, 231, 317–342. [Google Scholar] [CrossRef]
- Lan, G.; Ni, K.; Lin, W. Nanoscale metal–organic frameworks for phototherapy of cancer. Coord. Chem. Rev. 2019, 379, 65–81. [Google Scholar] [CrossRef]
- Rosi, N.L.; Eckert, J.; Eddaoudi, M.; Vodak, D.T.; Kim, J.; O’Keeffe, M.; Yaghi, O.M. Hydrogen Storage in Microporous Metal-Organic Frameworks. Science 2003, 300, 1127–1129. [Google Scholar] [CrossRef]
- Ahmed, A.; Liu, Y.; Purewal, J.; Tran, L.D.; Wong-Foy, A.G.; Veenstra, M.; Matzger, A.J.; Siegel, D.J. Balancing gravimetric and volumetric hydrogen density in MOFs. Energy Environ. Sci. 2017, 10, 2459–2471. [Google Scholar] [CrossRef]
- Choi, J.-S.; Son, W.-J.; Kim, J.; Ahn, W.-S. Metal–organic framework MOF-5 prepared by microwave heating: Factors to be considered. Microporous Mesoporous Mater. 2008, 116, 727–731. [Google Scholar] [CrossRef]
- Koizumi, K.; Nobusada, K.; Boero, M. Hydrogen storage mechanism and diffusion in metal–organic frameworks. Phys. Chem. Chem. Phys. 2019, 21, 7756–7764. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Wei, Z.; Deng, S. Equilibrium, kinetics and enthalpy of hydrogen adsorption in MOF-177. Int. J. Hydrogen Energy 2008, 33, 7479–7488. [Google Scholar] [CrossRef]
- Yuan, S.; Feng, L.; Wang, K.; Pang, J.; Bosch, M.; Lollar, C.; Sun, Y.; Qin, J.; Yang, X.; Zhang, P.; et al. Stable Metal-Organic Frameworks: Design, Synthesis, and Applications. Adv. Mater. 2018, 30, e1704303. [Google Scholar] [CrossRef] [PubMed]
- Wikipedia Contributors. MOF-5. Wikipedia, The Free Encyclopedia. Available online: https://de.wikipedia.org/wiki/MOF-5 (accessed on 13 February 2019).
- Chakraborty, A.; Kumar, S. Thermal management and desorption modeling of a cryo-adsorbent hydrogen storage system. Int. J. Hydrogen Energy 2013, 38, 3973–3986. [Google Scholar] [CrossRef]
- Richard, M.-A.; Benard, P.; Chahine, R. Gas adsorption process in activated carbon over a wide temperature range above the critical point. Part 1: Modified Dubinin-Astakhov model. Adsorpt 2009, 15, 43–51. [Google Scholar] [CrossRef]
- Richard, M.-A.; Bénard, P.; Chahine, R. Gas adsorption process in activated carbon over a wide temperature range above the critical point. Part 2: Conservation of mass and energy. Adsorption 2009, 15, 53–63. [Google Scholar] [CrossRef]
- Kumar, V.S.; Raghunathan, K.; Kumar, S. A lumped-parameter model for cryo-adsorber hydrogen storage tank. Int. J. Hydrogen Energy 2009, 34, 5466–5475. [Google Scholar] [CrossRef]
- Kumar, V.S.; Kumar, S. Generalized model development for a cryo-adsorber and 1-D results for the isobaric refueling period. Int. J. Hydrogen Energy 2010, 35, 3598–3609. [Google Scholar] [CrossRef]
- Ghosh, I.; Naskar, S.; Bandyopadhyay, S.S. Cryosorption storage of gaseous hydrogen for vehicular application–A conceptual design. Int. J. Hydrogen Energy 2010, 35, 161–168. [Google Scholar] [CrossRef]
- Hermosilla-Lara, G.; Momen, G.; Marty, P.; Le Neindre, B.; Hassouni, K. Hydrogen storage by adsorption on activated carbon: Investigation of the thermal effects during the charging process. Int. J. Hydrogen Energy 2007, 32, 1542–1553. [Google Scholar] [CrossRef]
- Momen, G.; Hermosilla, G.; Michau, A.; Pons, M.; Firdaous, M.; Marty, P.; Hassouni, K. Experimental and numerical investigation of the thermal effects during hydrogen charging in packed bed storage tank. Int. J. Heat Mass Transf. 2009, 52, 1495–1503. [Google Scholar] [CrossRef]
- Zhan, L.; Li, K.; Zhang, R.; Liu, Q.; Lü, C.; Ling, L. Improvements of the DA equation for application in hydrogen adsorption at supercritical conditions. J. Supercrit. Fluids 2004, 28, 37–45. [Google Scholar] [CrossRef]
- Paggiaro, R.; Michl, F.; Benard, P.; Polifke, W. Cryo-adsorptive hydrogen storage on activated carbon. II: Investigation of the thermal effects during filling at cryogenic temperatures. Int. J. Hydrogen Energy 2010, 35, 648–659. [Google Scholar] [CrossRef]
- Schütz, W.; Michl, F.; Polifke, W.; Paggiaro, R. Storage System for Storing a Medium and Method for Loading a Storage System with a Storage Medium and Emptying the Same Therefrom. U.S. Patent Application No. 10/578,172, 24 January 2008. [Google Scholar]
- Xiao, J.; Tong, L.; Deng, C.; Benard, P.; Chahine, R. Simulation of heat and mass transfer in activated carbon tank for hydrogen storage. Int. J. Hydrogen Energy 2010, 35, 8106–8116. [Google Scholar] [CrossRef]
- Vasiliev, L.L.; Kanonchik, L.E. Activated carbon fibres and composites on its base for high performance hydrogen storage system. Chem. Eng. Sci. 2010, 65, 2586–2595. [Google Scholar] [CrossRef]
- Huang, B.; Ni, Z.; Millward, A.; McGaughey, A.; Uher, C.; Kaviany, M.; Yaghi, O. Thermal conductivity of a metal-organic framework (MOF-5): Part II. Measurement. Int. J. Heat Mass Transf. 2007, 50, 405–411. [Google Scholar] [CrossRef]
- Hardy, B.; Tamburello, D.; Corgnale, C. Hydrogen storage adsorbent systems acceptability envelope. Int. J. Hydrogen Energy 2018, 43, 19528–19539. [Google Scholar] [CrossRef]
- Proch, S.; Herrmannsdörfer, J.; Kempe, R.; Kern, C.; Jess, A.; Seyfarth, L.; Senker, J. Pt@MOF-177: Synthesis, Room-Temperature Hydrogen Storage and Oxidation Catalysis. Chem. A Eur. J. 2019, 14, 8204–8212. [Google Scholar] [CrossRef]
- Rubio-Martinez, M.; Avci-Camur, C.; Thornton, A.W.; Imaz, I.; Maspoch, D.; Hill, M.R. New synthetic routes towards MOF production at scale. Chem. Soc. Rev. 2017, 46, 3453–3480. [Google Scholar] [CrossRef]
- DeSantis, D.; Mason, J.A.; James, B.D.; Houchins, C.; Long, J.R.; Veenstra, M. Techno-economic Analysis of Metal–Organic Frameworks for Hydrogen and Natural Gas Storage. Energy Fuels 2017, 31, 2024–2032. [Google Scholar] [CrossRef]
- Cheng, J.; Yuan, X.; Zhao, L.; Huang, D.; Zhao, M.; Dai, L.; Ding, R. GCMC simulation of hydrogen physisorption on carbon nanotubes and nanotube arrays. Carbon 2004, 42, 2019–2024. [Google Scholar] [CrossRef]
- Ariharan, A.; Viswanathan, B.; Nandhakumar, V. Nitrogen-incorporated carbon nanotube derived from polystyrene and polypyrrole as hydrogen storage material. Int. J. Hydrogen Energy 2018, 43, 5077–5088. [Google Scholar] [CrossRef]
- Kaskun, S.; Kayfeci, M. The synthesized nickel-doped multi-walled carbon nanotubes for hydrogen storage under moderate pressures. Int. J. Hydrogen Energy 2018, 43, 10773–10778. [Google Scholar] [CrossRef]
- Silambarasan, D.; Surya, V.; Iyakutti, K.; Asokan, K.; Vasu, V.; Kawazoe, Y.; Kandasami, A. Gamma (γ)-ray irradiated multi-walled carbon nanotubes (MWCNTs) for hydrogen storage. Appl. Surf. Sci. 2017, 418, 49–55. [Google Scholar] [CrossRef]
- Masika, E.; Mokaya, R. Preparation of ultrahigh surface area porous carbons templated using zeolite 13X for enhanced hydrogen storage. Prog. Nat. Sci. 2013, 23, 308–316. [Google Scholar] [CrossRef]
- Ahn, C. Enhanced Hydrogen Dipole Physisorption. 2006. Available online: https://www.hydrogen.energy.gov/pdfs/review06/stp_16_ahn.pdf (accessed on 25 March 2019).
- Broom, D.; Webb, C.; Fanourgakis, G.; Froudakis, G.; Trikalitis, P.; Hirscher, M. Concepts for improving hydrogen storage in nanoporous materials. Int. J. Hydrogen Energy 2019, 44, 7768–7779. [Google Scholar] [CrossRef]
- Shriniwasan, S.; Gor, N.; Tatiparti, S.S.V. Hydrogen Sorption Mechanism of Magnesium (Hydride). Mater. Today Proc. 2018, 5, 23235–23241. [Google Scholar] [CrossRef]
- Zuliani, D.; Reeson, D. Making Magnesium a More Cost and Environmentally Competitive Option. In Proceedings of the 9th International Conference on Magnesium Alloys and Their Applications, Vancouver, BC, Canada, 8–12 July 2012. [Google Scholar]
- Wu, G.; Zhang, J.; Li, Q.; Chou, K. A new model to describe absorption kinetics of Mg-based hydrogen storage alloys. Int. J. Hydrogen Energy 2011, 36, 12923–12931. [Google Scholar] [CrossRef]
- Li, J.; Zhou, C.; Fang, Z.Z.; Bowman, R.C., Jr.; Lu, J.; Ren, C. Isothermal hydrogenation kinetics of ball-milled nano-catalyzed magnesium hydride. Materials 2019, 5, 100227. [Google Scholar] [CrossRef]
- Vigelholm, B.; Kjøller, J.; Larsen, B.; Pedersen, A.S. Formation and decomposition of magnesium hydride. J. Less Common Met. 1983, 89, 135–144. [Google Scholar] [CrossRef]
- Paskevicius, M.; Sheppard, D.A.; Buckley, C.E. Thermodynamic Changes in Mechanochemically Synthesized Magnesium Hydride Nanoparticles. J. Am. Chem. Soc. 2010, 132, 5077–5083. [Google Scholar] [CrossRef] [PubMed]
- Zaluska, A.; Zaluski, L.; Ström–Olsen, J. Nanocrystalline magnesium for hydrogen storage. J. Alloy. Compd. 1999, 288, 217–225. [Google Scholar] [CrossRef]
- Yahya, M.; Ismail, M. Synergistic catalytic effect of SrTiO3 and Ni on the hydrogen storage properties of MgH2. Int. J. Hydrogen Energy 2018, 43, 6244–6255. [Google Scholar] [CrossRef]
- Bogdanović, B.; Felderhoff, M.; Pommerin, A.; Schüth, F.; Spielkamp, N. Advanced Hydrogen-Storage Materials Based on Sc-, Ce-, and Pr-Doped NaAlH4. Adv. Mater. 2006, 18, 1198–1201. [Google Scholar] [CrossRef]
- Rusman, N.; Dahari, M. A review on the current progress of metal hydrides material for solid-state hydrogen storage applications. Int. J. Hydrogen Energy 2016, 41, 12108–12126. [Google Scholar] [CrossRef]
- Garrier, S.; Chaise, A.; De Rango, P.; Marty, P.; Delhomme, B.; Fruchart, D.; Miraglia, S. MgH2 intermediate scale tank tests under various experimental conditions. Int. J. Hydrogen Energy 2011, 36, 9719–9726. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. Magnesium Hydride (CID=5486771). 2019. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5486771 (accessed on 13 February 2019).
- Sakintuna, B.; Lamaridarkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.-S.; Cho, Y.W.; Von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage–new perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Li, C.; Peng, P.; Zhou, D.; Wan, L. Research progress in LiBH4 for hydrogen storage: A review. Int. J. Hydrogen Energy 2011, 36, 14512–14526. [Google Scholar] [CrossRef]
- Li, H.-W.; Akiba, E.; Orimo, S.-I. Comparative study on the reversibility of pure metal borohydrides. J. Alloys Compd. 2013, 580, S292–S295. [Google Scholar] [CrossRef]
- Sun, T.; Xiao, F.; Tang, R.; Wang, Y.; Dong, H.; Li, Z.; Wang, H.; Liuzhang, O.; Zhu, M. Hydrogen storage performance of nano Ni decorated LiBH4 on activated carbon prepared through organic solvent. J. Alloys Compd. 2014, 612, 287–292. [Google Scholar] [CrossRef]
- Lombardo, L.; Yang, H.; Züttel, A. Study of borohydride ionic liquids as hydrogen storage materials. J. Energy Chem. 2019, 33, 17–21. [Google Scholar] [CrossRef]
- Salmi, T.; Russo, V. Reaction engineering approach to the synthesis of sodium borohydride. Chem. Eng. Sci. 2019, 199, 79–87. [Google Scholar] [CrossRef]
- Lee, J.; Shin, H.; Choi, K.S.; Lee, J.; Choi, J.-Y.; Yu, H.K. Carbon layer supported nickel catalyst for sodium borohydride (NaBH4) dehydrogenation. Int. J. Hydrogen Energy 2019, 44, 2943–2950. [Google Scholar] [CrossRef]
- Au, M.; Walters, R.T. Reversibility aspect of lithium borohydrides. Int. J. Hydrogen Energy 2010, 35, 10311–10316. [Google Scholar] [CrossRef]
- Skipper, C.V.J.; Hamaed, A.; Antonelli, D.M.; Kaltsoyannis, N. The Kubas interaction in M(II) (M = Ti, V, Cr) hydrazine-based hydrogen storage materials: A DFT study. Dalton Trans. 2012, 41, 8515–8523. [Google Scholar] [CrossRef]
- Kubas, G.J. Hydrogen activation on organometallic complexes and H2 production, utilization, and storage for future energy. J. Organomet. Chem. 2009, 694, 2648–2653. [Google Scholar] [CrossRef]
- Kubas, G.J. Metal–dihydrogen and σ-bond coordination: The consummate extension of the Dewar–Chatt–Duncanson model for metal–olefin π bonding. J. Organomet. Chem. 2001, 635, 37–68. [Google Scholar] [CrossRef]
- Morris, L.; Hales, J.J.; Trudeau, M.L.; Georgiev, P.; Embs, J.P.; Eckert, J.; Kaltsoyannis, N.; Antonelli, D.M.; Embs, J.P.P. A manganese hydride molecular sieve for practical hydrogen storage under ambient conditions. Energy Environ. Sci. 2019, 12, 1580–1591. [Google Scholar] [CrossRef]
- Hales, J.J.; Trudeau, M.L.; Antonelli, D.M.; Kaltsoyannis, N. Computational study of H2 binding to MH3 (M = Ti, V, or Cr). Dalton Trans. 2019, 48, 4921–4930. [Google Scholar] [CrossRef]
- Preuster, P.; Papp, C.; Wasserscheid, P. Liquid Organic Hydrogen Carriers (LOHCs): Toward a Hydrogen-free Hydrogen Economy. Acc. Chem. Res. 2017, 50, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Fogler, E.; Diskin-Posner, Y.; Iron, M.A.; Milstein, D. A novel liquid organic hydrogen carrier system based on catalytic peptide formation and hydrogenation. Nat. Commun. 2015, 6, 6859. [Google Scholar] [CrossRef] [PubMed]
- Taube, M.; Taube, P. A Liquid Organic Carrier of Hydrogen as a Fuel for Automobiles; Institut für Reaktorforschung: Würenlingen, Switzerland, 1979. [Google Scholar]
- Eblagon, K.M.; Rentsch, D.; Friedrichs, O.; Remhof, A.; Zuettel, A.; Ramirez-Cuesta, A.; Tsang, S.C.; Ramirez-Cuesta, A. Hydrogenation of 9-ethylcarbazole as a prototype of a liquid hydrogen carrier. Int. J. Hydrogen Energy 2010, 35, 11609–11621. [Google Scholar] [CrossRef]
- Geburtig, D.; Preuster, P.; Bösmann, A.; Müller, K.; Wasserscheid, P. Chemical utilization of hydrogen from fluctuating energy sources–Catalytic transfer hydrogenation from charged Liquid Organic Hydrogen Carrier systems. Int. J. Hydrogen Energy 2016, 41, 1010–1017. [Google Scholar] [CrossRef]
- He, T.; Pei, Q.; Chen, P. Liquid organic hydrogen carriers. J. Energy Chem. 2015, 24, 587–594. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P. Liquid Organic Hydrogen Carriers as an efficient vector for the transport and storage of renewable energy. Int. J. Hydrogen Energy 2012, 37, 18118–18132. [Google Scholar] [CrossRef]
- Teichmann, D.; Arlt, W.; Wasserscheid, P.; Freymann, R. A future energy supply based on Liquid Organic Hydrogen Carriers (LOHC). Energy Environ. Sci. 2011, 4, 2767–2773. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Smith, K.J. Structure sensitivity of dodecahydro-N-ethylcarbazole dehydrogenation over Pd catalysts. J. Catal. 2011, 279, 36–47. [Google Scholar] [CrossRef]
- Sotoodeh, F.; Zhao, L.; Smith, K.J. Kinetics of H2 recovery from dodecahydro-N-ethylcarbazole over a supported Pd catalyst. Appl. Catal. A Gen. 2009, 362, 155–162. [Google Scholar] [CrossRef]
- Gleichweit, C.; Schernich, S.; Lorenz, M.P.A.; Höfert, O.; Brückner, N.; Steinrück, H.-P.; Amende, M.; Zhao, W.; Wasserscheid, P.; Libuda, J.; et al. Dehydrogenation of Dodecahydro-N-ethylcarbazole on Pt(111). ChemSusChem 2013, 6, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Sobota, M.; Nikiforidis, I.; Amende, M.; Zanón, B.S.; Staudt, T.; Höfert, O.; Lykhach, Y.; Papp, C.; Hieringer, W.; Laurin, M.; et al. Dehydrogenation of Dodecahydro-N-ethylcarbazole on Pd/Al2O3 Model Catalysts. Chem. A Eur. J. 2011, 17, 11542–11552. [Google Scholar] [CrossRef] [PubMed]
- Amende, M.; Schernich, S.; Sobota, M.; Nikiforidis, I.; Hieringer, W.; Assenbaum, D.; Gleichweit, C.; Drescher, H.-J.; Papp, C.; Steinrück, H.-P.; et al. Dehydrogenation Mechanism of Liquid Organic Hydrogen Carriers: Dodecahydro- N -ethylcarbazole on Pd(111). Chem. A Eur. J. 2013, 19, 10854–10865. [Google Scholar] [CrossRef] [PubMed]
- Amende, M.; Gleichweit, C.; Werner, K.; Schernich, S.; Zhao, W.; Lorenz, M.P.A.; Höfert, O.; Papp, C.; Koch, M.; Wasserscheid, P.; et al. Model Catalytic Studies of Liquid Organic Hydrogen Carriers: Dehydrogenation and Decomposition Mechanisms of Dodecahydro-N-ethylcarbazole on Pt(111). ACS Catal. 2014, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, C.W.; Baker, R.T.; Staubitz, A.; Manners, I. B–N compounds for chemical hydrogen storage. Chem. Soc. Rev. 2009, 38, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Gong, X.; Wang, B.; Wu, Z.; Fang, T. A experimental study on the dehydrogenation performance of dodecahydro-N-ethylcarbazole on M/TiO2 catalysts. Int. J. Hydrogen Energy 2019, 44, 2951–2959. [Google Scholar] [CrossRef]
- Glaister, B.J.; Mudd, G.M. The environmental costs of platinum–PGM mining and sustainability: Is the glass half-full or half-empty? Miner. Eng. 2010, 23, 438–450. [Google Scholar] [CrossRef]
- He, Y.; Song, Y.; Cullen, D.A.; Laursen, S. Selective and Stable Non-Noble-Metal Intermetallic Compound Catalyst for the Direct Dehydrogenation of Propane to Propylene. J. Am. Chem. Soc. 2018, 140, 14010–14014. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. 9-Ethylcarbazole (CID=6836). PubChem Compound Database; 2019. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6836 (accessed on 13 February 2019).
- Sultan, O.; Shaw, H. Study of Automotive Storage of Hydrogen using Recyclable Liquid Chemical Carriers. 1975. Available online: http://adsabs.harvard.edu/abs/1975STIN. 7633642S (accessed on 13 February 2019).
- Chiyoda Corporation. What Is “SPERA HYDROGEN” System? 2017. Available online: https://www.chiyodacorp.com/en/service/spera-hydrogen/innovations/ (accessed on 1 February 2019).
- Chiyoda Corporation. Performance of 10,000 Hours of Operation in Chiyoda’s Demo Plant. 2017. Available online: https://www.chiyodacorp.com/en/service/spera-hydrogen/demo-plant/ (accessed on 1 February 2019).
- Kim, H.-Y.; Kang, M.-G.; Kim, T.-G.; Kang, C.-W. Toxicity of Methylcyclohexane and Its Effect on the Reproductive System in SD Rats. Saf. Health Work 2011, 2, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.W.; Neff, J.M.; Cox, B.A.; Tatem, H.E.; Hightower, G.M. Characteristics of dispersions and water-soluble extracts of crude and refined oils and their toxicity to estuarine crustaceans and fish. Mar. Boil. 1974, 27, 75–88. [Google Scholar] [CrossRef]
- Campo, P.; Lataye, R.; Cossec, B.; Placidi, V. Toluene-induced hearing loss: A mid-frequency location of the cochlear lesions. Neurotoxicol. Teratol. 1997, 19, 129–140. [Google Scholar] [CrossRef]
- Hydrogeit. Dibenzyltoluene: The Future of Hydrogen Storage. 2018. Available online: https://www.h2-international.com/2018/09/03/dibenzyltoluene-the-future-of-hydrogen-storage/ (accessed on 1 February 2019).
- Leinweber, A.; Müller, K. Hydrogenation of the Liquid Organic Hydrogen Carrier Compound Monobenzyl Toluene: Reaction Pathway and Kinetic Effects. Energy Technol. 2018, 6, 513–520. [Google Scholar] [CrossRef]
- Modisha, P.M.; Jordaan, J.H.; Bösmann, A.; Wasserscheid, P.; Bessarabov, D. Analysis of reaction mixtures of perhydro-dibenzyltoluene using two-dimensional gas chromatography and single quadrupole gas chromatography. Int. J. Hydrogen Energy 2018, 43, 5620–5636. [Google Scholar] [CrossRef]
- Heller, A.; Rausch, M.H.; Schulz, P.S.; Wasserscheid, P.; Fröba, A.P. Binary Diffusion Coefficients of the Liquid Organic Hydrogen Carrier System Dibenzyltoluene/Perhydrodibenzyltoluene. J. Chem. Eng. Data 2016, 61, 504–511. [Google Scholar] [CrossRef]
- Müller, K.; Stark, K.; Emel’Yanenko, V.N.; Varfolomeev, M.A.; Zaitsau, D.H.; Shoifet, E.; Schick, C.; Verevkin, S.P.; Arlt, W. Liquid Organic Hydrogen Carriers: Thermophysical and Thermochemical Studies of Benzyl- and Dibenzyl-toluene Derivatives. Ind. Eng. Chem. Res. 2015, 54, 7967–7976. [Google Scholar] [CrossRef]
- Müller, K.; Aslam, R.; Fischer, A.; Stark, K.; Wasserscheid, P.; Arlt, W. Experimental assessment of the degree of hydrogen loading for the dibenzyl toluene based LOHC system. Int. J. Hydrogen Energy 2016, 41, 22097–22103. [Google Scholar] [CrossRef]
- Brückner, N.; Obesser, K.; Bösmann, A.; Teichmann, D.; Arlt, W.; Dungs, J.; Wasserscheid, P. Evaluation of Industrially Applied Heat-Transfer Fluids as Liquid Organic Hydrogen Carrier Systems. ChemSusChem 2014, 7, 229–235. [Google Scholar]
- Arkema. GPS Safety Summary: Dibenzyltoluene. 2013. Available online: https://www.arkema.com/export/shared/.content/media/downloads/socialresponsability/safety-summuries/Hydrogen-Peroxide-Dibenzyltoluene-GPS-2013-02-10-V0.pdf (accessed on 1 February 2019).
- Shi, L.; Qi, S.; Qu, J.; Che, T.; Yi, C.; Yang, B. Integration of hydrogenation and dehydrogenation based on dibenzyltoluene as liquid organic hydrogen energy carrier. Int. J. Hydrogen Energy 2019, 44, 5345–5354. [Google Scholar] [CrossRef]
- Rönsch, S.; Schneider, J.; Matthischke, S.; Schluter, M.; Götz, M.; Lefebvre, J.; Prabhakaran, P.; Bajohr, S. Review on methanation–From fundamentals to current projects. Fuel 2016, 166, 276–296. [Google Scholar] [CrossRef]
- Götz, M.; Lefebvre, J.; Mörs, F.; Koch, A.M.; Graf, F.; Bajohr, S.; Reimert, R.; Kolb, T. Renewable Power-to-Gas: A technological and economic review. Renew. Energy 2016, 85, 1371–1390. [Google Scholar] [CrossRef]
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Genet. 2008, 6, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Seifert, A.; Rittmann, S.K.-M.R.; Herwig, C. Analysis of process related factors to increase volumetric productivity and quality of biomethane with Methanothermobacter marburgensis. Appl. Energy 2014, 132, 155–162. [Google Scholar] [CrossRef]
- Hashimoto, K.; Yamasaki, M.; Fujimura, K.; Matsui, T.; Izumiya, K.; Komori, M.; El-Moneim, A.; Akiyama, E.; Habazaki, H.; Kumagai, N.; et al. Global CO2 recycling—novel materials and prospect for prevention of global warming and abundant energy supply. Mater. Sci. Eng. A 1999, 267, 200–206. [Google Scholar] [CrossRef]
- Götz, M.; Bajohr, S.; Buchholz, D. Speicherung elektrischer Energie aus regenerativen Quellen im Erdgasnetz. Energ. Wasser Prax. 2011, 62, 72–76. [Google Scholar]
- Lefebvre, J.; Götz, M.; Bajohr, S.; Reimert, R.; Kolb, T. Improvement of three-phase methanation reactor performance for steady-state and transient operation. Fuel Process. Technol. 2015, 132, 83–90. [Google Scholar] [CrossRef]
- de Vasconcelos, B.R.; Minh, D.P.; Lyczko, N.; Phan, T.S.; Sharrock, P.; Nzihou, A. Upgrading greenhouse gases (methane and carbon dioxide) into syngas using nickel-based catalysts. Fuel 2018, 226, 195–203. [Google Scholar] [CrossRef]
- Weger, L.; Abánades, A.; Butler, T. Methane cracking as a bridge technology to the hydrogen economy. Int. J. Hydrogen Energy 2017, 42, 720–731. [Google Scholar] [CrossRef]
- Joglekar, M.; Nguyen, V.; Pylypenko, S.; Ngo, C.; Li, Q.; O’Reilly, M.E.; Gray, T.S.; Hubbard, W.A.; Gunnoe, T.B.; Herring, A.M.; et al. Organometallic Complexes Anchored to Conductive Carbon for Electrocatalytic Oxidation of Methane at Low Temperature. J. Am. Chem. Soc. 2016, 138, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Douvartzides, S.; Coutelieris, F.; Tsiakaras, P. Exergy analysis of a solid oxide fuel cell power plant fed by either ethanol or methane. J. Power Sources 2004, 131, 224–230. [Google Scholar] [CrossRef]
- Amiri, A.; Tang, S.; Steinberger-Wilckens, R.; Tadé, M.O. Evaluation of fuel diversity in Solid Oxide Fuel Cell system. Int. J. Hydrogen Energy 2018, 43, 23475–23487. [Google Scholar] [CrossRef]
- Valera-Medina, A.; Xiao, H.; Owen-Jones, M.; David, W.I.F.; Bowen, P.J. Ammonia for power. Prog. Energy Combust. Sci. 2018, 69, 63–102. [Google Scholar] [CrossRef]
- Lamb, K.E.; Dolan, M.D.; Kennedy, D.F. Ammonia for hydrogen storage; A review of catalytic ammonia decomposition and hydrogen separation and purification. Int. J. Hydrogen Energy 2019, 44, 3580–3593. [Google Scholar] [CrossRef]
- Christensen, C.H.; Johannessen, T.; Sørensen, R.Z.; Nørskov, J.K. Towards an ammonia-mediated hydrogen economy? Catal. Today 2006, 111, 140–144. [Google Scholar] [CrossRef]
- Perna, A.; Minutillo, M.; Jannelli, E.; Cigolotti, V.; Nam, S.; Han, J. Design and performance assessment of a combined heat, hydrogen and power (CHHP) system based on ammonia-fueled SOFC. Appl. Energy 2018, 231, 1216–1229. [Google Scholar] [CrossRef]
- Aziz, M.; Oda, T.; Morihara, A.; Kashiwagi, T. Combined nitrogen production, ammonia synthesis, and power generation for efficient hydrogen storage. Energy Procedia 2017, 143, 674–679. [Google Scholar] [CrossRef]
- Yapicioglu, A.; Dincer, I. A review on clean ammonia as a potential fuel for power generators. Renew. Sustain. Energy Rev. 2019, 103, 96–108. [Google Scholar] [CrossRef]
- Cinti, G.; Frattini, D.; Desideri, U.; Bidini, G.; Jannelli, E. Coupling Solid Oxide Electrolyser (SOE) and ammonia production plant. Appl. Energy 2017, 192, 466–476. [Google Scholar] [CrossRef]
- Lange, N.A.; Dean, J.A. Lange’s Handbook of Chemistry; McGraw-Hill: New York, NY, USA, 1967. [Google Scholar]
- Siddiqui, O.; Dincer, I. A review and comparative assessment of direct ammonia fuel cells. Therm. Sci. Eng. Prog. 2018, 5, 568–578. [Google Scholar] [CrossRef]
- Afif, A.; Radenahmad, N.; Cheok, Q.; Shams, S.; Kim, J.H.; Azad, A.K. Ammonia-fed fuel cells: A comprehensive review. Renew. Sustain. Energy Rev. 2016, 60, 822–835. [Google Scholar] [CrossRef]
- Cooper, S.J.; Brandon, N.P. Chapter 1 - An Introduction to Solid Oxide Fuel Cell Materials, Technology and Applications. In Solid Oxide Fuel Cell Lifetime and Reliability; Academic Press: Cambridge, MA, USA, 2017; pp. 1–18. [Google Scholar]
- Schmidt, O.; Gambhir, A.; Staffell, I.; Hawkes, A.; Nelson, J.; Few, S. Future cost and performance of water electrolysis: An expert elicitation study. Int. J. Hydrogen Energy 2017, 42, 30470–30492. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hayakawa, A.; Somarathne, K.D.K.A.; Okafor, E.C. Science and technology of ammonia combustion. Proc. Combust. Inst. 2018, 37, 109–133. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry; W.H. Freeman: New York, NY, USA, 2002. [Google Scholar]
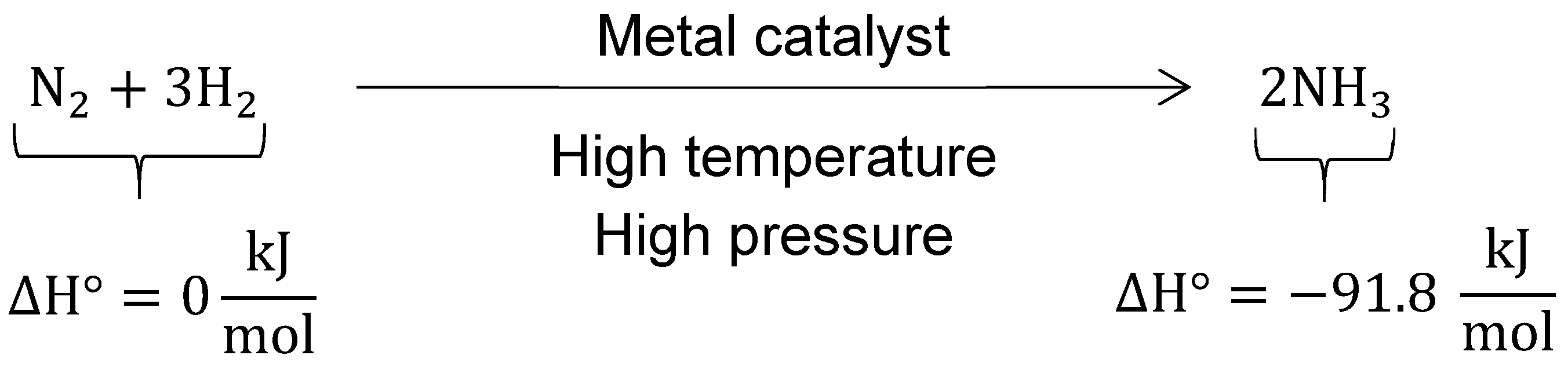
- Wikipedia Contributors. Haber Process. Wikipedia, The Free Encyclopedia. Available online: https://en.wikipedia.org/wiki/Haber_process (accessed on 19 February 2019).

| Property | Hydrogen | Natural Gas |
|---|---|---|
| Lower heating value (LHV, MJ/kg) | 120 [53] | 52 [53] |
| Higher heating value (HHV, MJ/kg) | 142 [53] | 47 [53] |
| Density at 273 K (kg/m3) | 0.09 [54] | 0.65 [54] |
| Boiling point at atmospheric pressure(K) | 20.3 [54] | 111.2 [55] |
| Liquid density (kg/m3) | 70.8 [54] | 450.0 [55] |
| Flammability concentration limits in air (vol %) | 4–75 [54] | 5–15 [54] |
| Diffusion coefficient in air (cm2/s) | 0.61 [54] | 0.16 [54] |
| Storage System Targets | Gravimetric Density System (wt %) | Volumetric Density System (MJ/L) | Cost ($/kWh) |
|---|---|---|---|
| Current status (700 bar compressed) | 4.2 | 2.9 | 15 |
| 2020 | 4.5 | 3.6 | 10 |
| Ultimate | 6.5 | 6.1 | 8 |
| Type | Materials | Typical Pressure (bar) | Cost ($/kg) | Gravimetric Density (wt %) |
|---|---|---|---|---|
| I | All-metal construction | 300 | 83 | 1.7 |
| II | Mostly metal, composite overwrap in the hoop direction | 200 | 86 | 2.1 |
| III | Metal liner, full composite overwrap | 700 | 700 [65] | 4.2 [66] |
| IV | All-composite construction | 700 | 633 [65] | 5.7 (Toyota Mirai) |
| Method | Gravimetric Energy Density (wt %) | Volumetric Energy Density (MJ/L) | Temperature (K) | Pressure (barg) | Remarks |
|---|---|---|---|---|---|
| Compressed | 5.7 | 4.9 | 293 | 700 | Current industry standard |
| Liquid | 7.5 | 6.4 | 20 | 0 | Boil-off constitues major disadvantage |
| Cold/cryo compressed | 5.4 | 4.0 | 40–80 | 300 | Boil-off constitues major disadvantage |
| MOF | 4.5 | 7.2 | 78 | 20–100 | Attractive densities only at very low temperatures. |
| Carbon nanostructures | 2.0 | 5.0 | 298 | 100 | Volumetric density based on powder density of 2.1 g/mL and 2.0 wt % storage capacity. |
| Metal hydrides | 7.6 | 13.2 | 260–425 | 20 | Requires thermal management system. |
| Metal borohydrides | 14.9–18.5 | 9.8–17.6 | 130 | 105 | Low temperature, high pressure thermal management required |
| Kubas-type | 10.5 | 23.6 | 293 | 120 | |
| LOHC | 8.5 | 7 | 293 | 0 | Highly endo/exothermal requires processing plant and catalyst. Not suitable for mobility |
| Chemical | 15.5 | 11.5 | 298 | 10 | Requires SOFC fuel cell. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rivard, E.; Trudeau, M.; Zaghib, K. Hydrogen Storage for Mobility: A Review. Materials 2019, 12, 1973. https://doi.org/10.3390/ma12121973
Rivard E, Trudeau M, Zaghib K. Hydrogen Storage for Mobility: A Review. Materials. 2019; 12(12):1973. https://doi.org/10.3390/ma12121973
Chicago/Turabian StyleRivard, Etienne, Michel Trudeau, and Karim Zaghib. 2019. "Hydrogen Storage for Mobility: A Review" Materials 12, no. 12: 1973. https://doi.org/10.3390/ma12121973
APA StyleRivard, E., Trudeau, M., & Zaghib, K. (2019). Hydrogen Storage for Mobility: A Review. Materials, 12(12), 1973. https://doi.org/10.3390/ma12121973







