Novel Ni/pHEMA-gr-PVP Composites Obtained by Polymerization with Simultaneous Metal Deposition: Structure and Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
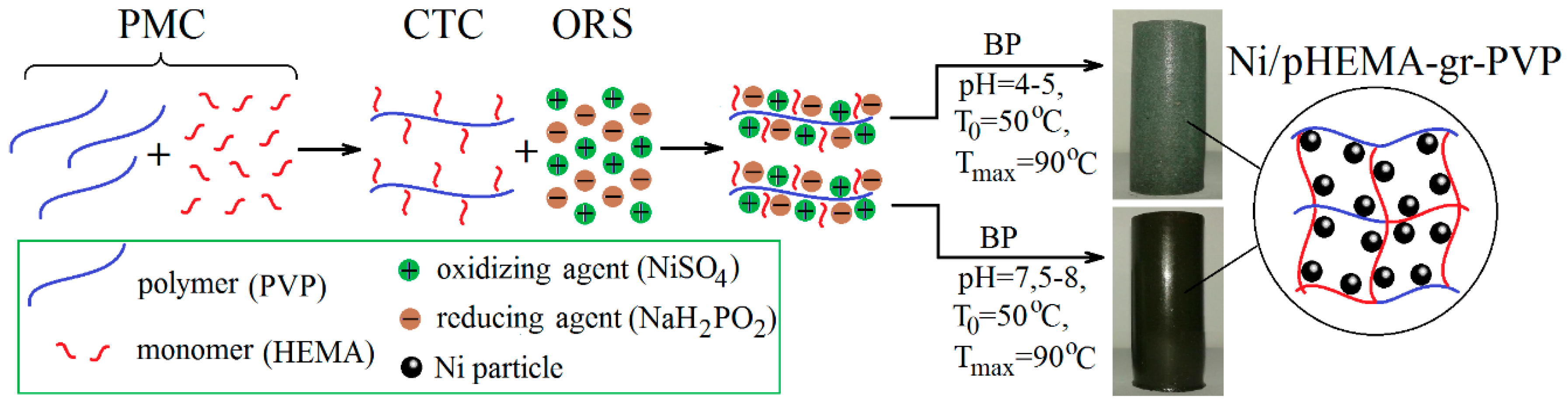
2.2. Synthesis Technique of Ni/pHEMA-gr-PVP Composites
2.3. Measurements and Characterization
2.3.1. Standard Methods of Instrumental Research
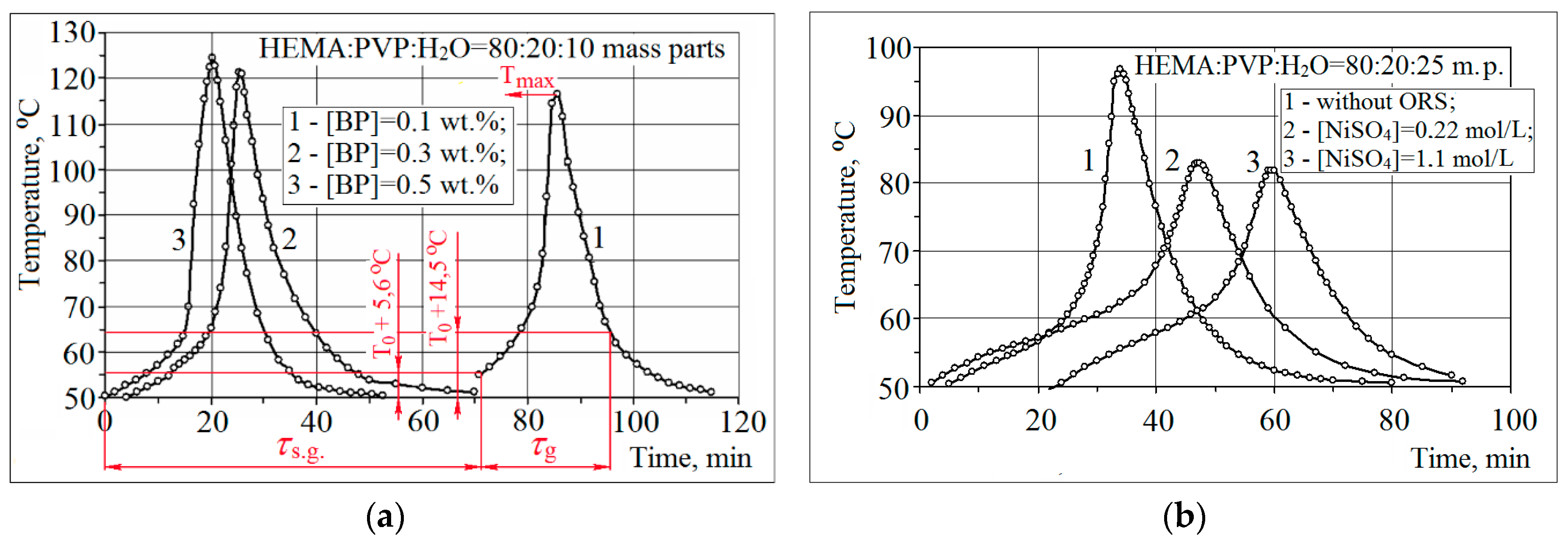
2.3.2. Thermometric Investigations of HEMA Copolymerization with PVP
2.3.3. Efficiency of PVP Grafting
2.3.4. The Molecular Weight between Cross-Links in the Polymer Network
2.3.5. Study of the Metal Content Obtained during the Polymerization Process
2.3.6. Physical–Mechanical Characteristics
2.3.7. Conductivity
2.3.8. Magnetic Properties
3. Results and Discussion
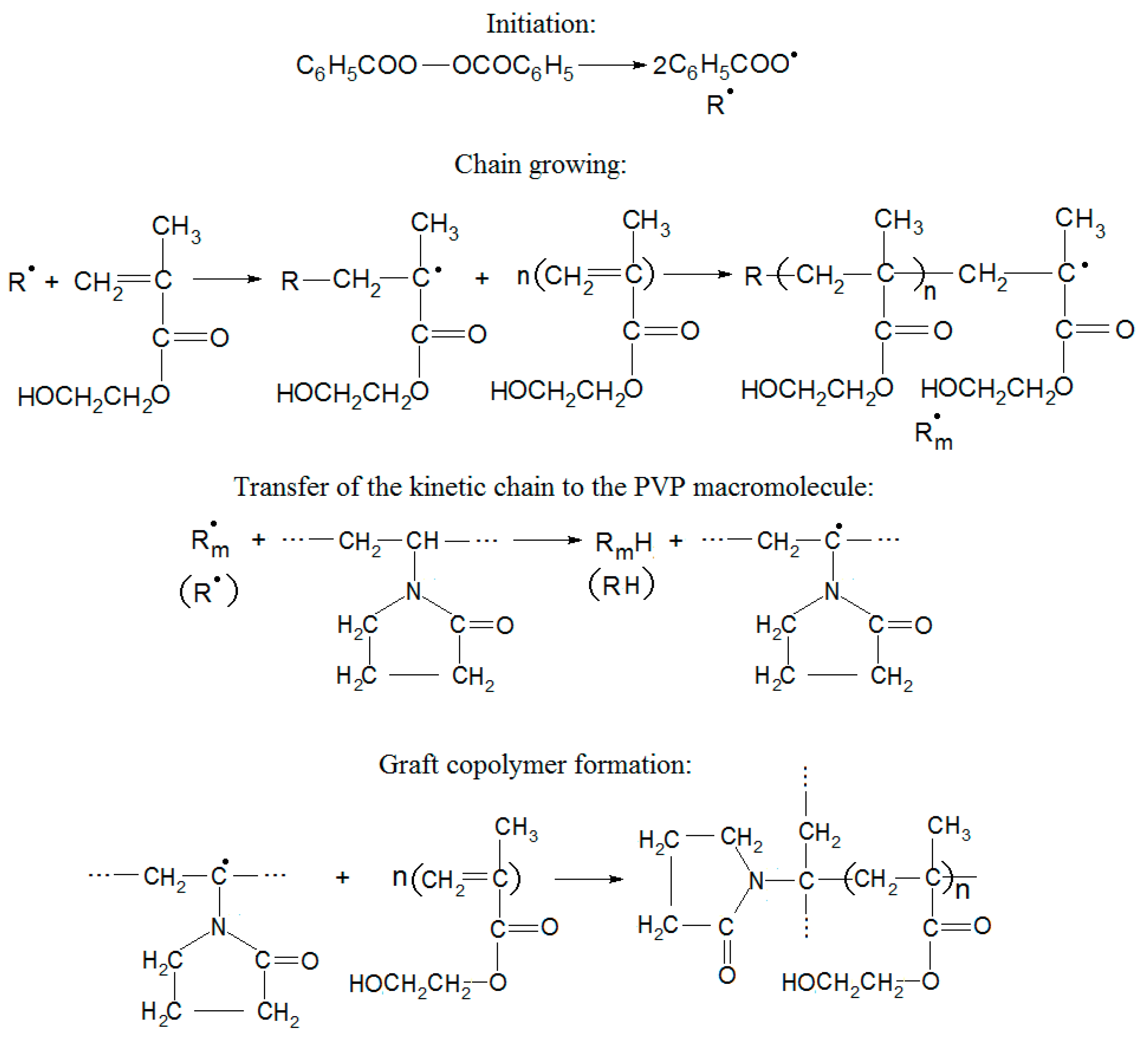
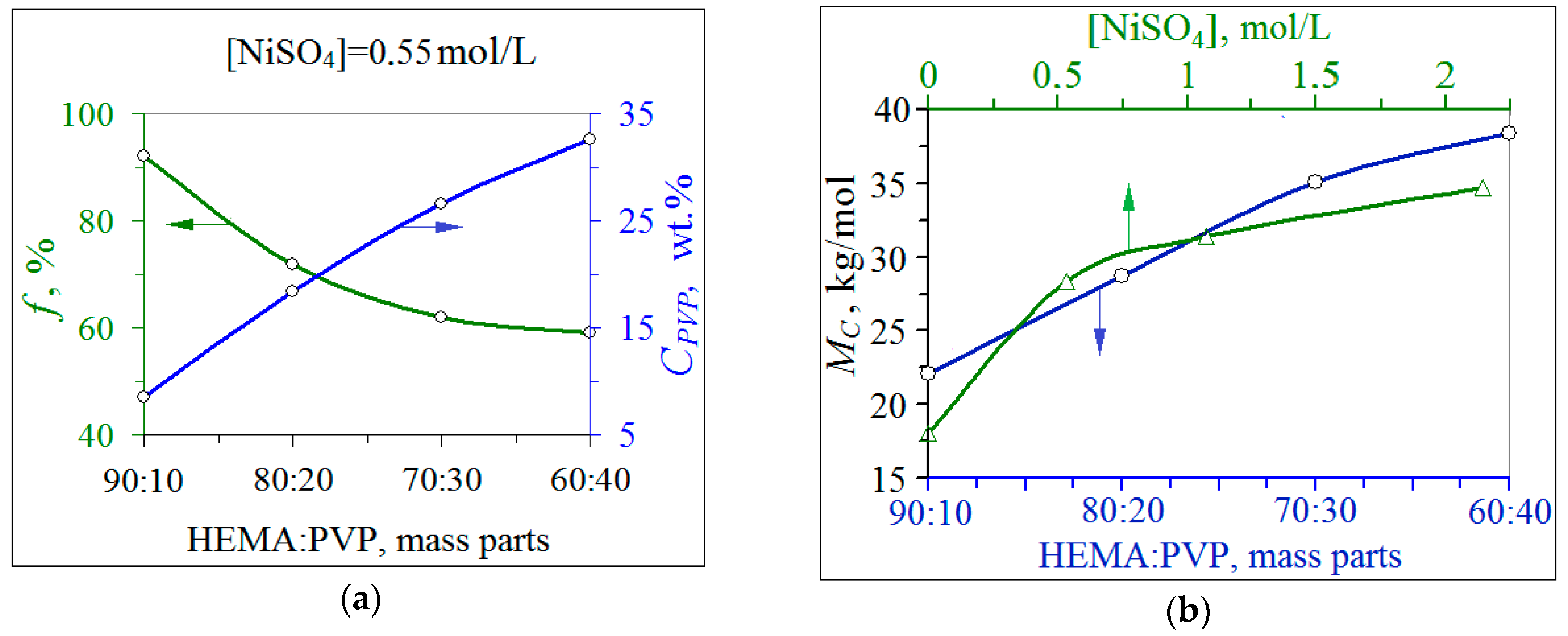
3.1. Synthesis of Ni/рHEMA-gr-PVP Composites
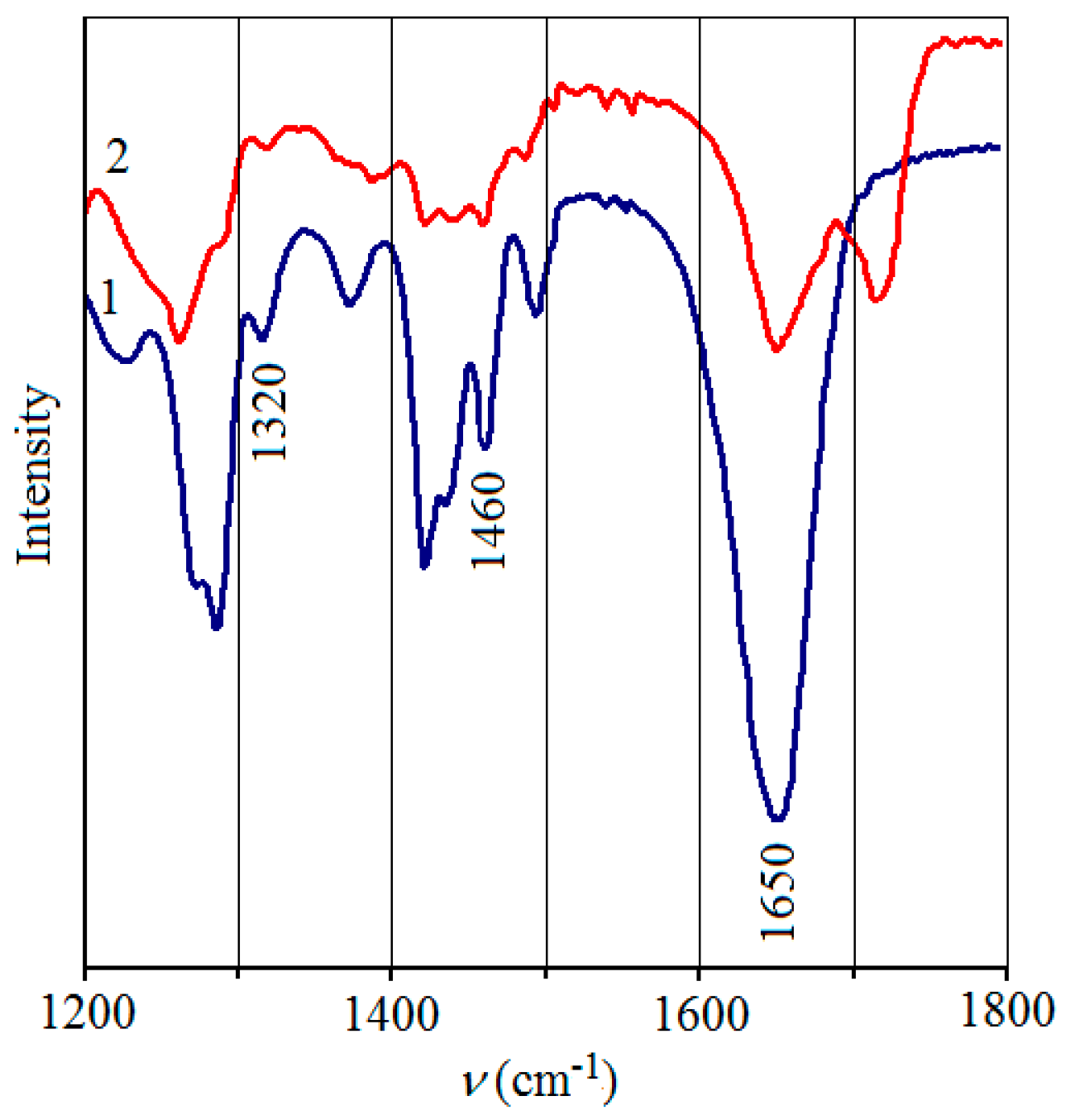
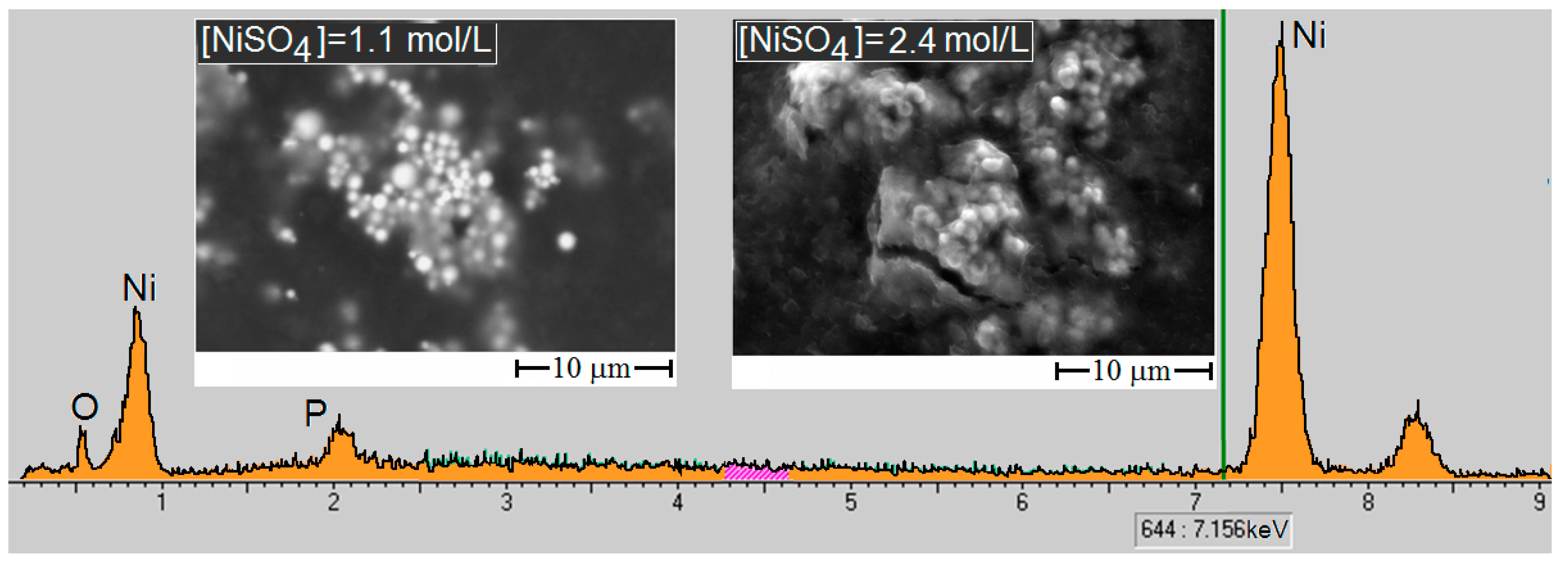
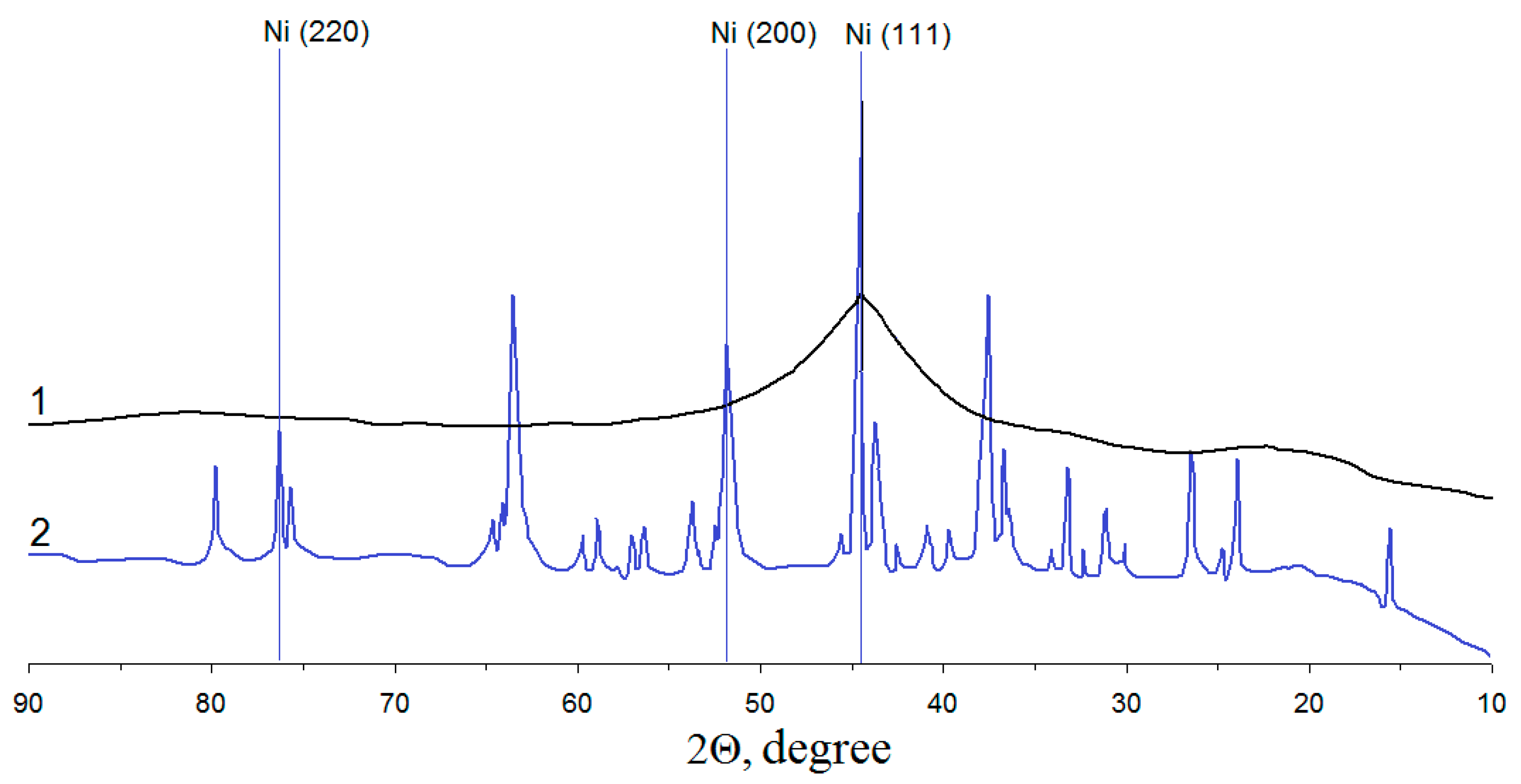
3.2. Study of the Ni/pHEMA-gr-PVP Composites’ Structure
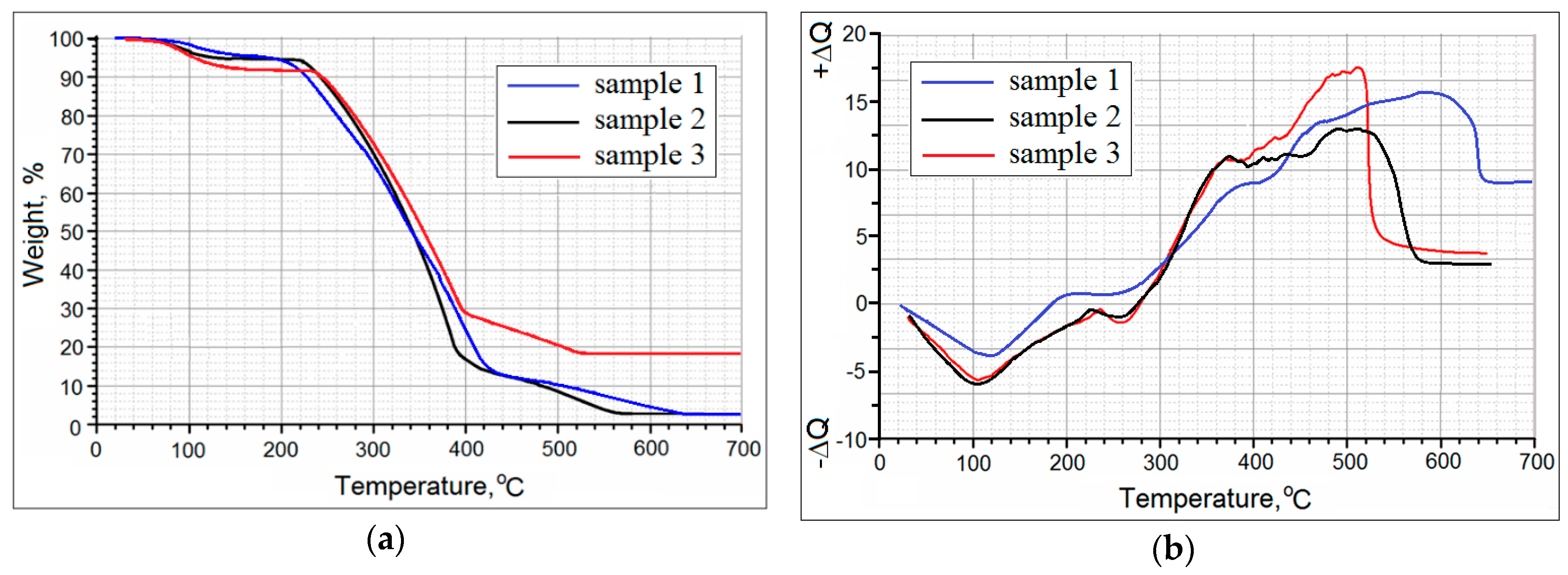
3.3. Properties of Metal-Filled Ni/pHEMA-gr-PVP Copolymers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moravskyi, V.; Dziaman, I.; Suberliak, S.; Grytsenko, O.; Kuznetsova, M. Features of the production of metal-filled composites by metallization of polymeric raw materials. In Proceedings of the 7th International Conference Nanomaterials: Application & Properties (NAP), Zatoka, Ukraine, 10–15 September 2017; IEEE: Odessa, Ukraine, 2017. [Google Scholar] [CrossRef]
- Moravskyi, V.; Dziaman, I.; Suberliak, S.; Kuznetsova, M.; Tsimbalista, T.; Dulebova, L. Research into kinetic patterns of chemical metallization of powderlike polyvinylchloride. East.-Eur. J. Enterp. Technol. 2017, 4, 50–57. [Google Scholar] [CrossRef]
- Nicolais, L.; Carotenuto, G. Metal-Polymer Nanocomposites; John Wiley & Sons: Hoboken, NJ, USA, 2005; p. 304. [Google Scholar] [CrossRef]
- Zare, Y.; Shabani, I. Polymer/metal nanocomposites for biomedical applications. Mater. Sci. Eng. 2016, 60, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Hule, R.A.; Pochan, D.J. Polymer Nanocomposites for biomedical applications. MRS Bull. 2007, 32, 354–358. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle composites: From synthesis to modern applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Thomas, V.; Namdeo, M.; Murali Mohan, Y.; Bajpai, S.K.; Bajpai, M. Review on Polymer, Hydrogel and microgel metal nanocomposites: A facile nanotechnological approach. J. Macromol. Sci. Part A 2007, 45, 107–119. [Google Scholar] [CrossRef]
- Varvarenko, S.; Voronov, A.; Samaryk, V.; Tarnavchyk, I.; Nosova, N.; Kohut, A.; Voronov, S. Covalent grafting of polyacrylamide-based hydrogels to a polypropylene surface activated with functional polyperoxide. React. Funct. Polym. 2010, 70, 647–655. [Google Scholar] [CrossRef]
- Samaryk, V.; Varvarenko, S.; Nosova, N.; Fihurka, N.; Musyanovych, A.; Landfester, K.; Popadyuk, N.; Voronov, S. Optical properties of hydrogels filled with dispersed nanoparticles. Chem. Chem. Technol. 2017, 11, 449–453. [Google Scholar] [CrossRef] [Green Version]
- Suberlyak, O.; Skorokhoda, V. Hydrogels based on polyvinylpyrrolidone copolymers. In Hydrogels; Haider, S., Haider, A., Eds.; IntechOpen: London, UK, 2018; pp. 136–214. [Google Scholar] [CrossRef]
- Kikuchi, A.; Okano, T. Pulsatile drug release control using hydrogels. Adv. Drug Deliv. Rev. 2002, 54, 53–77. [Google Scholar] [CrossRef]
- Skorokhoda, V.; Melnyk, Y.; Shalata, V.; Skorokhoda, T.; Suberliak, S. An investigation of obtaining patterns, structure and diffusion properties of biomedical purpose hydrogel membranes. East.-Eur. J. Enterp. Technol. 2017, 1, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Schexnailder, P.; Schmidt, G. Nanocomposite polymer hydrogels. Colloid Polym. Sci. 2009, 287, 1–11. [Google Scholar] [CrossRef]
- Spanoudaki, A.; Fragiadakis, D.; Vartzeli-Nikaki, K.; Pissis, P.; Hernandez, J.C.R.; Pradas, M.M. Nanostructured and nanocomposite hydrogels for biomedical applications. In Surface Chemistry in Biomedical and Environmental Science; Blitz, J.P., Gun’ko, V.M., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 229–240. [Google Scholar] [CrossRef]
- Palza, H. Antimicrobial Polymers with metal nanoparticles. Int. J. Mol. Sci. 2015, 16, 2099–2116. [Google Scholar] [CrossRef] [PubMed]
- Nebesnyi, R.; Ivasiv, V.; Pikh, Z.; Kharandiuk, T.; Shpyrka, I.; Voronchak, T.; Shatan, A. Low temperature acrolein to acrylic acid oxidation with hydrogen peroxide on se-organic catalysts. Chem. Chem. Technol. 2019, 13, 38–45. [Google Scholar] [CrossRef]
- Tan, N.P.B.; Lee, C.H.; Li, P. Green synthesis of smart metal/polymer nanocomposite particles and their tuneable catalytic activities. Polymers 2016, 8, 105. [Google Scholar] [CrossRef] [PubMed]
- Urban, G.A.; Weiss, T. Hydrogels for biosensors. Hydrogel Sensors and Actuators. Springer Series on Chemical Sensors and Biosensors (Methods and Applications); Gerlach, G., Arndt, K.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; Volume 6, pp. 197–220. [Google Scholar] [CrossRef]
- Zheng, Y.; Wang, A. Ag nanoparticle-entrapped hydrogel as promising material for catalytic reduction of organic dyes. J. Mater. Chem. 2012, 22, 16552–16559. [Google Scholar] [CrossRef]
- Hapiot, F.; Menuel, S.; Monflier, E. Thermoresponsive Hydrogels in Catalysis. ACS Catal. 2013, 3, 1006–1010. [Google Scholar] [CrossRef]
- Sahiner, N. Soft and flexible hydrogel templates of different sizes and various functionalities for metal nanoparticle preparation and their use in catalysis. Prog. Polym. Sci. 2013, 38, 1329–1356. [Google Scholar] [CrossRef]
- Ajmal, M.; Aftab, F.; Bibi, I.; Iqbal, M.; Ambreen, J.; Ahmad, H.B.; Akhtar, N.; Haleem, A.; Siddiq, M. Facile synthesis of porous anionic hydrogel embedded with nickel nanoparticles and evaluation of its catalytic performance for the rapid reduction of 4-nitrophenol. J. Porous Mater. 2019, 26, 281–290. [Google Scholar] [CrossRef]
- Sahiner, N.; Ozay, H.; Ozay, O.; Aktas, N. New catalytic route: Hydrogels as templates and reactors for in situ Ni nanoparticle synthesis and usage in the reduction of 2- and 4-nitrophenols. Appl. Catal. A Gen. 2010, 385, 201–207. [Google Scholar] [CrossRef]
- Ozay, O.; Aktas, N.; Inger, E.; Sahiner, N. Hydrogel assisted nickel nanoparticle synthesis and their use in hydrogen production from sodium boron hydride. Int. J. Hydrog. Energy 2011, 36, 1998–2006. [Google Scholar] [CrossRef]
- Cai, H.; Lu, P.; Dong, J. Robust nickel–polymer nanocomposite particles for hydrogen generation from sodium borohydride. Fuel 2016, 166, 297–301. [Google Scholar] [CrossRef]
- Suberlyak, О.; Grytsenko, O.; Hischak, K.; Hnatchuk, N. Researching influence the nature of metal on mechanism of synthesis polyvinilpyrrolidone metal copolymers. Chem. Chem. Technol. 2013, 7, 289–294. [Google Scholar] [CrossRef]
- Grytsenko, O.; Spiśak, Е.; Dulebová, L.; Moravskii, V.; Suberlyak, О. Sorption capable film coatings with variable conductivity. Mater. Sci. Forum 2015, 818, 97–101. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Wang, Y.; Liu, C.; Jin, M.; Xu, Y.; Li, L.; Guo, X.; Hu, A.; Liu, T.; et al. A thermosensitive hydrogel carrier for nickel nanoparticles. Colloids Interface Sci. Commun. 2015, 4, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Tarnavchyk, I.; Voronov, A.; Kohut, A.; Nosova, N.; Varvarenko, S.; Samaryk, V.; Voronov, S. Reactive hydrogel networks for the fabrication of metal-polymer nanocomposites. Macromol. Rapid Commun. 2009, 30, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.B.; Shi, Y.; Liu, X.J.; Zhang, Y.F. Preparation of polyvinylidene fluoride–nickel hollow fiber catalytic membranes for hydrogen generation from sodium borohydride. Fuel 2015, 140, 685–692. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle-hydrogel composites. Concept, design, and applications of these promising, multifunctional materials. Adv. Sci. 2015, 12, 1–12. [Google Scholar] [CrossRef]
- Montheard, J.P.; Chatzopoulos, M.; Chappard, D. 2-Hydroxyethyl Methacrylate (HEMA): Chemical properties and applications in biomedical fields. J. Macromol. Sci. Part C 1992, 32, 1–34. [Google Scholar] [CrossRef]
- Yanez, F.; Concheiro, A.; Alvarez-Lorenzo, C. Macromolecule release and smoothness of semiinterpenetrating PVP–pHEMA networks for comfortable soft contact lenses. Eur. J. Pharm. Biopharm. 2008, 69, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Teodorescu, M.; Bercea, M. Poly(vinylpyrrolidone)—A versatile polymer for biomedical and beyond medical applications. Polym.-Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Reverberi, A.P.; Salerno, M.; Lauciello, S.; Fabiano, B. Synthesis of copper nanoparticles in ethylene glycol by chemical reduction with vanadium (+2) Salts. Materials 2016, 9, 809. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez, G.; Gonzalez, G.; Silva, P. Synthesis and characterization of metallics nanoparticles stabilized with polyvinylpyrrolidone. Microsc. Microanal. 2005, 11, 1944–1945. [Google Scholar] [CrossRef]
- Suberlyak, O.V.; Krasins’kyi, V.V.; Shapoval, I.M.; Hrytsenko, O.M. Influence of the mechanism and parameters of hardening of modified novolac phenol-formaldehyde resins on the physicomechanical properties of the composite. Mater. Sci. 2011, 46, 669–678. [Google Scholar] [CrossRef]
- Krasinskyi, V.; Suberlyak, O.; Dulebová, L.; Antoniuk, V. Nanocomposites on the basis of thermoplastics and montmorillonite modified by polyvinylpyrrolidone. Key Eng. Mater. 2017, 756, 3–10. [Google Scholar] [CrossRef]
- Li, J.; Inukai, K.; Takahashi, Y.; Tsuruta, A.; Shin, W. Thin film coating with highly dispersible barium titanate-polyvinylpyrrolidone nanoparticles. Materials 2018, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Uchida, T.; Izu, N.; Shin, W. Effect of core-shell ceria/poly(vinylpyrrolidone) (PVP) nanoparticles incorporated in polymer films and their optical properties (2): Increasing the refractive index. Materials 2017, 10, 710. [Google Scholar] [CrossRef]
- Del Sorbo, G.R.; Truda, G.; Bifulco, A.; Passaro, J.; Petrone, G.; Vitolo, B.; Ausanio, G.; Vergara, A.; Marulo, F.; Branda, F. Non monotonous effects of noncovalently functionalized graphene addition on the structure and sound absorption properties of polyvinylpyrrolidone (1300 kDa) electrospun mats. Materials 2019, 12, 108. [Google Scholar] [CrossRef]
- Suberlyak, О.V.; Baran, N.M.; Yatsul’chak, H.V. Physicomechanical properties of the films based on polyamide–polyvinylpyrrolidone mixtures. Mater. Sci. 2017, 53, 392–397. [Google Scholar] [CrossRef]
- Suberlyak, O.; Baran, N.; Gnatowski, A.; Jaruga, T.; Melnyk, Y. Regularities of films forming on the basis of polyamide-polyvinylpyrrolidone mixtures. Chem. Chem. Technol. 2012, 6, 73–76. [Google Scholar] [CrossRef]
- Suberlyak, O.V.; Melnyk, Y.Y.; Baran, N.M. Formation of membranes from aliphatic polyamide-polyvinylpyrrolidone blends. Russ. J. Appl. Chem. 2009, 82, 1746–1749. [Google Scholar] [CrossRef]
- Grytsenko, О.; Pokhmurska, A.; Suberliak, S.; Kushnirchuk, M.; Panas, M.; Moravskyi, V.; Kovalchuk, R. Technological features in obtaining highly effective hydrogel dressings for medical purposes. East.-Eur. J. Enterp. Technol. 2018, 6, 6–13. [Google Scholar] [CrossRef]
- Grytsenko, O.M.; Suberlyak, O.V.; Moravskyi, V.S.; Gayduk, A.V. Investigation of nickel chemical precipitation kinetics. East.-Eur. J. Enterp. Technol. 2016, 1, 26–31. [Google Scholar] [CrossRef] [Green Version]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Suberlyak, O.V.; Hrytsenko, O.M.; Hishchak, K.Y. Influence of the metal surface of powder filler om the structure and properties of composite materials based on the co-polymers of methacrylates with polyvinylpyrrolidone. Mater. Sci. 2016, 52, 155–164. [Google Scholar] [CrossRef]
- Dulebova, L.; Garbacz, T.; Krasinskyi, V.; Duleba, B. The influence of modifying HDPE on properties of the surface. Mater. Sci. Forum 2015, 818, 101–104. [Google Scholar] [CrossRef]
- Suberlyak, О.V.; Mel’nyk, Y.Y.; Skorokhoda, V.I. Regularities of preparation and properties of hydrogel membrane. Mater. Sci. 2015, 50, 889–896. [Google Scholar] [CrossRef]
- Suberlyak, O.; Grytsenko, O.; Kochubei, V. The role of FeSO4 in the obtaining of polyvinylpirolidone copolymers. Chem. Chem. Technol. 2015, 9, 429–434. [Google Scholar] [CrossRef]
- Skorokhoda, V. Matrix polymerization of 2-Hydroxyethylmethacrylate in the presence of polyvinylpyrrolidone in permanent magnetic field. Chem. Chem. Technol. 2010, 4, 191–196. [Google Scholar]
- Bühler, V. Polyvinylpyrrolidone Excipients for Pharmaceuticals: Povidone, Crospovidone and Copovidone; Springer: Berlin/Heidelberg, Germany, 2005; pp. 1–254. [Google Scholar] [CrossRef]
- Semko, L.S.; Kruchek, O.I.; Dzubenko, L.S.; Gorbik, P.P.; Oranska, O.I. Transformations in nanostructured powders of nickel and nickel/dextrane nanocomposite. Nanosyst. Nanomater. Nanotechnol. 2008, 6, 137–146. [Google Scholar]
- Liu, T.Y.; Hu, S.H.; Liu, T.Y.; Liu, D.M.; Chen, S.Y. Magnetic-sensitive behavior of intelligent ferrogels for controlled release of drug. Langmuir 2006, 22, 5974–5978. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grytsenko, O.; Gajdoš, I.; Spišák, E.; Krasinskyi, V.; Suberlyak, O. Novel Ni/pHEMA-gr-PVP Composites Obtained by Polymerization with Simultaneous Metal Deposition: Structure and Properties. Materials 2019, 12, 1956. https://doi.org/10.3390/ma12121956
Grytsenko O, Gajdoš I, Spišák E, Krasinskyi V, Suberlyak O. Novel Ni/pHEMA-gr-PVP Composites Obtained by Polymerization with Simultaneous Metal Deposition: Structure and Properties. Materials. 2019; 12(12):1956. https://doi.org/10.3390/ma12121956
Chicago/Turabian StyleGrytsenko, Oleksandr, Ivan Gajdoš, Emil Spišák, Volodymyr Krasinskyi, and Oleh Suberlyak. 2019. "Novel Ni/pHEMA-gr-PVP Composites Obtained by Polymerization with Simultaneous Metal Deposition: Structure and Properties" Materials 12, no. 12: 1956. https://doi.org/10.3390/ma12121956




