In-Situ Growth of Au on KTaO3 Sub-Micron Cubes via Wet Chemical Approach for Enhanced Photodegradation of p-Nitrophenol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Synthesis of KTaO3 and KTaO3/Au Nanocrystals
2.3. Characterization
2.4. Photodegradation Measurement
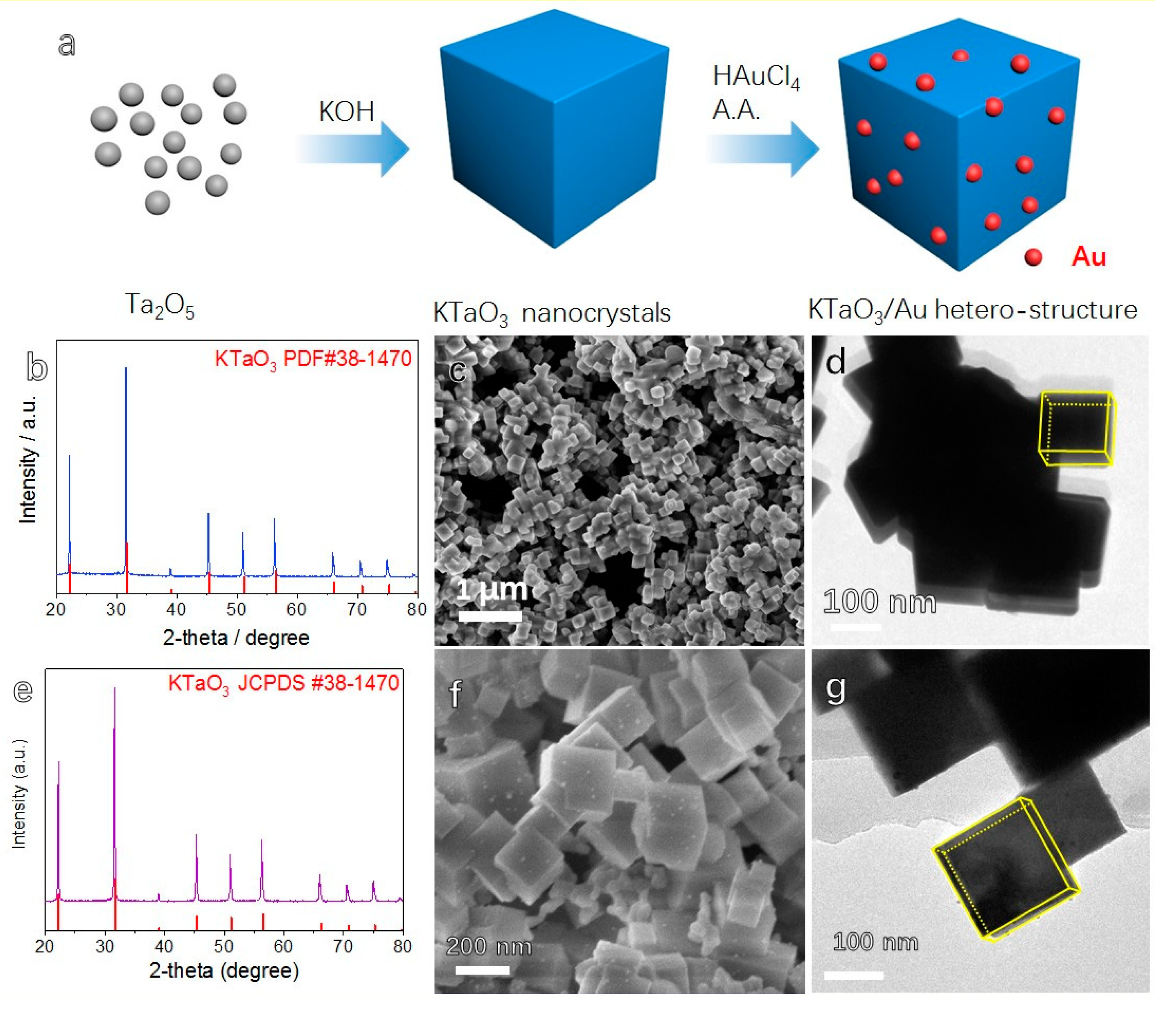
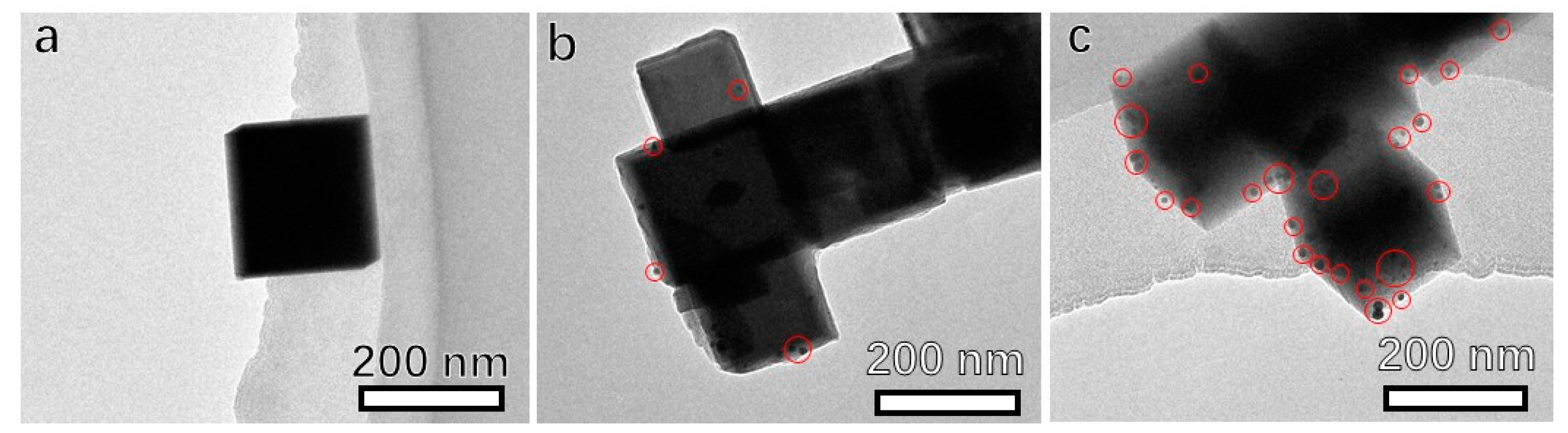
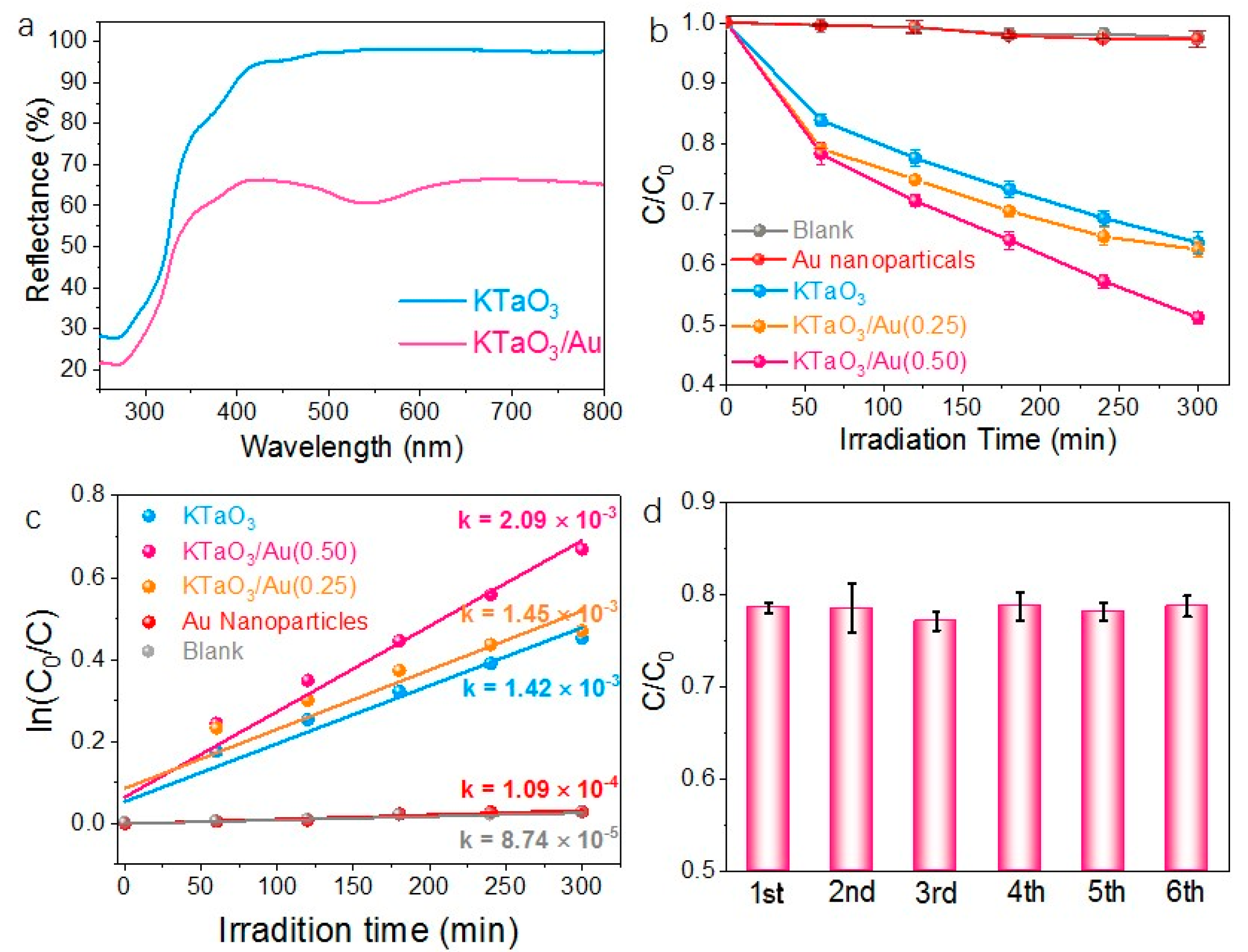
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Marchelek, M.; Bajorowicz, B.; Mazierski, P.; Cybula, A.; Klimczuk, T.; Winiarski, M.; Fijałkowska, N.; Zaleska, A. KTaO3-based nanocomposites for air treatment. Catal. Today 2015, 252, 47–53. [Google Scholar] [CrossRef]
- Yucel, I.; Cakmak, S. Effect of oxygen vacancy on Sb3+, Nb3+ and V3+ doped KTaO3 compounds. Optik 2019, 178, 467–477. [Google Scholar] [CrossRef]
- Rossella, F.; Perucchini, L.; Galinetto, P.; Sarnoggia, G.; Mozzati, M.C.; Azzoni, C.B.; Badalyan, A.G.; Trepakov, V.A.; Syrnikov, P.P. Influence of Cu ions on the photo-transport properties in KTaO3: (Cu,V) single crystals. In Physica Status Solidi C—Current Topics in Solid State Physics; Stutzmann, M., Ed.; Wiley: Hoboken, NJ, USA, 2007; Volume 4, p. 1101. [Google Scholar]
- Uniyal, M.; Bhatt, S.C.; Kashyap, S. Preparation and ultrasonic study of sodium potassium tantalate (Na1−xKxTaO3) mixed system. Indian J. Pure Appl. Phys. 2019, 57, 212–216. [Google Scholar]
- Sudrajat, H.; Thushari, I.; Babel, S. Chemical state and coordination structure of La cations doped in KTaO3 photocatalysts. J. Phys. Chem. Solids 2019, 127, 94–100. [Google Scholar] [CrossRef]
- Marchena, C.L.; Pecchi, G.A.; Pierella, L.B. Selective styrene oxidation on alkaline tantalates ATaO3 (A = Li, Na, K) as heterogeneous catalysts. Catal. Commun. 2019, 119, 28–32. [Google Scholar] [CrossRef]
- Zhu, W.; Chao, J.-H.; Chen, C.-J.; Shang, A.; Lee, Y.G.; Yin, S.; Dubinskii, M.; Hoffman, R.C. Photon excitation enabled large aperture space-charge-controlled potassium tantalate niobate (KTN) beam deflector. Appl. Phys. Lett. 2018, 112, 132901. [Google Scholar] [CrossRef]
- Hagiwara, H.; Higashi, K.; Watanabe, M.; Kakigi, R.; Ida, S.; Ishihara, T. Effect of Porphyrin Molecular Structure on Water Splitting Activity of a KTaO3 Photocatalyst. Catalysts 2016, 6, 42–51. [Google Scholar] [CrossRef]
- Shao, X.; Yin, X.; Wang, J. Nanoheterostructures of potassium tantalate and nickel oxide for photocatalytic reduction of carbon dioxide to methanol in isopropanol. J. Colloid Interf. Sci. 2018, 512, 466–473. [Google Scholar] [CrossRef]
- Krukowska, A.; Winiarski, M.J.; Strychalska-Nowak, J.; Klimczuk, T.; Lisowski, W.; Mikolajczyk, A.; Pinto, H.P.; Puzyn, T.; Grzyb, T.; Zaleska-Medynska, A. Rare earth ions doped K2Ta2O6 photocatalysts with enhanced UV-vis light activity. Appl. Catal. B-Environ. 2018, 224, 451–468. [Google Scholar] [CrossRef]
- Rao, M.P.; Nandhini, V.P.; Wu, J.J.; Syed, A.; Ameen, F.; Anandan, S. Synthesis of N-doped potassium tantalate perovskite material for environmental applications. J. Solid State Chem. 2018, 258, 647–655. [Google Scholar] [CrossRef]
- Huang, F.; Tian, H.; Meng, X.; Tan, P.; Cao, X.; Bai, Y.; Hu, C.; Zhou, Z. Large Room Temperature Electrocaloric Effect in KTa1−xNbxO3 Single Crystal. Phys. Status Solidi-Rapid Res. Lett. 2019, 13, 1800515. [Google Scholar] [CrossRef]
- Yong, Z.; Ren, J.; Hu, H.; Li, P.; Ouyang, S.; Xu, H.; Wang, D. Synthesis, Characterization, and Photocatalytic Activity of g-C3N4/KTaO3 Composites under Visible Light Irradiation. J. Nanomater. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Hu, S.; Liu, X.; Wang, C.; Camargo, P.H.C.; Wang, J. Tuning Thermal Catalytic Enhancement in Doped MnO2–Au Nano-Heterojunctions. ACS Appl. Mater. Interf. 2019, 11, 17444–17451. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xing, P.; Chen, P.; Chen, Q.; Wang, Y.; Yu, J.; He, Y. Synthesis of carbon doped KTaO3 and its enhanced performance in photocatalytic H2 generation. Catal. Commun. 2018, 109, 6–9. [Google Scholar] [CrossRef]
- Reza Gholipour, M.; Dinh, C.-T.; Béland, F.; Do, T.-O. Nanocomposite heterojunctions as sunlight-driven photocatalysts for hydrogen production from water splitting. Nanoscale 2015, 7, 8187–8208. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xiao, C.; Wu, X.; Zhang, G. Potassium Tantalate K6Ta10.8O30 with Tungsten Bronze Structure and Its Photocatalytic Property. Chin. J. Chem. 2017, 35, 189–195. [Google Scholar] [CrossRef]
- Wang, H.; Gao, Y.; Liu, J.; Li, X.; Ji, M.; Zhang, E.; Cheng, X.; Xu, M.; Liu, J.; Rong, H.; et al. Efficient Plasmonic Au/CdSe Nanodumbbell for Photoelectrochemical Hydrogen Generation beyond Visible Region. Adv. Energy Mater. 2019, 9, 1803889. [Google Scholar] [CrossRef]
- Lin, S.; Lin, X.; Han, S.; Zhao, H.Y.; Hasi, W.; Wang, L. Highly monodisperse Au@Ag nanospheres: synthesis by controlled etching route and size-dependent SERS performance of their surperlattices. Nanotechnology 2019, 30, 215601. [Google Scholar] [CrossRef]
- Kumar, R.; Badilescu, S.; Packirisamy, M. Tuning of Morphology and Stability of Gold Nanostars Through pH Adjustment. J. Nanosci. Nanotechnol. 2019, 19, 4617–4622. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, Z.; Li, Z.; Xiao, J.; Shan, H.; Fang, Z.; Qi, L. Controlled growth and shape-directed self-assembly of gold nanoarrows. Sci. Adv. 2017, 3, e1701183. [Google Scholar] [CrossRef]
- Wu, Y.; Cai, S.; Wang, D.; He, W.; Li, Y. Syntheses of water-soluble octahedral, truncated octahedral, and cubic Pt-Ni nanocrystals and their structure-activity study in model hydrogenation reactions. J. Am. Chem. Soc. 2012, 134, 8975–8981. [Google Scholar] [CrossRef]
- Dej-Adisai, S.; Pitakbut, T. Determination of alpha-glucosidase inhibitory activity from selected Fabaceae plants. Pak. J. Pharm. Sci. 2015, 28, 1679–1683. [Google Scholar] [PubMed]
- Wang, Z.P.; Ma, B.K.; Shen, C.; Cheong, L.Z. Direct, selective and ultrasensitive electrochemical biosensing of methyl parathion in vegetables using Burkholderia cepacia lipase@MOF nanofibers-based biosensor. Talanta 2019, 197, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Mohan, A.M.V.; Soto, F.; Chrostowski, R.; Wang, J. A microneedle biosensor for minimally-invasive transdermal detection of nerve agents. Analyst 2017, 142, 918–924. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-G.; Sai-Anand, G.; Komathi, S.; Gopalan, A.I.; Kang, S.-W.; Lee, K.-P. Efficient visible-light-driven photocatalytic degradation of nitrophenol by using graphene-encapsulated TiO2 nanowires. J. Hazard. Mater. 2015, 283, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Mou, H.; Song, C.; Zhou, Y.; Zhang, B.; Wang, D. Design and synthesis of porous Ag/ZnO nanosheets assemblies as super photocatalysts for enhanced visible-light degradation of 4-nitrophenol and hydrogen evolution. Appl. Catal. B-Environ. 2018, 221, 565–573. [Google Scholar] [CrossRef]
- Naraginti, S.; Stephen, F.B.; Radhakrishnan, A.; Sivakumar, A. Zirconium and silver co-doped TiO2 nanoparticles as visible light catalyst for reduction of 4-nitrophenol, degradation of methyl orange and methylene blue. Spectrochim Acta A 2015, 135, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Bajorowicz, B.; Reszczyńska, J.; Lisowski, W.; Klimczuk, T.; Winiarski, M.; Słoma, M.; Zaleska-Medynska, A. Perovskite-type KTaO3–reduced graphene oxide hybrid with improved visible light photocatalytic activity. RSC Adv. 2015, 5, 91315–91325. [Google Scholar] [CrossRef]
- Ji, M.W.; Xu, M.; Zhang, W.; Yang, Z.Z.; Huang, L.; Liu, J.J.; Zhang, Y.; Gu, L.; Yu, Y.X.; Hao, W.C.; et al. Structurally Well-Defined Au@Cu2−xS Core-Shell Nanocrystals for Improved Cancer Treatment Based on Enhanced Photothermal Efficiency. Adv. Mater. 2016, 28, 3094–3101. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, S.; Ji, M.; Yan, C.; Zhang, K.; Deng, Q.; Xu, J.; Zhu, C.; Li, B.; Wang, J. In-Situ Growth of Au on KTaO3 Sub-Micron Cubes via Wet Chemical Approach for Enhanced Photodegradation of p-Nitrophenol. Materials 2019, 12, 1950. https://doi.org/10.3390/ma12121950
Chang S, Ji M, Yan C, Zhang K, Deng Q, Xu J, Zhu C, Li B, Wang J. In-Situ Growth of Au on KTaO3 Sub-Micron Cubes via Wet Chemical Approach for Enhanced Photodegradation of p-Nitrophenol. Materials. 2019; 12(12):1950. https://doi.org/10.3390/ma12121950
Chicago/Turabian StyleChang, Shengding, Muwei Ji, Changxu Yan, Kai Zhang, Qian Deng, Jian Xu, Caizhen Zhu, Bo Li, and Jin Wang. 2019. "In-Situ Growth of Au on KTaO3 Sub-Micron Cubes via Wet Chemical Approach for Enhanced Photodegradation of p-Nitrophenol" Materials 12, no. 12: 1950. https://doi.org/10.3390/ma12121950
APA StyleChang, S., Ji, M., Yan, C., Zhang, K., Deng, Q., Xu, J., Zhu, C., Li, B., & Wang, J. (2019). In-Situ Growth of Au on KTaO3 Sub-Micron Cubes via Wet Chemical Approach for Enhanced Photodegradation of p-Nitrophenol. Materials, 12(12), 1950. https://doi.org/10.3390/ma12121950



