The Effect of Cellulose Nanocrystal Coatings on the Glass Fiber–Epoxy Interphase
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. CNC-Dispersion and Emulsion Preparation
2.3. Addition of CNCs on the GF Surface
2.4. Fiber and Composite Imaging
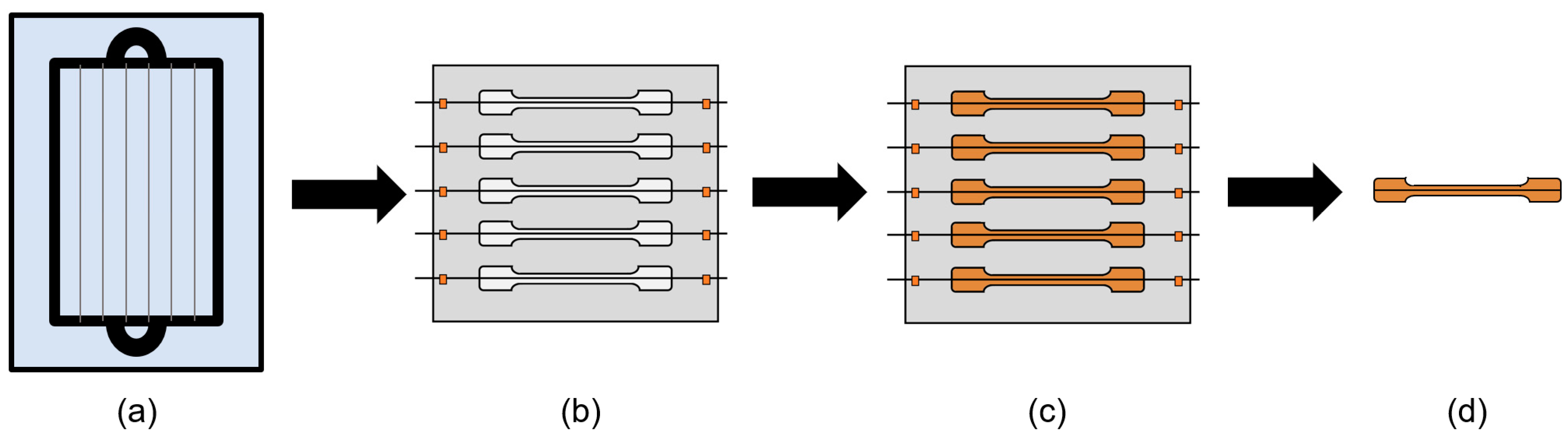
2.5. Characterization of Interfacial Shear Strength (IFSS)
2.6. CNC–Fiber Interactions
3. Results
3.1. Probing the Coatings on GF Using FTIR
3.2. Coating Characterization by SEM and TGA
3.3. Fragmentation Behavior
3.4. Photoelastic Features at Fragmentation
4. Discussion
4.1. Effect of Dispersion Media on Fragmentation
4.2. Effect of CNC Surface Functionalization on Fragmentation
4.3. Effect of CNC Concentration on Fragmentation
5. Conclusions
Author Contributions
Disclaimer
Funding
Acknowledgments
Conflicts of Interest
References
- Gattesco, N.; Amadio, C.; Bedon, C. Experimental and numerical study on the shear behavior of stone masonry walls strengthened with GFRP reinforced mortar coating and steel-cord reinforced repointing. Eng. Struct. 2015, 90, 143–157. [Google Scholar] [CrossRef] [Green Version]
- Valluzzi, M.R.; Modena, C.; de Felice, G. Current practice and open issues in strengthening historical buildings with composites. Mater. Struct. 2014, 47, 1971–1985. [Google Scholar] [CrossRef]
- Siwowski, T.; Rajchel, M.; Kaleta, D.; Własak, L. The First Polish Road Bridge Made of FRP Composites. Struct. Eng. Int. 2017, 27, 308–314. [Google Scholar] [CrossRef]
- Bedon, C.; Agullo, C.P.; Luna-Navarro, A.; Overend, M.; Favoino, F. Thermo-mechanical Investigation of Novel GFRP-glass Sandwich Facade Components. Chall. Glass Conf. Proc. 2018, 6, 501–512. [Google Scholar] [CrossRef]
- Friedrich, K.; Almajid, A.A. Manufacturing Aspects of Advanced Polymer Composites for Automotive Applications. Appl. Compos. Mater. 2013, 20, 107–128. [Google Scholar] [CrossRef]
- Gu, Y.; Li, M.; Wang, J.; Zhang, Z. Characterization of the interphase in carbon fiber/polymer composites using a nanoscale dynamic mechanical imaging technique. Carbon 2010, 48, 3229–3235. [Google Scholar] [CrossRef]
- Hodzic, A.; Stachurski, Z.H.; Kim, J.K. Nano-indentation of polymer–glass interfaces Part I. Experimental and mechanical analysis. Polymer 2000, 41, 6895–6905. [Google Scholar] [CrossRef]
- Thomason, J.L.; Adzima, L.J. Sizing up the interphase: An insider’s guide to the science of sizing. Compos. Part A Appl. Sci. Manuf. 2001, 32, 313–321. [Google Scholar] [CrossRef]
- Mäder, E.; Gao, S.L.; Plonka, R. Static and dynamic properties of single and multi-fiber/epoxy composites modified by sizings. Compos. Sci. Technol. 2007, 67, 1105–1115. [Google Scholar] [CrossRef]
- Thomason, J.L. The interface region in glass fibre-reinforced epoxy resin composites: 3. Characterization of fibre surface coatings and the interphase. Composites 1995, 26, 487–498. [Google Scholar] [CrossRef]
- Zhang, J.; Zhuang, R.; Liu, J.; Mäder, E.; Heinrich, G.; Gao, S. Functional interphases with multi-walled carbon nanotubes in glass fibre/epoxy composites. Carbon 2010, 48, 2273–2281. [Google Scholar] [CrossRef]
- Zinck, P.; Mäder, E.; Gerard, J.F. Role of silane coupling agent and polymeric film former for tailoring glass fiber sizings from tensile strength measurements. J. Mater. Sci. 2001, 36, 5245–5252. [Google Scholar] [CrossRef]
- Mahmood, H.; Tripathi, M.; Pugno, N.; Pegoretti, A. Enhancement of interfacial adhesion in glass fiber/epoxy composites by electrophoretic deposition of graphene oxide on glass fibers. Compos. Sci. Technol. 2016, 126, 149–157. [Google Scholar] [CrossRef]
- Qin, W.; Vautard, F.; Drzal, L.T.; Yu, J. Mechanical and electrical properties of carbon fiber composites with incorporation of graphene nanoplatelets at the fiber–matrix interphase. Compos. Part B Eng. 2015, 69, 335–341. [Google Scholar] [CrossRef]
- Gao, X.; Jensen, R.E.; McKnight, S.H.; Gillespie, J.W. Effect of colloidal silica on the strength and energy absorption of glass fiber/epoxy interphases. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1738–1747. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Brinchi, L.; Cotana, F.; Fortunati, E.; Kenny, J.M. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr. Polym. 2013, 94, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Moon, R.J.; Schueneman, G.T.; Simonsen, J. Overview of Cellulose Nanomaterials, Their Capabilities and Applications. JOM 2016, 68, 2383–2394. [Google Scholar] [CrossRef]
- Börjesson, M.; Westman, G. Crystalline nanocellulose—Preparation, modification, and properties. In Cellulose-Fundamental Aspects and Current Trends; InTech: London, UK, 2015. [Google Scholar]
- Lu, P.; Hsieh, Y.L. Preparation and properties of cellulose nanocrystals: Rods, spheres, and network. Carbohydr. Polym. 2010, 82, 329–336. [Google Scholar] [CrossRef]
- Habibi, Y. Key advances in the chemical modification of nanocelluloses. Chem. Soc. Rev. 2014, 43, 1519–1542. [Google Scholar] [CrossRef]
- De Assis, C.A.; Houtman, C.; Phillips, R.; Bilek, E.; Rojas, O.J.; Pal, L.; Peresin, M.S.; Jameel, H.; Gonzalez, R. Conversion Economics of Forest Biomaterials: Risk and Financial Analysis of CNC Manufacturing. Biofuels Bioprod. Biorefining 2017, 11, 682–700. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, J.Y.; Baez, C.; Kitin, P.; Elder, T. Highly thermal-stable and functional cellulose nanocrystals and nanofibrils produced using fully recyclable organic acids. Green Chem. 2016, 18, 3835–3843. [Google Scholar] [CrossRef]
- Fox, D.M.; Rodriguez, R.S.; Devilbiss, M.N.; Woodcock, J.; Davis, C.S.; Sinko, R.; Keten, S.; Gilman, J.W. Simultaneously Tailoring Surface Energies and Thermal Stabilities of Cellulose Nanocrystals Using Ion Exchange: Effects on Polymer Composite Properties for Transportation, Infrastructure, and Renewable Energy Applications. ACS Appl. Mater. Interfaces 2016, 8, 27270–27281. [Google Scholar] [CrossRef] [PubMed]
- Asadi, A.; Miller, M.; Moon, R.; Kalaitzidou, K. Improving the interfacial and mechanical properties of short glass fiber/epoxy composites by coating the glass fibers with cellulose nanocrystals. Express Polym. Lett. 2016, 10, 587. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, X.; Yin, X.; Lin, Q.; Zhu, M. A Novel Route to Modify the Interface of Glass Fiber-Reinforced Epoxy Resin Composite via Bacterial Cellulose. Int. J. Polym. Mater. Polym. Biomater. 2014, 63, 221–227. [Google Scholar] [CrossRef]
- Reid, M.S.; Villalobos, M.; Cranston, E.D. Benchmarking Cellulose Nanocrystals: From the Laboratory to Industrial Production. Langmuir 2017, 33, 1583–1598. [Google Scholar] [CrossRef]
- Kelly, A.; Tyson, W.R. Tensile properties of fibre-reinforced metals: Copper/tungsten and copper/molybdenum. J. Mech. Phys. Solids 1965, 13, 329–350. [Google Scholar] [CrossRef]
- Holmes, G.A.; Peterson, R.C.; Hunston, D.L.; McDonough, W.G.; Schutte, C.L. The Effect of Nonlinear Viscoelasticity on Interfacial Shear Strength Measurements. In Time Dependent and Nonlinear Effects in Polymers and Composites; Schapery, R.A., Ed.; ASTM: West Conshohocken, PA, USA, 2000; Volume ASTM STP 1357, pp. 98–117. [Google Scholar]
- Hui, C.Y.; Phoenix, S.; Shia, D. The single-filament-composite test: A new statistical theory for estimating the interfacial shear strength and Weibull parameters for fiber strength. Compos. Sci. Technol. 1998, 57, 1707–1725. [Google Scholar] [CrossRef]
- Zhandarov, S.; Pisanova, E.; Dovgyalo, V. Fragmentation of a single filament during tension in a matrix as a method of determining adhesion. Mech. Compos. Mater. 1992, 28, 270–286. [Google Scholar] [CrossRef]
- Kargarzadeh, H.; MSheltami, R.; Ahmad, I.; Abdullah, I.; Dufresne, A. Cellulose nanocrystal: A promising toughening agent for unsaturated polyester nanocomposite. Polymer 2015, 56, 346–357. [Google Scholar] [CrossRef]
- Xu, X.; Liu, F.; Jiang, L.; Zhu, J.Y.; Haagenson, D.; Wiesenborn, D.P. Cellulose Nanocrystals vs. Cellulose Nanofibrils: A Comparative Study on Their Microstructures and Effects as Polymer Reinforcing Agents. ACS Appl. Mater. Interfaces 2013, 5, 2999–3009. [Google Scholar] [CrossRef] [PubMed]
- Stoch, L.; Środa, M. Infrared spectroscopy in the investigation of oxide glasses structure. J. Mol. Struct. 1999, 511–512, 77–84. [Google Scholar] [CrossRef]
- Basic Analysis. In JMP® 12 Documentation Library; SAS Institute Inc.: Cary, NC, USA, 2015; pp. 169–171, 189–196.
- Drzal, L.T.; Madhukar, M. Fibre-matrix adhesion and its relationship to composite mechanical properties. J. Mater. Sci. 1993, 28, 569–610. [Google Scholar] [CrossRef]
- Kim, B.W.; Nairn, J.A. Observations of Fiber Fracture and Interfacial Debonding Phenomena Using the Fragmentation Test in Single Fiber Composites. J. Compos. Mater. 2002, 36, 1825–1858. [Google Scholar] [CrossRef]
- Zhou, X.F.; Nairn, J.A.; Wagner, H.D. Fiber–matrix adhesion from the single-fiber composite test: Nucleation of interfacial debonding. Compos. Part A Appl. Sci. Manuf. 1999, 30, 1387–1400. [Google Scholar] [CrossRef]
- Michael, T.P.; András, V.; John, D.; Natalia, F.; Bin, M.; Ryan, W.; Arvind, R.; Robert, J.M.; Ronald, S.; Theodore, H.W.; et al. Development of the metrology and imaging of cellulose nanocrystals. Meas. Sci. Technol. 2011, 22, 024005. [Google Scholar]
- Mo, Z.L.; Zhao, Z.L.; Chen, H.; Niu, G.P.; Shi, H.F. Heterogeneous preparation of cellulose-polyaniline conductive composites with cellulose activated by acids and its electrical properties. Carbohydr. Polym. 2009, 75, 660–664. [Google Scholar] [CrossRef]
- Zhang, X.; Fan, X.; Yan, C.; Li, H.; Zhu, Y.; Li, X.; Yu, L. Interfacial microstructure and properties of carbon fiber composites modified with graphene oxide. ACS Appl. Mater. Interfaces 2012, 4, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Karger-Kocsis, J.; Mahmood, H.; Pegoretti, A. Recent advances in fiber/matrix interphase engineering for polymer composites. Prog. Mater. Sci. 2015, 73, 1–43. [Google Scholar] [CrossRef]
- Li, Q.; Church, J.S.; Naebe, M.; Fox, B.L. Interfacial characterization and reinforcing mechanism of novel carbon nanotube—Carbon fibre hybrid composites. Carbon 2016, 109, 74–86. [Google Scholar] [CrossRef]
- Zhu, J.; Imam, A.; Crane, R.; Lozano, K.; Khabashesku, V.N.; Barrera, E.V. Processing a glass fiber reinforced vinyl ester composite with nanotube enhancement of interlaminar shear strength. Compos. Sci. Technol. 2007, 67, 1509–1517. [Google Scholar] [CrossRef]
- Gao, S.L.; Mäder, E.; Plonka, R. Nanocomposite coatings for healing surface defects of glass fibers and improving interfacial adhesion. Compos. Sci. Technol. 2008, 68, 2892–2901. [Google Scholar] [CrossRef] [Green Version]
- Ansari, F.; Galland, S.; Johansson, M.; Plummer, C.J.G.; Berglund, L.A. Cellulose nanofiber network for moisture stable, strong and ductile biocomposites and increased epoxy curing rate. Compos. Part A Appl. Sci. Manuf. 2014, 63, 35–44. [Google Scholar] [CrossRef] [Green Version]







| 1st Part | 2nd Part | 3rd Part | 4th Part | 5th Part | |
|---|---|---|---|---|---|
| Variable: | Fiber type | Dispersant concentration% | Dispersion medium | CNC% in solution | CNC functionality |
| Values: |
|
|
|
|
|
| Sample | Coating Type | Sample | Coating Type |
|---|---|---|---|
| G0 | As-received | G5e | GF coat 5%Emulsion |
| Ga1CI | GF coat 1%CNCS | G5e1CII | GF coat 5%Emulsion 1%CNCPh |
| Ga1CII | GF coat 1%CNCPh | G5e2CII | GF coat 5%Emulsion 2%CNCPh |
| Ga2CII | GF coat 2%CNCPh | G1e1CII | GF coat 1%Emulsion 1%CNCPh |
| Gp | GF coat 0.01%PLL | G5e1CI | GF coat 5%Emulsion 1%CNCS |
| Gp0.1CI | GF coat 0.01%PLL 0.1%CNCS | G5s | GF coat 5%EP871 |
| Gp0.1CII | GF coat 0.01%PLL 0.1%CNCPh | G5s1CII | GF coat 5%EP871 1%CNCPh |
| Gp1CI | GF coat 0.01%PLL 1%CNCS | ||
| Gp1CII | GF coat 0.01%PLL 1%CNCPh |
| Coating Type | G0 | Ga1CII | G5e | G5e1CII | G5s1CII |
|---|---|---|---|---|---|
| Avg. mass% | 0.75 (0.03) | 2.15 (0.38) | 3.92 (0.13) | 6.66 (0.71) | 7.44 (0.91) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goswami, J.; Haque, E.; Fox, D.M.; Gilman, J.W.; Holmes, G.A.; Moon, R.J.; Kalaitzidou, K. The Effect of Cellulose Nanocrystal Coatings on the Glass Fiber–Epoxy Interphase. Materials 2019, 12, 1951. https://doi.org/10.3390/ma12121951
Goswami J, Haque E, Fox DM, Gilman JW, Holmes GA, Moon RJ, Kalaitzidou K. The Effect of Cellulose Nanocrystal Coatings on the Glass Fiber–Epoxy Interphase. Materials. 2019; 12(12):1951. https://doi.org/10.3390/ma12121951
Chicago/Turabian StyleGoswami, Joyanta, Ejaz Haque, Douglas M. Fox, Jeffrey W. Gilman, Gale A. Holmes, Robert J. Moon, and Kyriaki Kalaitzidou. 2019. "The Effect of Cellulose Nanocrystal Coatings on the Glass Fiber–Epoxy Interphase" Materials 12, no. 12: 1951. https://doi.org/10.3390/ma12121951
APA StyleGoswami, J., Haque, E., Fox, D. M., Gilman, J. W., Holmes, G. A., Moon, R. J., & Kalaitzidou, K. (2019). The Effect of Cellulose Nanocrystal Coatings on the Glass Fiber–Epoxy Interphase. Materials, 12(12), 1951. https://doi.org/10.3390/ma12121951






