Three-Dimensional Characterization of Hardened Paste of Hydrated Tricalcium Silicate by Serial Block-Face Scanning Electron Microscopy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
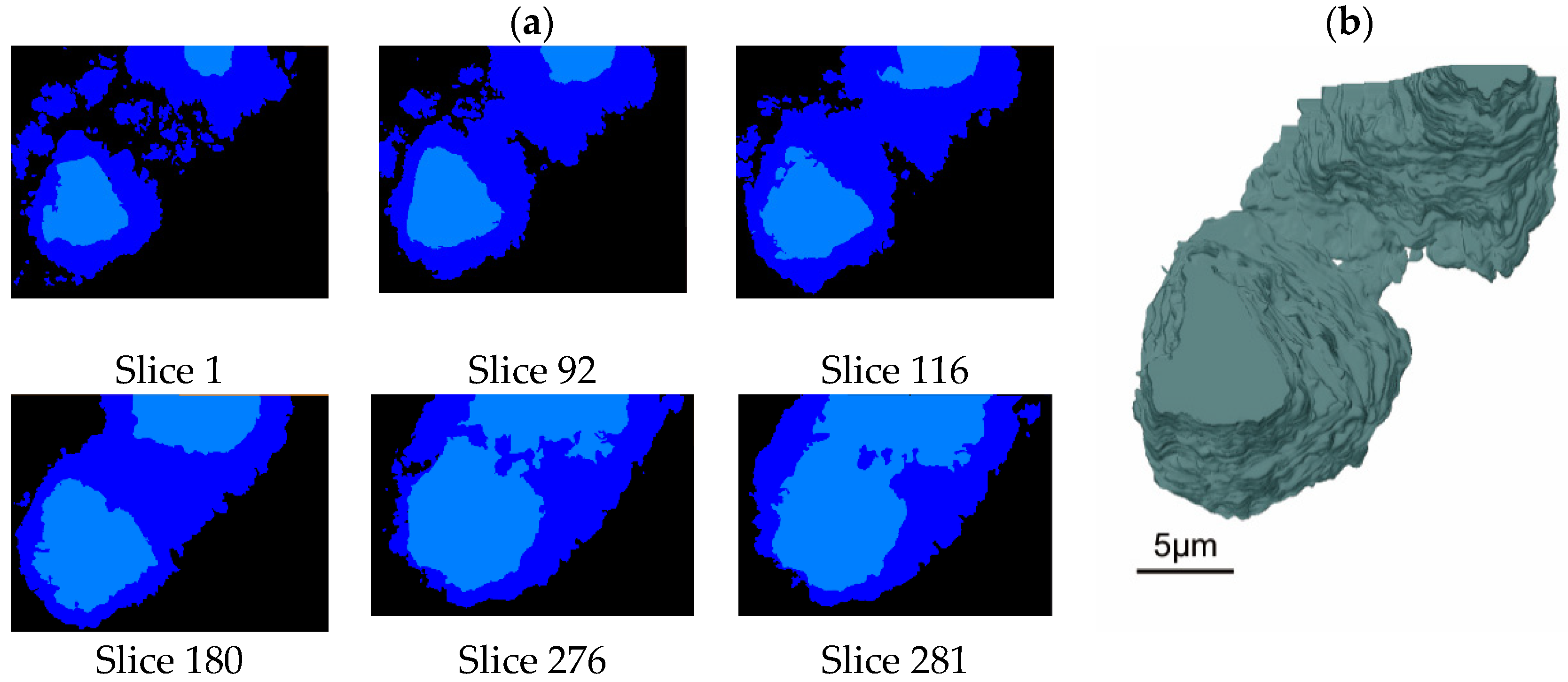
3.1. The 3D Microstructure of Hardened Paste of Hydrated C3S
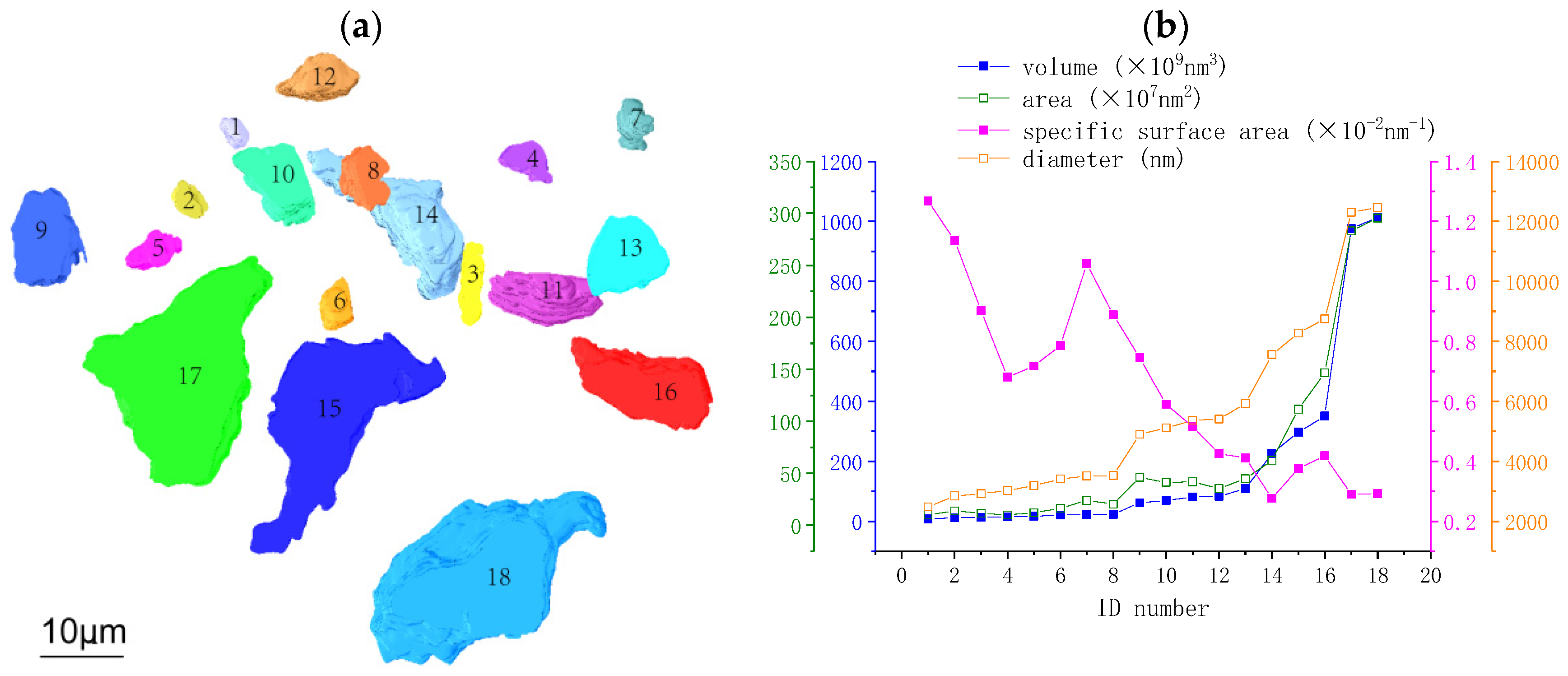
3.2. Analysis of Anhydrous C3S Particles in the Hydrated C3S Paste
3.2.1. Morphological Parameters of Anhydrous C3S
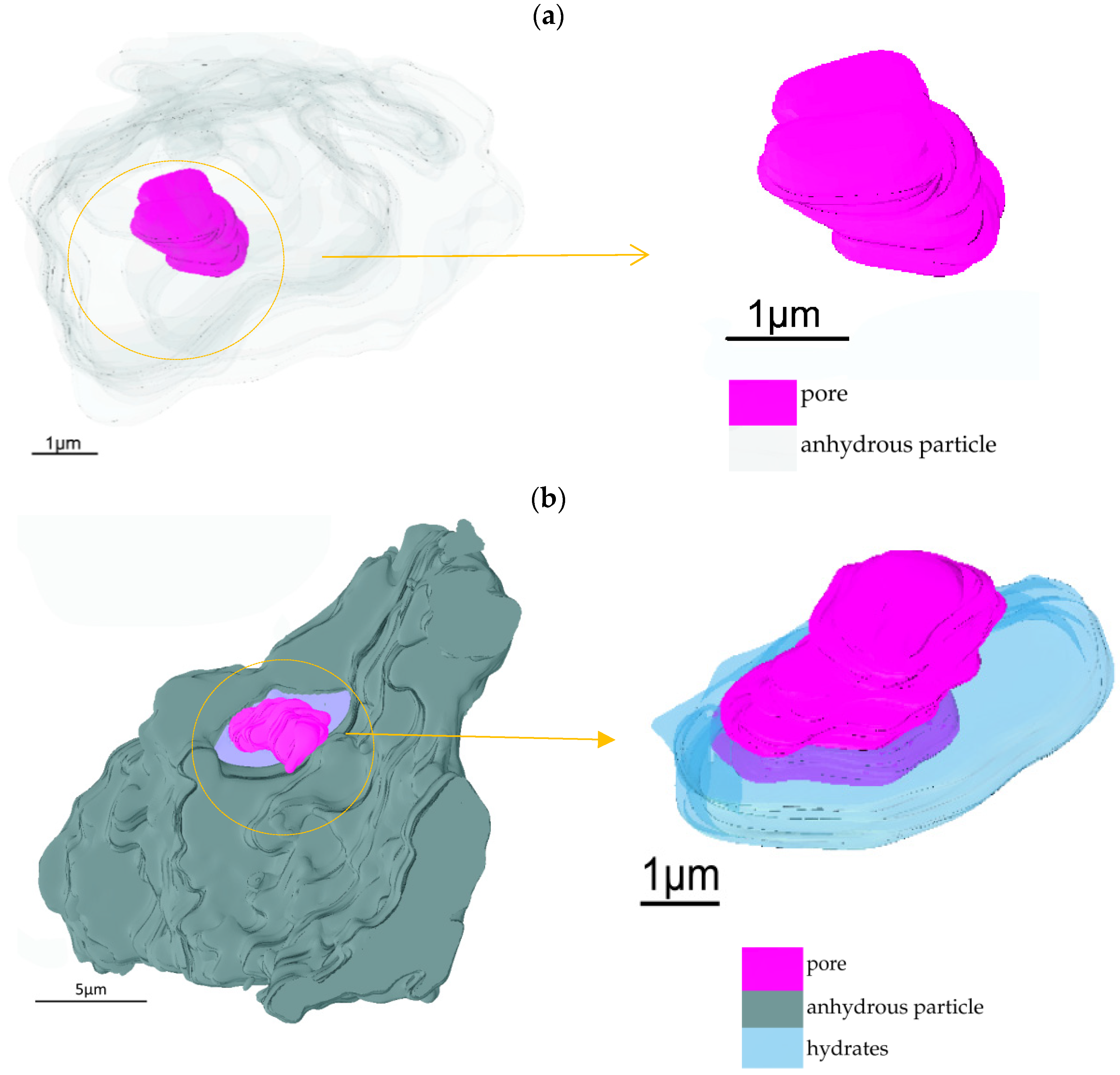
3.2.2. Interface Structure between Anhydrous C3S Particles and Hydrates
3.3. Analysis of Pore Structure in the Hydrated C3S Paste
3.3.1. Quantitative Analysis of Pores
3.3.2. The Spatial Distribution of Pores
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Richardson, I. Cement Chemistry, 3rd ed.; ICE Publishing: London, UK, 2018. [Google Scholar]
- Hu, Q.; Aboustait, M.; Kim, T.; Ley, M.T.; Bullard, J.W.; Scherer, G.; Hanan, J.C.; Rose, V.; Winarski, R.; Gelb, J. Direct measurements of 3d structure, chemistry and mass density during the induction period of C3S hydration. Cem. Concr. Res. 2016, 89, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.F.W.; Barret, P.; Brown, P.W.; Double, D.D.; Frohnsdorff, G.; Johansen, V.; Ménétrier-Sorrentino, D.; Odler, I.; Parrott, L.J.; Pommersheim, J.M.; et al. The hydration of tricalcium silicate. Mater. Struct. 1984, 17, 457–468. [Google Scholar] [CrossRef]
- Bullard, J.W.; Jennings, H.M.; Livingston, R.A.; Nonat, A.; Scherer, G.W.; Schweitzer, J.S.; Scrivener, K.L.; Thomas, J.J. Mechanisms of cement hydration. Cem. Concr. Res. 2011, 41, 1208–1223. [Google Scholar] [CrossRef]
- Stark, J. Recent advances in the field of cement hydration and microstructure analysis. Cem. Concr. Res. 2011, 41, 666–678. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Nonat, A. Hydration of cementitious materials, present and future. Cem. Concr. Res. 2011, 41, 651–665. [Google Scholar] [CrossRef]
- Esteves, L.P. On the hydration of water-entrained cement–silica systems: Combined SEM, XRD and thermal analysis in cement pastes. Thermochim. Acta 2011, 518, 27–35. [Google Scholar] [CrossRef]
- Feng, X.; Garboczi, E.J.; Bentz, D.P.; Stutzman, P.E.; Mason, T.O. Estimation of the degree of hydration of blended cement pastes by a scanning electron microscope point-counting procedure. Cem. Concr. Res. 2004, 34, 1787–1793. [Google Scholar] [CrossRef]
- Franus, W.; Panek, R.; Wdowin, M. SEM Investigation of Microstructures in Hydration Products of Portland Cement. In 2nd International Multidisciplinary Microscopy and Microanalysis Congress; Polychroniadis, E.K., Oral, A.Y., Ozer, M., Eds.; Springer International Publishing: Cham, Denmark, 2015; pp. 105–112. [Google Scholar]
- Igarashi, S.; Kawamura, M.; Watanabe, A. Analysis of cement pastes and mortars by a combination of backscatter-based SEM image analysis and calculations based on the Powers model. Cem. Concr. Compos. 2004, 26, 977–985. [Google Scholar] [CrossRef] [Green Version]
- Ylmén, R.; Jäglid, U.; Steenari, B.-M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- Groves, G.W. TEM Studies of Cement Hydration. Symp. M Microstruct. Dev. Hydration Cem. 2011, 85, 3. [Google Scholar] [CrossRef]
- Richardson, I.G.; Skibsted, J.; Black, L.; Kirkpatrick, R.J. Characterisation of cement hydrate phases by TEM, NMR and Raman spectroscopy. Adv. Cem. Res. 2010, 22, 233–248. [Google Scholar] [CrossRef]
- Han, S.; Yan, P.; Liu, R. Study on the hydration product of cement in early age using TEM. Sci. China Technol. Sci. 2012, 55, 2284–2290. [Google Scholar] [CrossRef]
- Papadakis, V.G.; Pedersen, E.J.; Lindgreen, H. An AFM-SEM investigation of the effect of silica fume and fly ash on cement paste microstructure. J. Mater. Sci. 1999, 34, 683–690. [Google Scholar] [CrossRef]
- Peled, A.; Castro, J.; Weiss, W.J. Atomic force and lateral force microscopy (AFM and LFM) examinations of cement and cement hydration products. Cem. Concr. Compos. 2013, 36, 48–55. [Google Scholar] [CrossRef]
- Chotard, T.J.; Boncoeur-Martel, M.P.; Smith, A.; Dupuy, J.P.; Gault, C. Application of X-ray computed tomography to characterise the early hydration of calcium aluminate cement. Cem. Concr. Compos. 2003, 25, 145–152. [Google Scholar] [CrossRef]
- Hu, Q.; Aboustait, M.; Kim, T.; Ley, M.T.; Hanan, J.C.; Bullard, J.; Winarski, R.; Rose, V. Direct three-dimensional observation of the microstructure and chemistry of C3S hydration. Cem. Concr. Res. 2016, 88, 157–169. [Google Scholar] [CrossRef] [PubMed]
- Gastaldi, D.; Canonico, F.; Capelli, L.; Boccaleri, E.; Milanesio, M.; Palin, L.; Croce, G.; Marone, F.; Mader, K.; Stampanoni, M. In situ tomographic investigation on the early hydration behaviors of cementing systems. Constr. Build. Mater. 2012, 29, 284–290. [Google Scholar] [CrossRef]
- Helfen, L.; Dehn, F.; Mikulík, P.; Baumbach, T. Three-dimensional imaging of cement microstructure evolution during hydration. Adv. Cem. Res. 2005, 17, 103–111. [Google Scholar] [CrossRef]
- Liu, X.; Aranda, M.A.G.; Chen, B.; Wang, P.; Harder, R.; Robinson, I. In Situ Bragg Coherent Diffraction Imaging Study of a Cement Phase Microcrystal during Hydration. Cryst. Growth Des. 2015, 15, 3087–3091. [Google Scholar] [CrossRef] [Green Version]
- Hesse, C.; Goetz-Neunhoeffer, F.; Neubauer, J. A new approach in quantitative in-situ XRD of cement pastes: Correlation of heat flow curves with early hydration reactions. Cem. Concr. Res. 2011, 41, 123–128. [Google Scholar] [CrossRef]
- Mendes, A.; Gates, W.P.; Sanjayan, J.G.; Collins, F. NMR, XRD, IR and synchrotron NEXAFS spectroscopic studies of OPC and OPC/slag cement paste hydrates. Mater. Struct. 2011, 44, 1773–1791. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Füllmann, T.; Gallucci, E.; Walenta, G.; Bermejo, E. Quantitative study of Portland cement hydration by X-ray diffraction/Rietveld analysis and independent methods. Cem. Concr. Res. 2004, 34, 1541–1547. [Google Scholar] [CrossRef]
- Soin, A.V.; Catalan, L.J.J.; Kinrade, S.D. A combined QXRD/TG method to quantify the phase composition of hydrated Portland cements. Cem. Concr. Res. 2013, 48, 17–24. [Google Scholar] [CrossRef]
- Vedalakshmi, R.; Sundara Raj, A.; Srinivasan, S.; Ganesh Babu, K. Quantification of hydrated cement products of blended cements in low and medium strength concrete using TG and DTA technique. Thermochim. Acta 2003, 407, 49–60. [Google Scholar] [CrossRef]
- Winslow, D.N.; Diamond, S. Specific Surface of Hardened Portland Cement Paste as Determined by Small-Angle X-Ray Scattering. J. Am. Ceram. Soc. 1974, 57, 193–197. [Google Scholar] [CrossRef]
- Guryanov, A.M. Nanoscale investigation by small angle neutron scattering of modified Portland cement compositions. In XXIV R-S-P Seminar, Theoretical Foundation of Civil Engineering; Jemiolo, S., Gajewski, M., Krzeminski, M., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2015; Volume 111, pp. 283–289. [Google Scholar]
- Allen, A.J.; Thomas, J.J. Analysis of C–S–H gel and cement paste by small-angle neutron scattering. Cem. Concr. Res. 2007, 37, 319–324. [Google Scholar] [CrossRef]
- Parisatto, M.; Dalconi, M.C.; Valentini, L.; Artioli, G.; Rack, A.; Tucoulou, R.; Cruciani, G.; Ferrari, G. Examining microstructural evolution of Portland cements by in-situ synchrotron micro-tomography. J. Mater. Sci. 2015, 50, 1805–1817. [Google Scholar] [CrossRef]
- Cook, R.A.; Hover, K.C. Mercury porosimetry of cement-based materials and associated correction factors. Constr. Build. Mater. 1993, 7, 231–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Zhou, J.; Ye, G.; Shui, Z. Pore structure of blended cement paste by means of pressurization¿ depressurization cycling mercury intrusion porosimetry. In Proceedings of the 1st Ageing of Materials & Structures Conference, Delft, The Netherlands, 26–28 May 2014. [Google Scholar]
- Ibrahim, M.; Arockiaraj, J.; Amritanand, R.; Venkatesh, K.; David, K.S. Recurrent Lumbar Disc Herniation: Results of Revision Surgery and Assessment of Factors that May Affect the Outcome. A Non-Concurrent Prospective Study. Asian Spine J. 2015, 9, 728–736. [Google Scholar] [CrossRef] [Green Version]
- Leighton, S.B. SEM images of block faces, cut by a miniature microtome within the SEM—A technical note. Scan. Electron. Microsc. 1981, 1981 Pt 2, 73–76. [Google Scholar]
- Denk, W.; Horstmann, H. Serial Block-Face Scanning Electron Microscopy to Reconstruct Three-Dimensional Tissue Nanostructure. PLoS Biol. 2004, 2, e329. [Google Scholar] [CrossRef] [PubMed]
- Zankel, A.; Kraus, B.; Poelt, P.; Schaffer, M.; Ingolic, E. Ultramicrotomy in the ESEM, a versatile method for materials and life sciences. J. Microsc. 2009, 233, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.; Milhet, X.; Gadaud, P.; Boyer, S.A.E.; Thompson, G.E.; Lee, P. Quantitative characterization of porosity and determination of elastic modulus for sintered micro-silver joints. J. Mater. Process. Technol. 2015, 225, 19–23. [Google Scholar] [CrossRef]
- Chen, B.; Guizar-Sicairos, M.; Xiong, G.; Shemilt, L.; Diaz, A.; Nutter, J.; Burdet, N.; Huo, S.; Mancuso, J.; Monteith, A.; et al. Three-Dimensional Structure Analysis and Percolation Properties of a Barrier Marine Coating. Sci. Rep. 2013, 3, 1177. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Curioni, M.; Zhou, X.; Mancuso, J.; Skeldon, P.; Thompson, G.E. Investigation of dealloying by ultra-high-resolution nanotomography. Surf. Interface Anal. 2012, 45, 1548–1552. [Google Scholar] [CrossRef]
- Lu, J.; Chen, B.; Liu, X.; Yang, F.; Robinson, I.K. 3D microstructure reconstruction of casting aluminum alloy based on serial block-face scanning electron microscopy. J. Alloys Compd. 2019, 778, 721–730. [Google Scholar] [CrossRef]
- Bai, X.; Chen, B.; Yang, F.; Liu, X.P.; Silva-Nunes, D.; Robinson, I. Three-dimensional imaging and analysis of the internal structure of SAPO-34 zeolite crystals. RSC Adv. 2018, 8, 33631–33636. [Google Scholar] [CrossRef] [Green Version]
- Kubota, Y. New developments in electron microscopy for serial image acquisition of neuronal profiles. Microscopy 2015, 64, 27–36. [Google Scholar] [CrossRef]
- Peddie, C.J.; Collinson, L.M. Exploring the third dimension: Volume electron microscopy comes of age. Micron 2014, 61, 9–19. [Google Scholar] [CrossRef]
- Yang, F.; Liu, X.; Zhao, Y.; Zhang, Y.; Wang, P.; Robinson, I.; Chen, B. Investigation of Three-Dimensional Microstructure of Tricalcium Silicate (C3S) by Electron Microscopy. Materials 2018, 11, 1110. [Google Scholar] [CrossRef]
- De la Torre, A.G.; Aranda, M.A.G. Accuracy in Rietveld quantitative phase analysis of Portland cements. J. Appl. Crystallogr. 2003, 36, 1169–1176. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Dai, J.-G. X-ray computed tomography for pore-related characterization and simulation of cement mortar matrix. NDT E Int. 2017, 86, 28–35. [Google Scholar] [CrossRef]
- Bullard, J.W.; Hagedorn, J.; Ley, M.T.; Hu, Q.; Griffin, W.; Terrill, J.E. A Critical Comparison of 3D Experiments and Simulations of Tricalcium Silicate Hydration. J. Am. Ceram. Soc. 2018, 101, 1453–1470. [Google Scholar] [CrossRef] [PubMed]


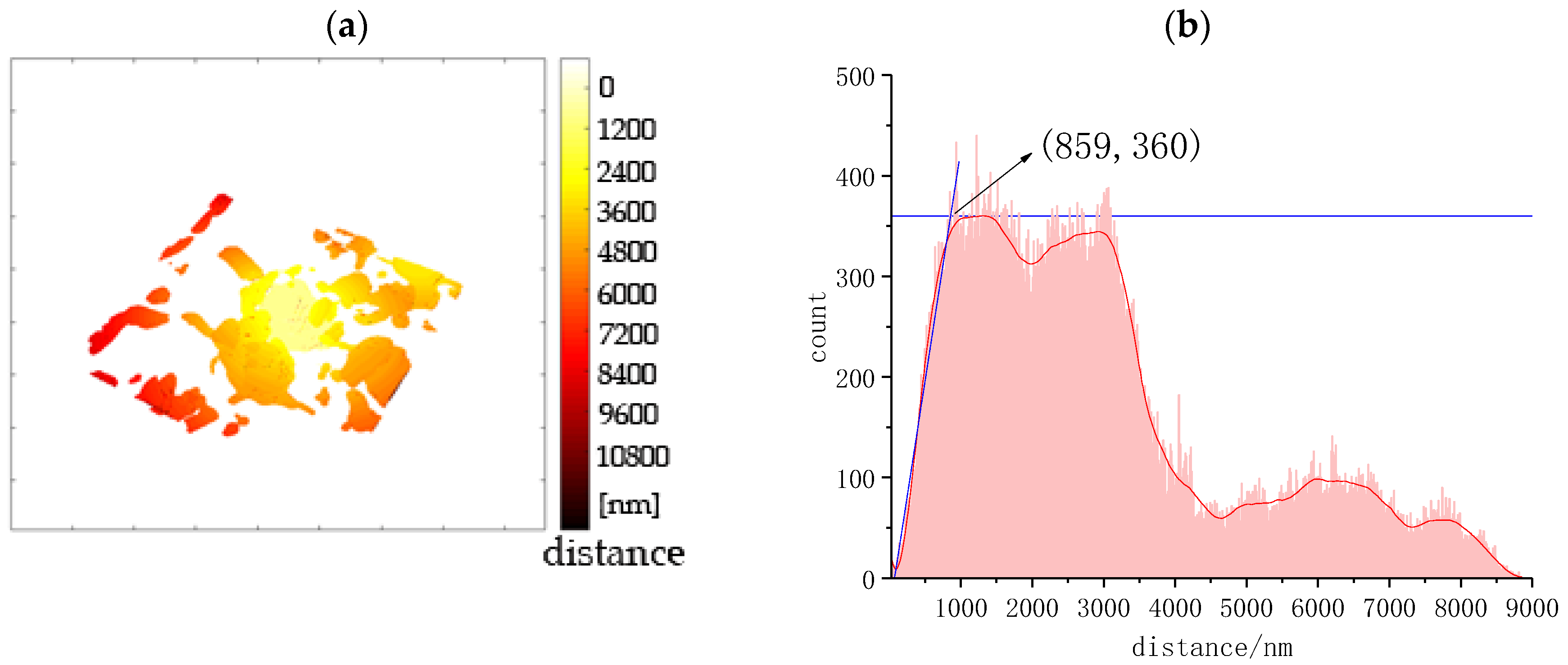
| Min | Mean | Max | Inflexion | |
|---|---|---|---|---|
| Distance/nm | 30 | 4400 | 9000 | 860 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Liu, X.; Chen, B.; Yang, F.; Zhang, Y.; Wang, P.; Robinson, I. Three-Dimensional Characterization of Hardened Paste of Hydrated Tricalcium Silicate by Serial Block-Face Scanning Electron Microscopy. Materials 2019, 12, 1882. https://doi.org/10.3390/ma12121882
Zhao Y, Liu X, Chen B, Yang F, Zhang Y, Wang P, Robinson I. Three-Dimensional Characterization of Hardened Paste of Hydrated Tricalcium Silicate by Serial Block-Face Scanning Electron Microscopy. Materials. 2019; 12(12):1882. https://doi.org/10.3390/ma12121882
Chicago/Turabian StyleZhao, Yongjuan, Xianping Liu, Bo Chen, Fei Yang, Yongming Zhang, Peiming Wang, and Ian Robinson. 2019. "Three-Dimensional Characterization of Hardened Paste of Hydrated Tricalcium Silicate by Serial Block-Face Scanning Electron Microscopy" Materials 12, no. 12: 1882. https://doi.org/10.3390/ma12121882
APA StyleZhao, Y., Liu, X., Chen, B., Yang, F., Zhang, Y., Wang, P., & Robinson, I. (2019). Three-Dimensional Characterization of Hardened Paste of Hydrated Tricalcium Silicate by Serial Block-Face Scanning Electron Microscopy. Materials, 12(12), 1882. https://doi.org/10.3390/ma12121882





