Characterization of Photoactive Fe-TiO2 Lime Coatings for Building Protection: The Role of Iron Content
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Fe-TiO2 Nanoparticles and Pastes with Air Lime and Fe-TiO2
2.2. Characterization of the Photoactive Fe-doped TiO2 Nanoparticles
2.3. Assessment of the Photocatalytic Activity of the Photoactive Nanoparticles
2.4. Evaluation of the Carbonation Process and Photocatalytic Performance of the Photoactive Fe-TiO2 Lime Pastes
3. Results and Discussion
3.1. Characterization of the Photoactive Fe-doped TiO2 Nanoparticles with FTIR, XRD, BET, UV-Vis, and XPS
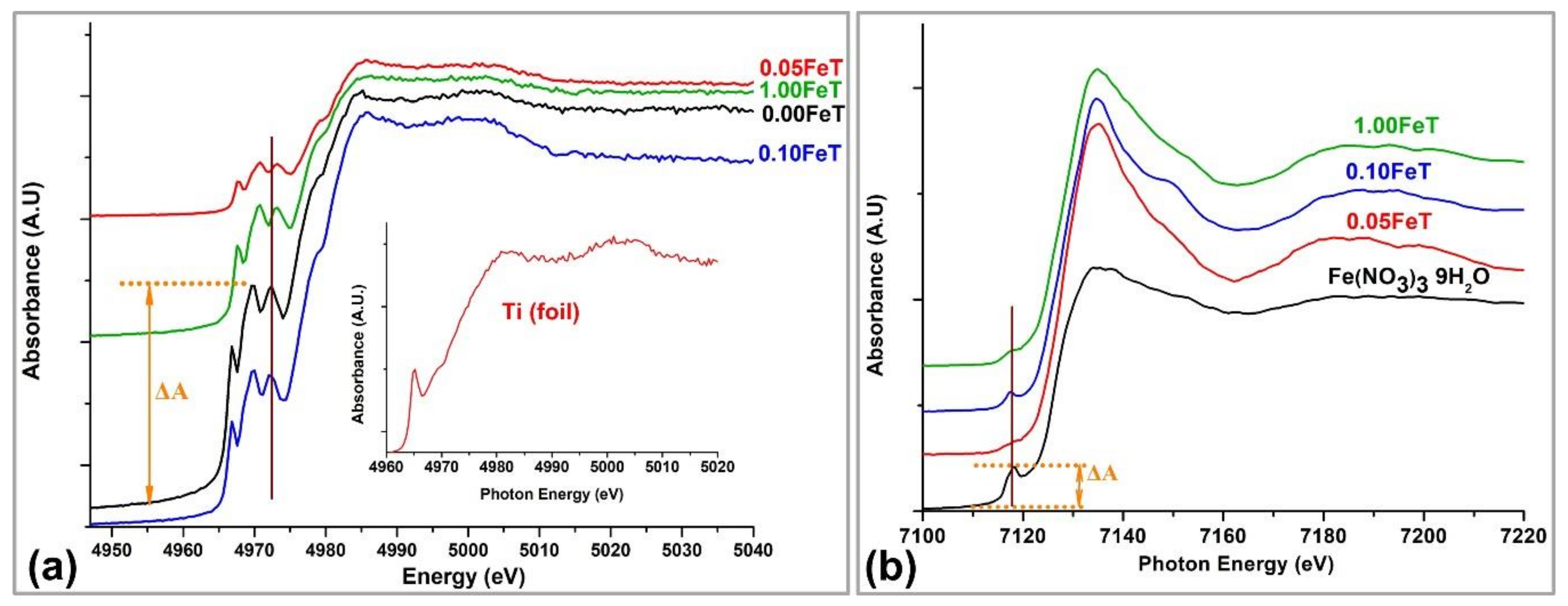
3.2. XANES Analysis of Samples
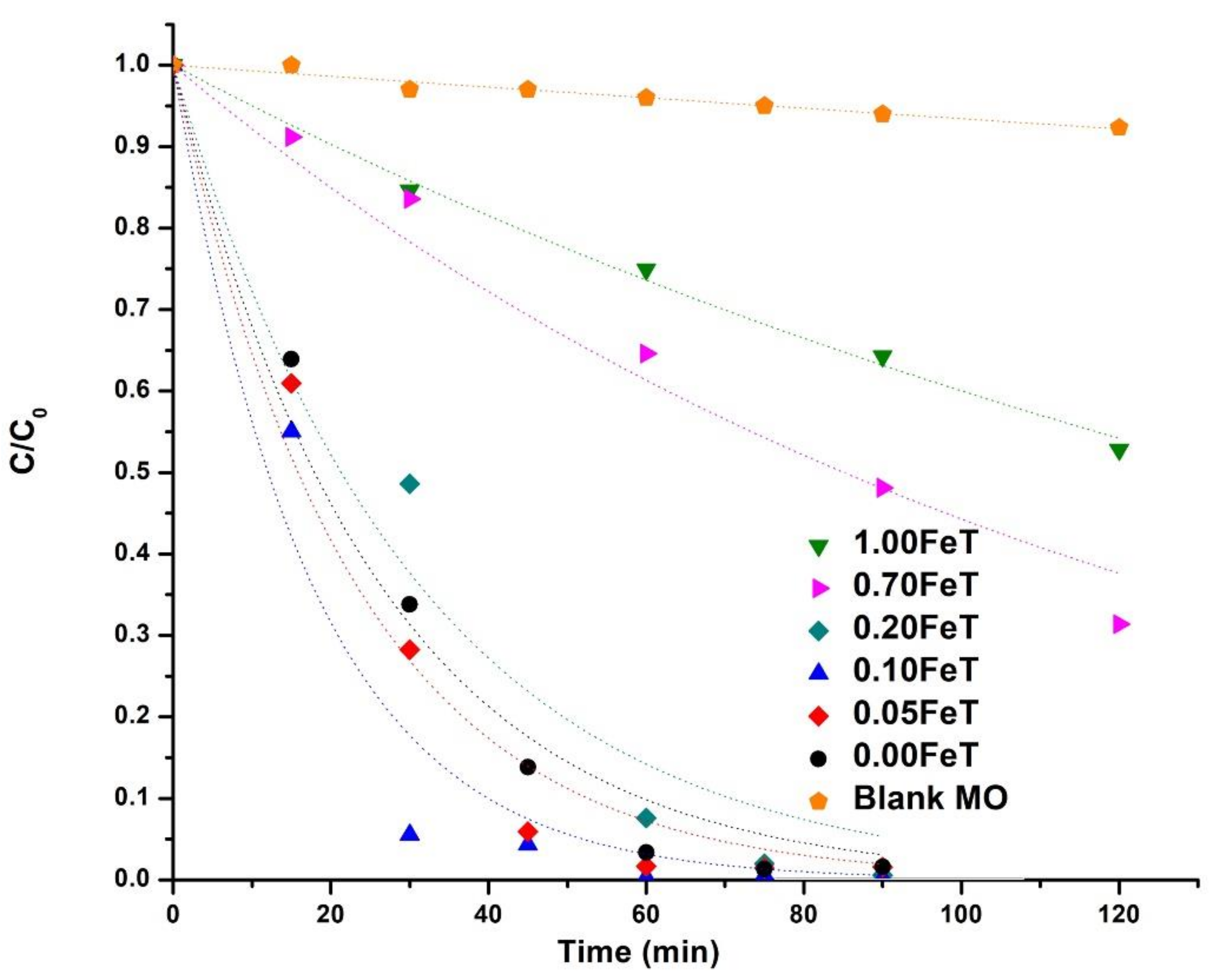
3.3. Photocatalytic Activity of the Fe-doped TiO2 Nanoparticles
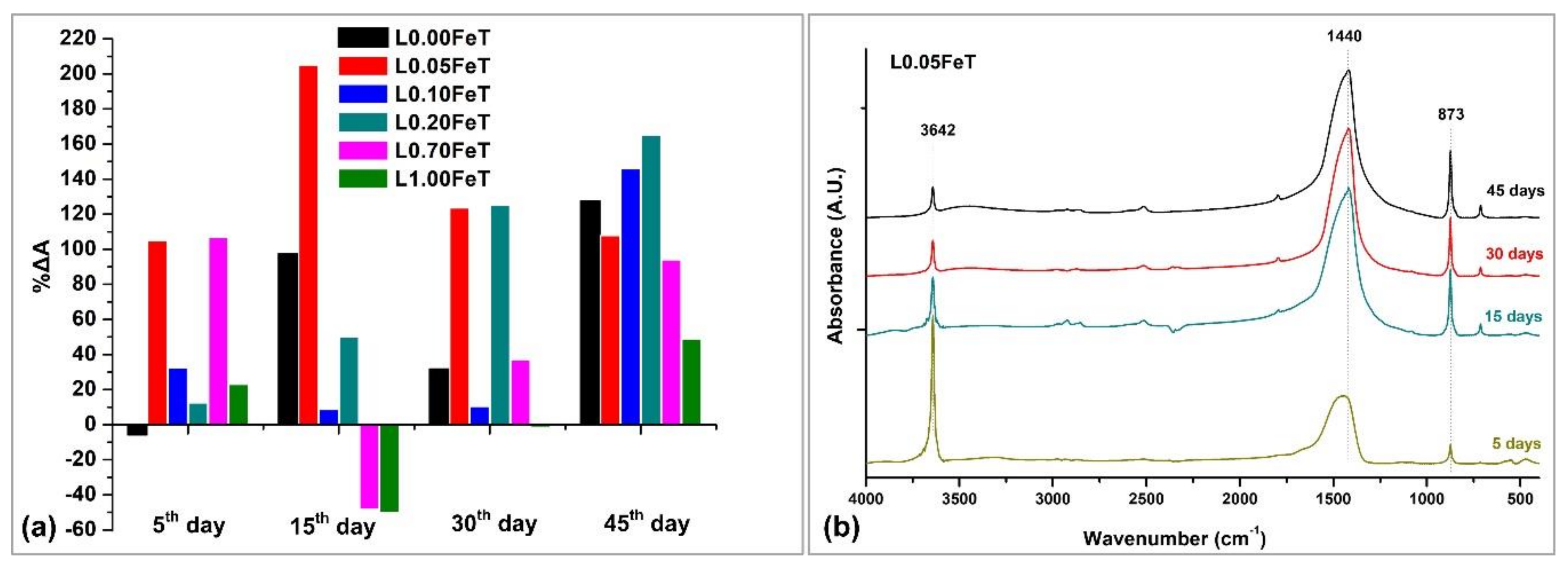
3.4. Assessment of the Carbonation Process and the Self-Cleaning Properties of the Lime Pastes
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Maravelaki-Kalaitzaki, P.; Agioutantis, Z.; Lionakis, E.; Stavroulaki, M.; Perdikatsis, V. Physico-chemical and mechanical characterization of hydraulic mortars containing nano-titania for restoration applications. Cem. Concr. Compos. 2013, 36, 33–41. [Google Scholar] [CrossRef]
- Pozo-Antonio, J.S.; Dionísio, A. Physical-mechanical properties of mortars with addition of TiO2 nanoparticles. Constr. Build. Mater. 2017, 148, 261–272. [Google Scholar] [CrossRef]
- Karatasios, I.; Katsiotis, M.; Likodimos, V.; Kontos, A.I.; Papavassiliou, G.; Falaras, P.; Kilikoglou, V. Photo-induced carbonation of lime-TiO2 mortars. Appl. Catal. B Environ. 2010, 95, 78–86. [Google Scholar] [CrossRef]
- Pérez-Nicolás, M.; Navarro-Blasco, I.; Fernández, J.M.; Alvarez, J.I. Atmospheric NOx removal: Study of cement mortars with iron- and vanadium-doped TiO2 as visible light–sensitive photocatalysts. Constr. Build. Mater. 2017, 2017 149, 257–271. [Google Scholar] [CrossRef]
- Lucas, S. Influence of operating parameters and ion doping on the photocatalytic activity of mortars containing titanium dioxide nanoparticles. Mater. Today Proc. 2017, 4, 11588–11593. [Google Scholar] [CrossRef]
- Delekar, S.D.; Yadav, H.M.; Achary, S.N.; Meena, S.S.; Pawar, S.H. Structural refinement and photocatalytic activity of Fe-doped anatase TiO2 nanoparticles. Appl. Surf. Sci. 2012, 263, 536–545. [Google Scholar] [CrossRef]
- Vijayan, P.; Mahendiran, C.; Suresh, C.; Shanthi, K. Photocatalytic activity of iron doped nanocrystalline titania for the oxidative degradation of 2,4,6-trichlorophenol. Catal. Today 2009, 141, 220–224. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, J.; Chen, F.; Iino, K.; Anpo, M. High activity TiO2 Photocatalysts Prepared by a Modified Sol–gel Method: Characterization and their Photocatalytic Activity for the Degradation of XRG and X-GL. Top. Catal. 2005, 35, 261–268. [Google Scholar] [CrossRef]
- Nadzirah, S.; Foo, K.L.; Hashim, U. Morphological Reaction on the Different Stabilizers of Titanium Dioxide Nanoparticles. Int. J. Electrochem. Sci. 2015, 10, 5498–5512. [Google Scholar]
- Spectragryph - optical spectroscopy software. Available online: http://www.effemm2.de/ (accessed on 1 April 2019).
- Karydas, A.G.; Czyzycki, M.; Leani, J.J.; Migliori, A.; Osan, J.; Bogovac, M.; Wrobel, P.; Vakula, N.; Padilla-Alvarez, R.; Menk, R.H.; Gol, M.G.; Antonelli, M.; Tiwari, M.K.; Caliri, C.; Vogel-Mikuš, K.; Darby, I.; Kaiser, R.B. An IAEA multi-technique X-ray spectrometry endstation at Elettra Sincrotrone Trieste: Benchmarking results and interdisciplinary applications. J. Synchrotron Radiat. 2018, 25, 189–203. [Google Scholar] [PubMed]
- Kapridaki, C.; Pinho, L.; Mosquera, M.J.; Maravelaki-Kalaitzaki, P. Producing photoactive, transparent and hydrophobic SiO2-crystalline TiO2 nanocomposites at ambient conditions with application as self-cleaning coatings. Appl. Catal. B Environ. 2014, 156–157, 416–427. [Google Scholar] [CrossRef]
- Velasco, M.J.; Rubio, F.; Rubio, J.; Oteo, J.L. Hydrolysis of titanium tetrabutoxide study by FT-IR spectroscopy. Spectroscy Lett. 1999, 32, 289–304. [Google Scholar] [CrossRef]
- Castro, C.A.; Centeno, A.; Giraldo, S.A. Iron promotion of the TiO2 photosensitization process towards the photocatalytic oxidation of azo dyes under solar-simulated light irradiation. Mater. Chem. Phys. 2011, 129, 1176–1183. [Google Scholar] [CrossRef]
- Marami, M.B.; Farahmandjou, M.; Khoshnevisan, B. Sol–Gel Synthesis of Fe-Doped TiO2 Nanocrystals. J. Electron. Mater. 2018, 47, 3741–3748. [Google Scholar] [CrossRef]
- Cheng, H.H.; Chen, S.S.; Yang, S.Y.; Liu, H.M.; Lin, K.S. Sol-Gel hydrothermal synthesis and visible light photocatalytic degradation performance of Fe/N codoped TiO2 catalysts. Materials 2018, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Kandori, K.; Shigetomi, T.; Ishikawa, T. Study on forced hydrolysis reaction of acidic Fe2(SO4)3 solution—structure and properties of precipitates. Colloids Surf. A 2004, 232, 19–28. [Google Scholar] [CrossRef]
- Ali, T.; Tripathi, P.; Azam, A.; Raza, W.; Ahmed, A.S.; Ahmed, A.; Muneer, M. Photocatalytic performance of Fe-doped TiO2 nanoparticles under visible-light irradiation. Mater. Res. Express. 2017, 4. [Google Scholar] [CrossRef]
- Xie, Y.; Heo, S.; Yoo, S.; Ali, G.; Cho, S. Synthesis and photocatalytic activity of anatase TiO2 nanoparticles-coated carbon nanotubes. Nanoscale Res. Lett. 2010, 5, 603–607. [Google Scholar] [CrossRef]
- Elghniji, K.; Atyaoui, A.; Livraghi, S.; Bousselmi, L.; Giamello, E.; Ksibi, M. Synthesis and characterization of Fe3+ doped TiO2 nanoparticles and films and their performance for photocurrent response under UV illumination. J. Alloys Compounds 2012, 541, 421–427. [Google Scholar] [CrossRef]
- Lei, X.F.; Zhang, Z.N.; Wu, Z.X.; Piao, Y.J.; Chen, C.; Li, X.; Xue, X.X.; Yang, H. Synthesis and characterization of Fe, N and C tri-doped polymorphic TiO2 and the visible light photocatalytic reduction of Cr(VI). Sep. Purif. Technol. 2017, 174, 66–74. [Google Scholar] [CrossRef]
- Butler, E.B.; Chen, C.C.; Hung, Y.T.; Al Ahmand, M.S. Effect of Fe-doped TiO2 photocatalysts on the degradation of acid orange 7. Integr. Ferroelectr. 2016, 168, 1–9. [Google Scholar] [CrossRef]
- Abidov, A.; Allabergenov, B.; Lee, J.; Jeon, H.W.; Jeong, S.W.; Kim, S. X-Ray Photoelectron Spectroscopy Characterization of Fe Doped TiO2 Photocatalyst. Int. J. Mater. Mech. Manuf. 2013, 1, 294–296. [Google Scholar] [CrossRef]
- Crisan, M.; Rãileanua, M.; Drãgana, N.; Crişan, D.; Ianculescu, A.; Niţoi, I.; Oancea, P.; Şomăcescu, S.; Stănică, N.; Vasile, B.; Stan, C. Sol-gel iron-doped TiO2 nanopowders with photocatalytic activity. App. Catal. A Gen. 2015, 504, 130–142. [Google Scholar] [CrossRef]
- Pokrovski, G.S.; Schott, J.; Farges, F.; Hazemann, J.L. Iron (III)-silica interactions in aqueous dolution: Insights from X-ray absorption fine structure spectroscopy. Geochimica et Cosmochimica Acta 2003, 67, 3559–3573. [Google Scholar] [CrossRef]
- Yamamoto, T. Assignment of pre-edge peaks in K-edge x-ray absorption spectra of 3d transition metal compounds: electric dipole or quadrupole? X-Ray Spectrometry 2008, 37, 572–584. [Google Scholar] [CrossRef]
- Farges, F.; Brown, G.E. Rehr, J.J. Ti K-edge XANES studies of Ti coordination and disorder in oxide compounds: Comparison between theory and experiment. Phys. Rev. B 1997, 56, 1809. [Google Scholar]
- Wang, D.; Liu, L.; Sun, X.; Sham, T.K. Observation of lithiation-induced structural variations in TiO2 nanotube arrays by X-ray absorption fine structure. J. Mater. Chem. A 2015, 3, 412–419. [Google Scholar] [CrossRef]
- Farges, F.; Brown, G.E.; Navrotsky, A.; Gan, H.; Rehr, J.R. Coordination chemistry of Ti(IV) in silicate glasses and melts: III. Glasses and melts from ambient to high temperatures. Geochim. Cosmochim. Acta 1996, 60, 3055–3065. [Google Scholar] [CrossRef]
- Hs, T.F.; Hsiung, T.L.; Wang, J.; Huang, C.H.; Wang, P.H. In situ XANES studies of TiO2/Fe3O4@C during photocatalytic degradation of trichloroethylene. Nucl. Instrum. Methods Phys. Res. Sect. A 2010, 619, 98–101. [Google Scholar] [CrossRef]
- Wilke, M.; Farges, F.; Petit, P.E.; Brown, G.E.; Martin, F. Oxidation state and coordination of Fe in minerals: An Fe K-XANES spectroscopic study. Am. Mineral. 2001, 86, 714–730. [Google Scholar] [CrossRef]
- Combes, J.M.; Manceau, A.; Galas, G. Formation of ferric oxides from aqueous solutions: A polyhedral approach by X-ray Absorption Spectroscopy: II. Hematite formation from ferric gels. Geochimica et Cosmochimica Acta 1990, 54, 1083–1091. [Google Scholar] [CrossRef]
- Methods of Soil Analysis, Part 5, Mineralogical Methods. Available online: https://dl.sciencesocieties.org/publications/books/pdfs/sssabookseries/methodsofsoilan5/frontmatter (accessed on 1 April 2019).
- Ajiboye, B.; Akinremi, O.; Jurgensen, A. Experimental Validation of Quantitative XANES Analysis for Phosphorus Speciation. Soil Sci. Soc. Am. J. 2007, 71, 1288–1291. [Google Scholar] [CrossRef]
- Gaur, A.; Shrivastava, B.D. A Comparative study of the methods of speciation using X-Ray Absorption Fine Structure. Acta Physica Polonica A 2012, 121, 647–652. [Google Scholar]
- Rozman, N.; Tobaldi, M.D.; Cvelbar, U.; Puliyalil, H.; Labrincha, A.J.; Legat, A.; Škapin, S.A. Hydrothermal Synthesis of Rare-Earth Modified Titania: Influence on Phase Composition, Optical Properties, and Photocatalytic Activity. Materials 2019, 12, 713. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Xiang, Q.; Zhou, M. Preparation, characterization and visible-light-driven photocatalytic activity of Fe-doped titania nanorods and first-principles study for electronic structures. Appl. Catal. B Environ. 2009, 90, 595–602. [Google Scholar] [CrossRef]
- Tong, T.; Zhang, J.; Tian, B.; Chen, F.; He, D. Preparation of Fe3+-doped TiO2 catalysts by controlled hydrolysis of titanium alkoxide and study on their photocatalytic activity for methyl orange degradation. J. Hazard. Mater. 2008, 155, 572–579. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Sánchez, I.; Bandala, E. Photocatalytic Degradation of Estriol Using Iron-Doped TiO2 under High and Low UV Irradiation. Catalysts 2018, 8, 625. [Google Scholar] [CrossRef]
- Theodoridou, M.; Charalambous, E.; Maravelaki-Kalaitzaki, P.; Ioannou, I. Amelioration of crushed brick-lime composites using nano-additives. Cem. Concr. Compos. 2016, 68, 77–87. [Google Scholar] [CrossRef]
- Nuño, M.; Pesce, G.L.; Bowen, C.R.; Xenophontos, P.; Ball, R.J. Environmental performance of nano-structured Ca(OH)2/TiO2 photocatalytic coatings for buildings. Build. Environ. 2015, 92, 734–742. [Google Scholar] [CrossRef]
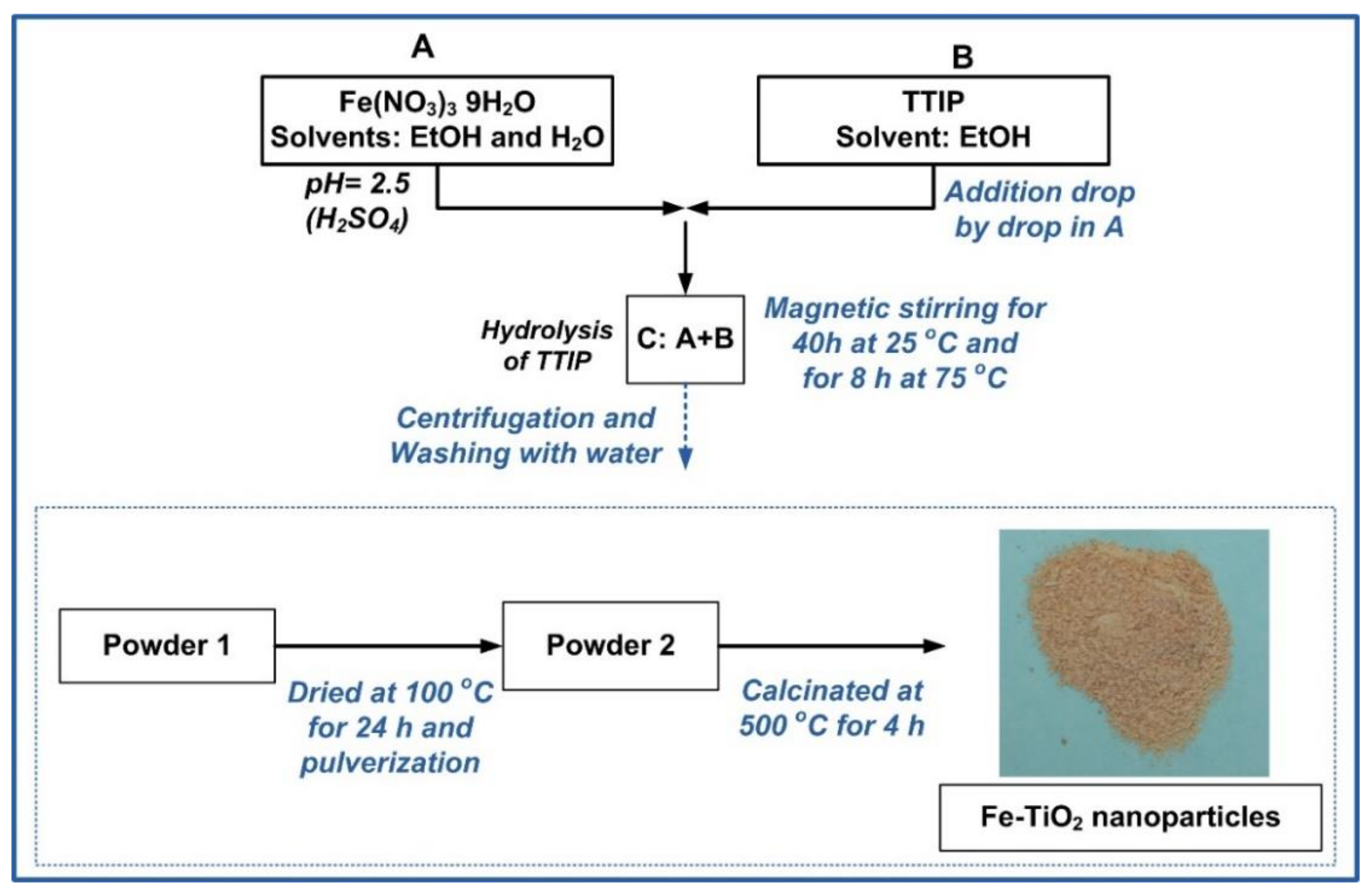
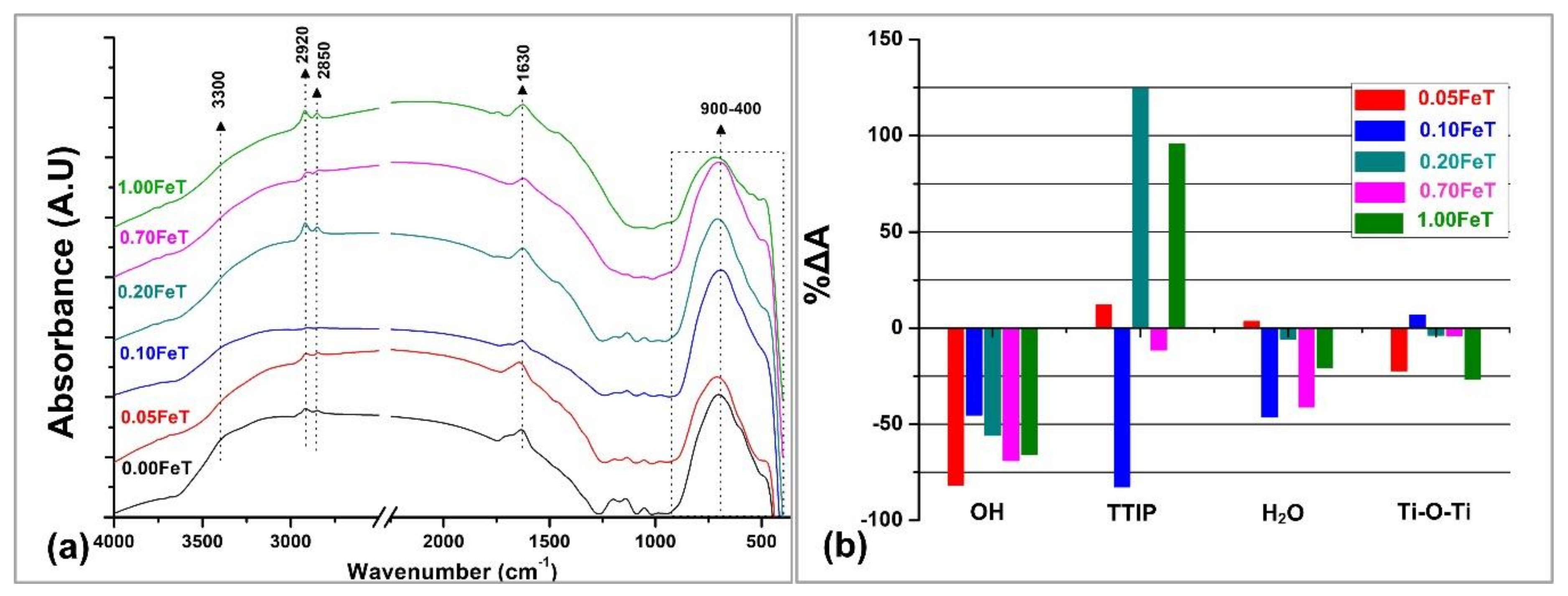
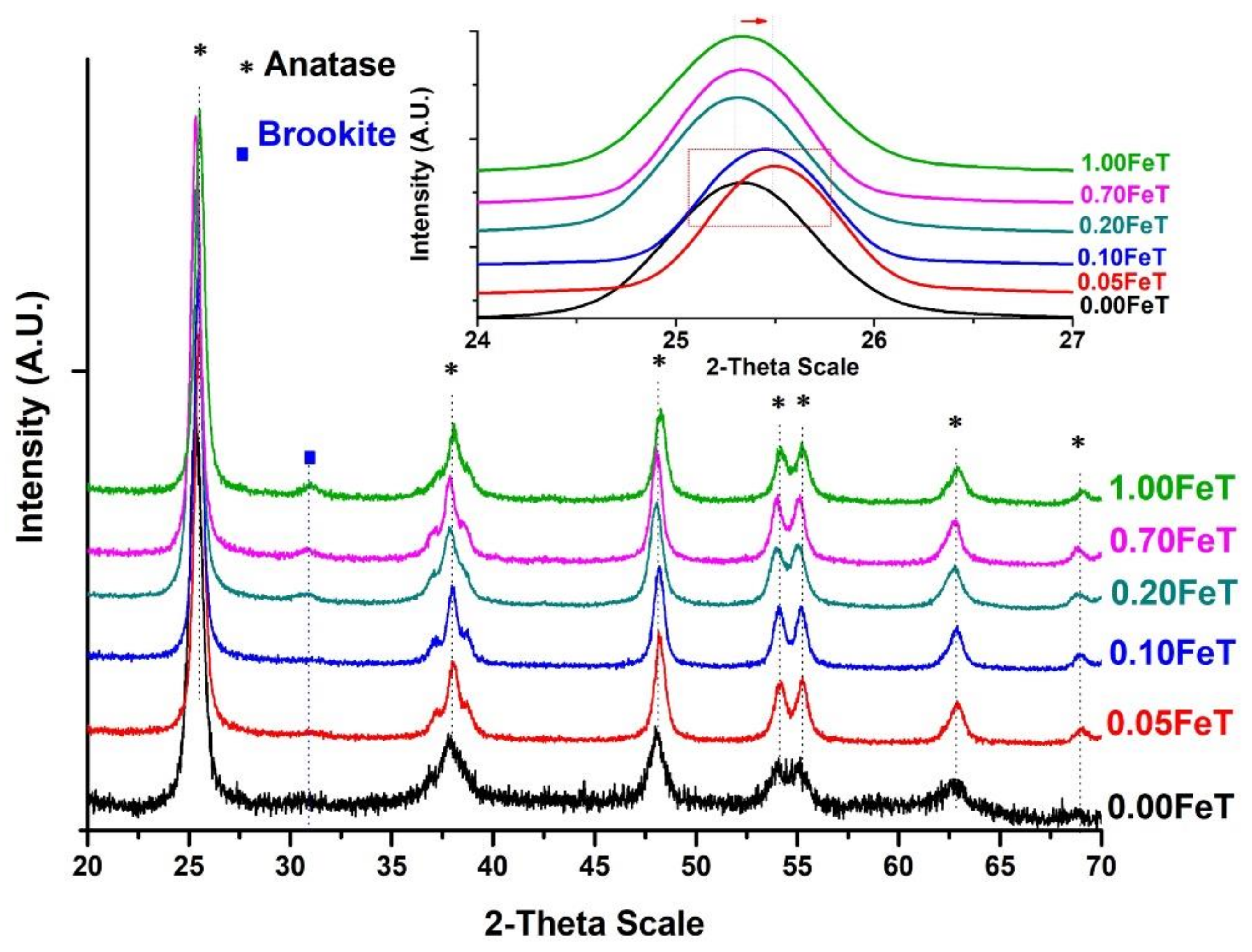
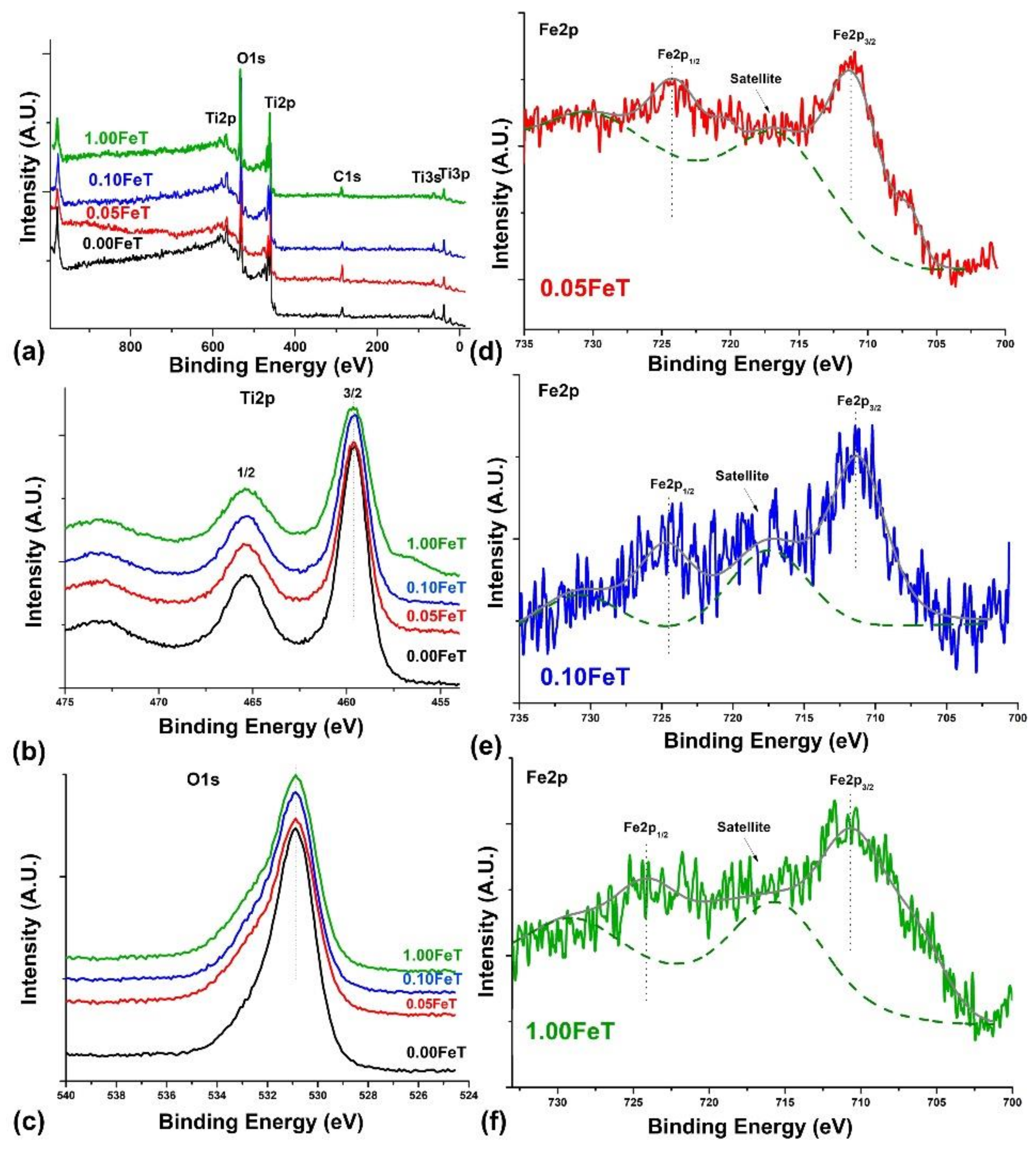

| Fe-TiO2 (T) Synthesized Nanoparticles | Pastes of Lime (L) and Fe-TiO2 Nanoparticles (3% w/w) |
|---|---|
| - | Lime (Blank) |
| 0.00FeT | L0.00FeT |
| 0.05FeT | L0.05FeT |
| 0.10FeT | L0.10FeT |
| 0.20FeT | L0.20FeT |
| 0.70FeT | L0.70FeT |
| 1.00FeT | L1.00FeT |
| TiO2 Nanoparticles | % Anatase | % Brookite | Crystallite Size of Anatase (nm) | Surface Area (m2 g−1) | Band Gap (eV) |
|---|---|---|---|---|---|
| 0.00FeT | 100 | - | 20.4 | 52 | 3.13 |
| 0.05FeT | 98 | 2 | 12.9 | 65 | 3.06 |
| 0.10FeT | 98 | 2 | 13.1 | 76 | 2.95 |
| 0.20FeT | 97 | 3 | 11.5 | n.a. | 2.95 |
| 0.70FeT | 96 | 4 | 12.6 | n.a. | 2.64 |
| 1.00FeT | 92 | 8 | 12.5 | 59 | 2.61 |
| Sample | k (10−3 min−1) | r2 | % Dis. (30 min) * | % Dis. (90 min) * |
|---|---|---|---|---|
| Blank | 0.7 | 0.97 | 3 | 5 |
| 0.00FeT | 39 | 0.98 | 66 | 98 |
| 0.05FeT | 44 | 0.98 | 71 | 98 |
| 0.10FeT | 58 | 0.96 | 94 | 100 |
| 0.20FeT | 33 | 0.97 | 51 | 100 |
| 0.70FeT | 8 | 0.98 | 16 | 69 |
| 1.00FeT | 5 | 0.99 | 15 | 36 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kapridaki, C.; Xynidis, N.; Vazgiouraki, E.; Kallithrakas-Kontos, N.; Maravelaki-Kalaitzaki, P. Characterization of Photoactive Fe-TiO2 Lime Coatings for Building Protection: The Role of Iron Content. Materials 2019, 12, 1847. https://doi.org/10.3390/ma12111847
Kapridaki C, Xynidis N, Vazgiouraki E, Kallithrakas-Kontos N, Maravelaki-Kalaitzaki P. Characterization of Photoactive Fe-TiO2 Lime Coatings for Building Protection: The Role of Iron Content. Materials. 2019; 12(11):1847. https://doi.org/10.3390/ma12111847
Chicago/Turabian StyleKapridaki, Chrysi, Nikolaos Xynidis, Eleftheria Vazgiouraki, Nikolaos Kallithrakas-Kontos, and Pagona Maravelaki-Kalaitzaki. 2019. "Characterization of Photoactive Fe-TiO2 Lime Coatings for Building Protection: The Role of Iron Content" Materials 12, no. 11: 1847. https://doi.org/10.3390/ma12111847
APA StyleKapridaki, C., Xynidis, N., Vazgiouraki, E., Kallithrakas-Kontos, N., & Maravelaki-Kalaitzaki, P. (2019). Characterization of Photoactive Fe-TiO2 Lime Coatings for Building Protection: The Role of Iron Content. Materials, 12(11), 1847. https://doi.org/10.3390/ma12111847






