Synthetic Calcium Phosphate Ceramics as a Potential Treatment for Bisphosphonate-Related Osteonecrosis of the Jaw
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ZOL-Ceramics Adsorption
2.3. Primary Cell Cultures
2.4. ZOL and ZOL–BCP Treatment
2.5. Cell Citotoxicity
2.6. Cell Migration Studies/Scratch Assay
2.7. Viability Assay
2.8. Cell Cycle Analysis
2.9. Types of Cell Death
2.10. Statistical Analysis
3. Results
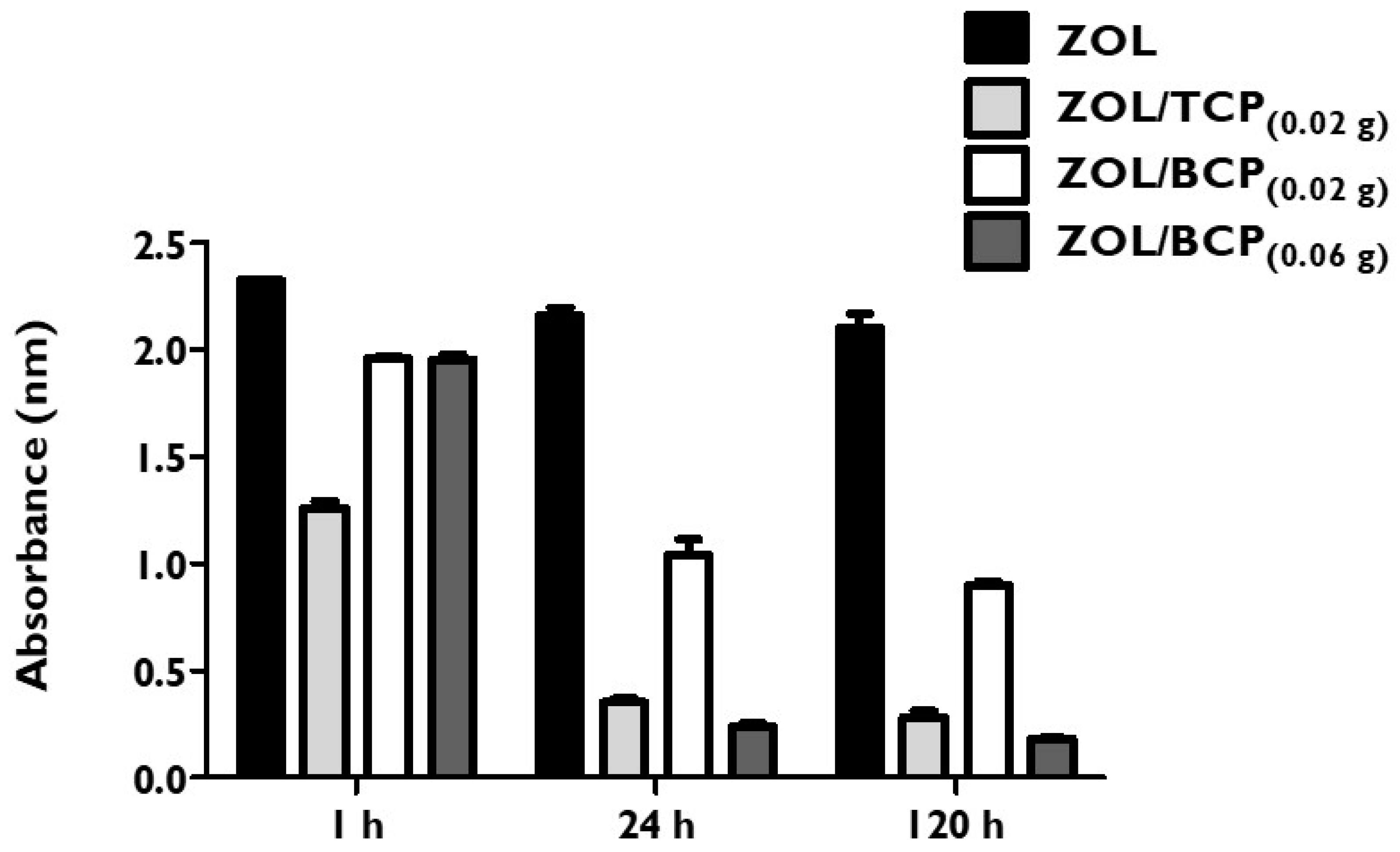
3.1. ZOL-Ceramics Adsorption
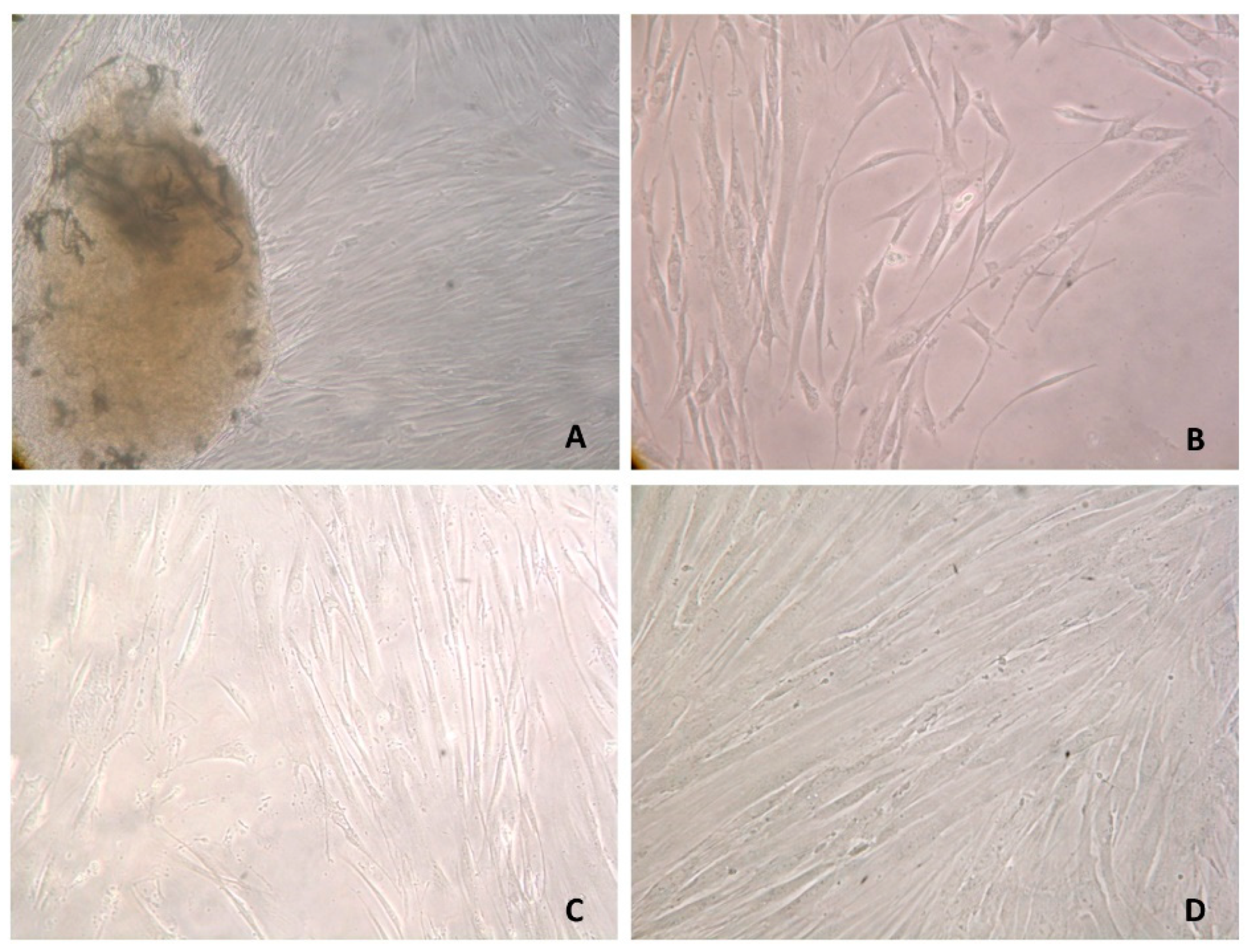
3.2. Primary Cell Cultures
3.3. ZOL Citotoxicity
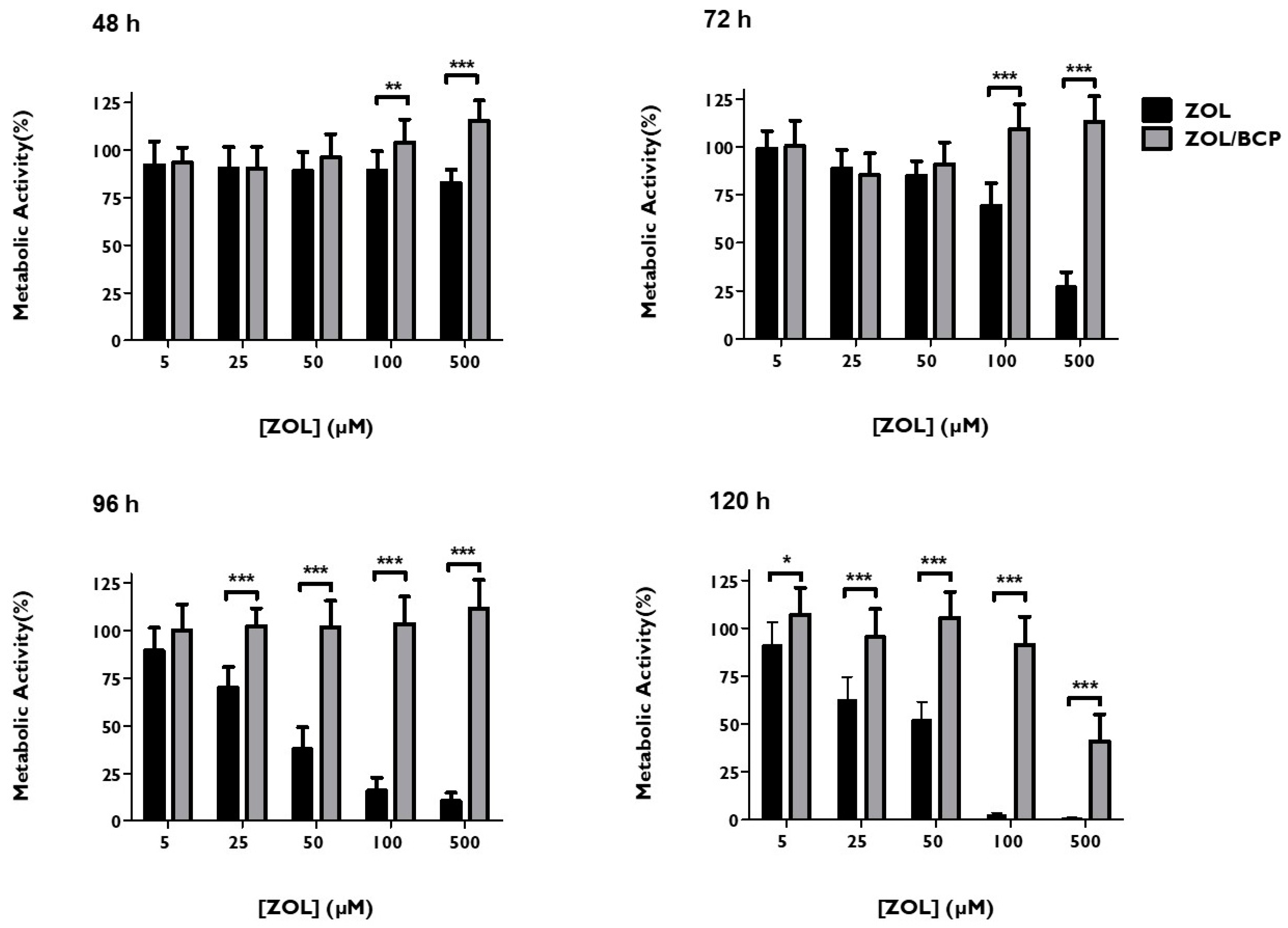
3.4. ZOL–BCP Citotoxicity
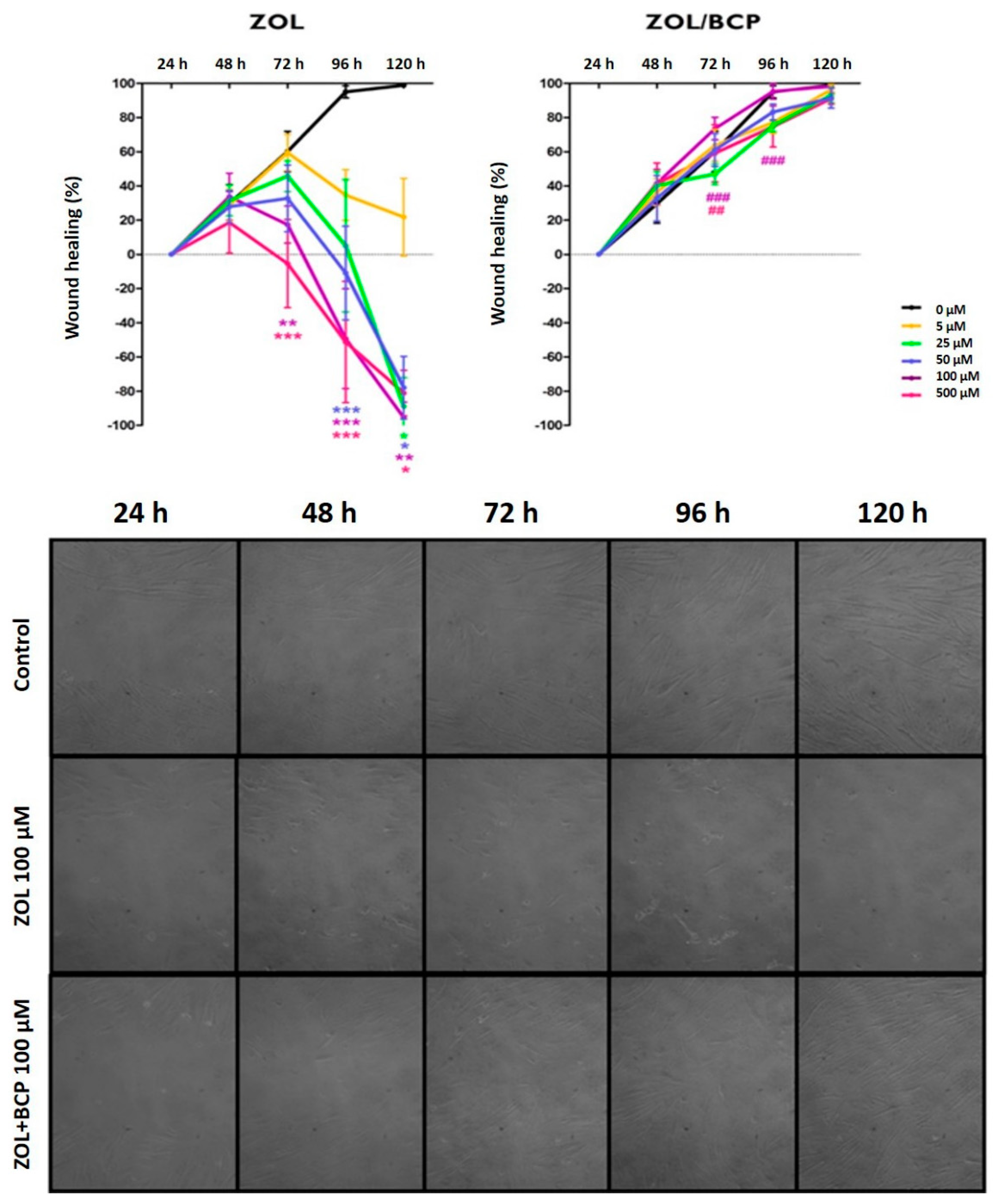
3.5. Cell Migration Studies/Scratch Assay
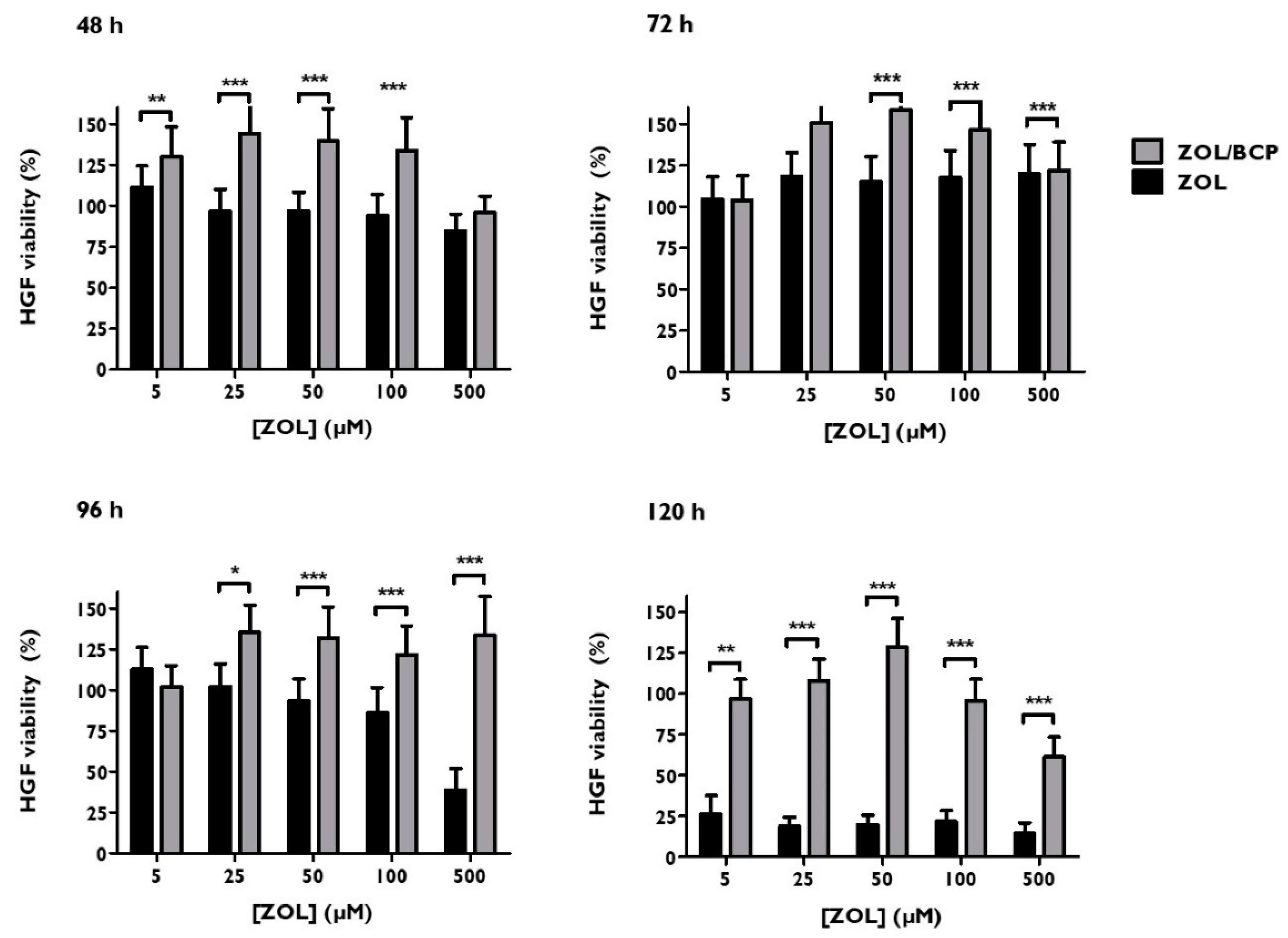
3.6. Viability
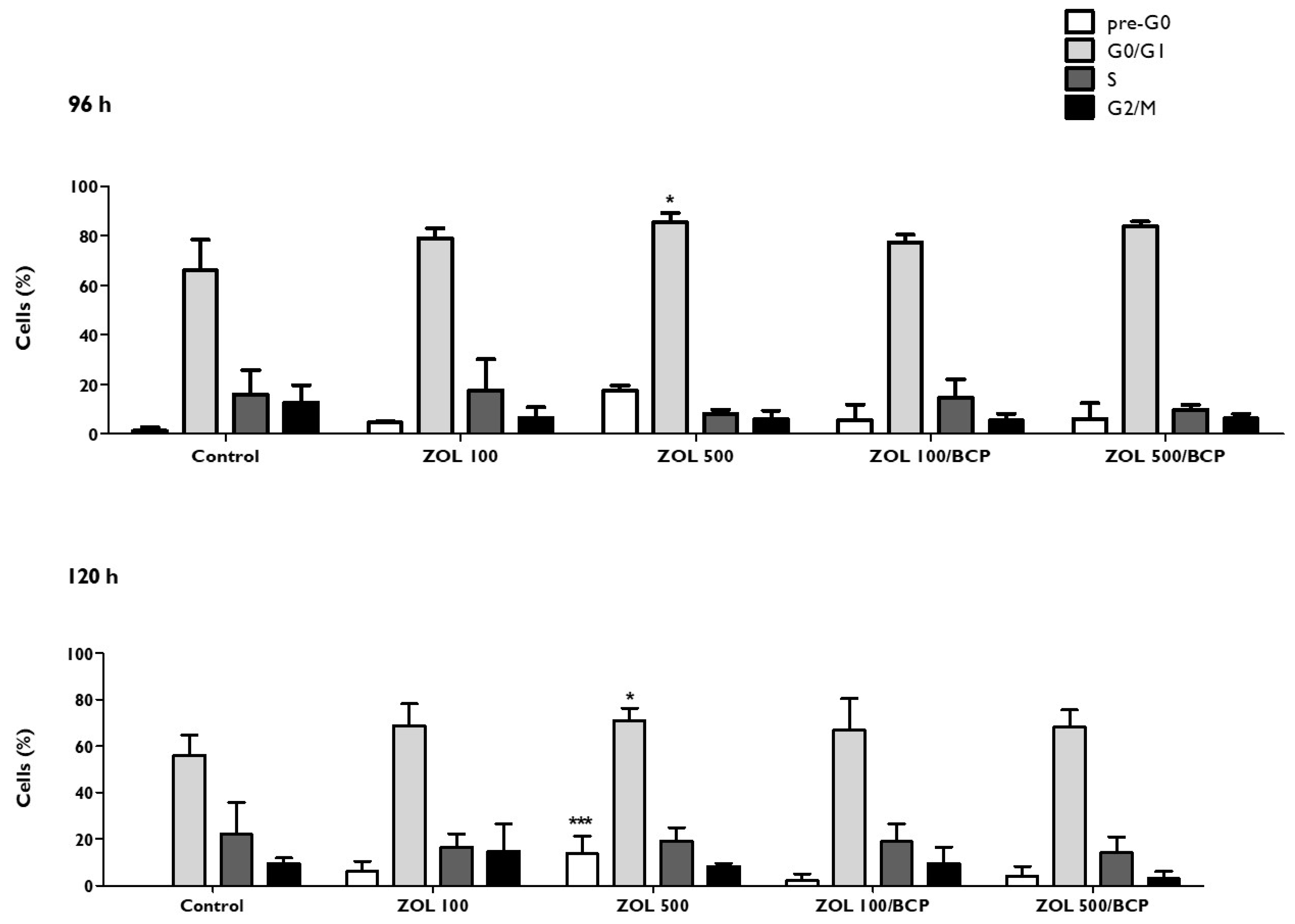
3.7. Cell Cycle
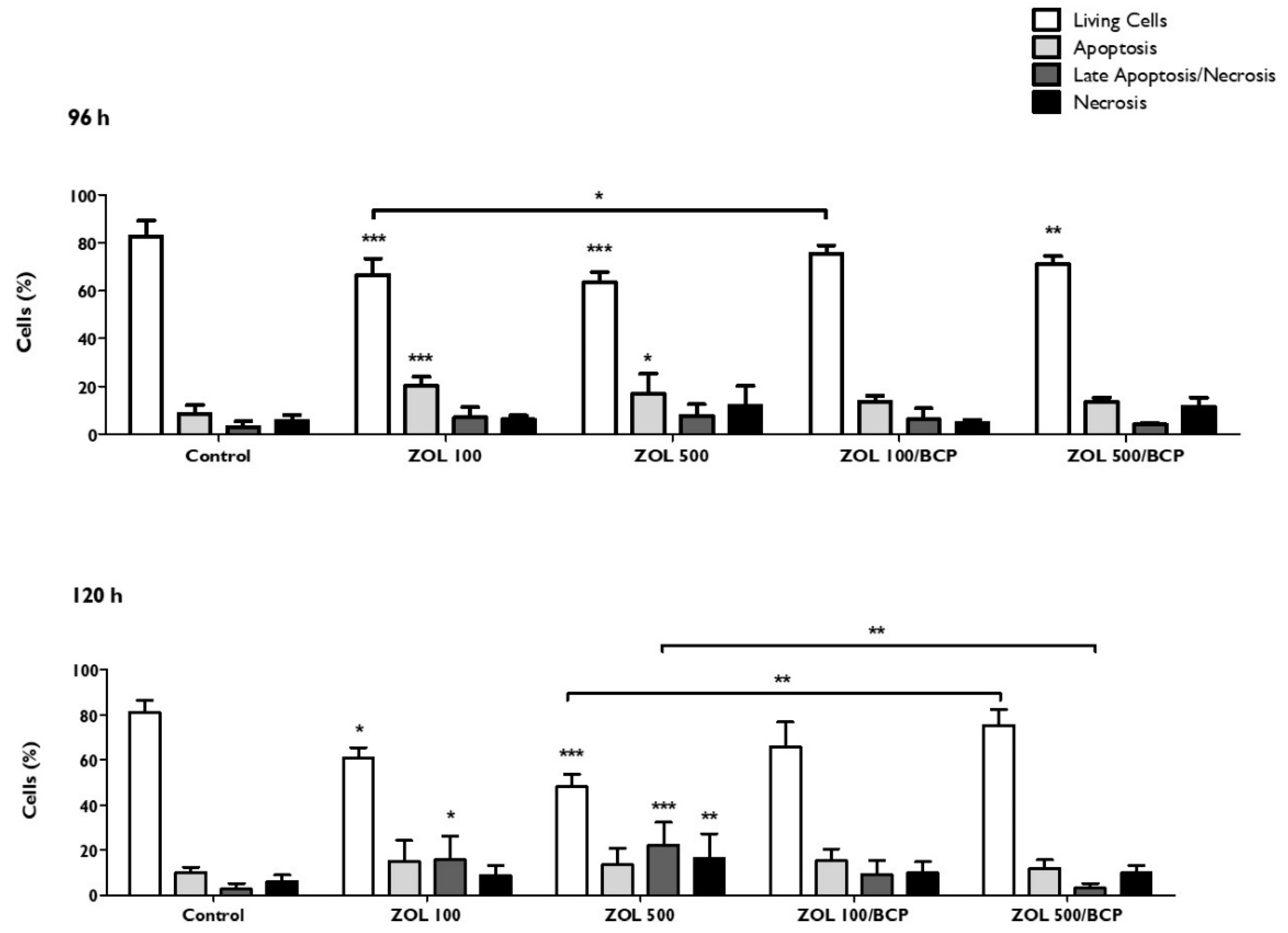
3.8. Types of Cell Death
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [PubMed]
- Coleman, R.E.; Lipton, A.; Costa, L.; Cook, R.J.; Lee, K.-A.; Saad, F.; Brown, J.E.; Terpos, E.; Major, P.P.; Kohno, N.; et al. Possible survival benefits from zoledronic acid treatment in patients with bone metastases from solid tumours and poor prognostic features—An exploratory analysis of placebo-controlled trials. J. Bone Oncol. 2013, 2, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Zhou, H.; Dunstan, C.R.; Sutherland, R.L.; Seibel, M.J. The role of the bone microenvironment in skeletal metastasis. J. Bone Oncol. 2013, 2, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Weilbaecher, K.N.; Guise, T.A.; McCauley, L.K. Cancer to bone: A fatal attraction. Nat. Rev. Cancer 2011, 11, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Russell, R.G.G.; Watts, N.B.; Ebetino, F.H.; Rogers, M.J. Mechanisms of action of bisphosphonates: Similarities and differences and their potential influence on clinical efficacy. Osteoporos. Int. 2008, 19, 733–759. [Google Scholar] [CrossRef] [PubMed]
- Maurizi, A.; Rucci, N. The Osteoclast in Bone Metastasis: Player and Target. Cancers 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Ikebe, T. Pathophysiology of BRONJ: Drug-related osteoclastic disease of the jaw. Oral Sci. Int. 2013, 10, 1–8. [Google Scholar] [CrossRef]
- Lim, S.S.; Lee, B.; Kim, I.S.; Hwang, S.J. Differential modulation of zoledronate and etidronate in osseous healing of an extracted socket and tibia defect. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2017, 123, 8–19. [Google Scholar] [CrossRef]
- Sarin, J.; DeRossi, S.S.; Akintoye, S.O. Updates on bisphosphonates and potential pathobiology of bisphosphonate-induced jaw osteonecrosis. Oral Dis. 2008, 14, 277–285. [Google Scholar] [CrossRef]
- Lesclous, P.; Abi Najm, S.; Carrel, J.-P.; Baroukh, B.; Lombardi, T.; Willi, J.-P.; Rizzoli, R.; Saffar, J.-L.; Samson, J. Bisphosphonate-associated osteonecrosis of the jaw: A key role of inflammation? Bone 2009, 45, 843–852. [Google Scholar] [CrossRef]
- Hewitt, C.; Farah, C.S. Bisphosphonate-related osteonecrosis of the jaws: A comprehensive review. J. Oral Pathol. Med. 2007, 36, 319–328. [Google Scholar] [CrossRef] [PubMed]
- Kuroshima, S.; Sasaki, M.; Sawase, T. Medication-related osteonecrosis of the jaw: A literature review. J. Oral Biosci. 2019. [Google Scholar] [CrossRef] [PubMed]
- Rollason, V.; Laverrière, A.; MacDonald, L.C.I.; Walsh, T.; Tramèr, M.R.; Vogt-Ferrier, N.B. Interventions for treating bisphosphonate-related osteonecrosis of the jaw (BRONJ). Cochrane Database Syst. Rev. 2016, 2, CD008455. [Google Scholar] [CrossRef] [PubMed]
- Ravosa, M.J.; Ning, J.; Liu, Y.; Stack, M.S. Bisphosphonate effects on the behaviour of oral epithelial cells and oral fibroblasts. Arch. Oral Biol. 2011, 56, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Gavaldá, C.; Bagán, J.-V. Concept, diagnosis and classification of bisphosphonate-associated osteonecrosis of the jaws. A review of the literature. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e260–e270. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.G.; Pansani, T.N.; Soares, D.G.; Cardoso, L.M.; Hebling, J.; de Souza Costa, C.A. Influence of bisphosphonates on the adherence and metabolism of epithelial cells and gingival fibroblasts to titanium surfaces. Clin. Oral Investig. 2018, 22, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef]
- Verron, E.; Khairoun, I.; Guicheux, J.; Bouler, J.-M. Calcium phosphate biomaterials as bone drug delivery systems: A review. Drug Discov. Today 2010, 15, 547–552. [Google Scholar] [CrossRef]
- Cattalini, J.P.; Boccaccini, A.R.; Lucangioli, S.; Mouriño, V. Bisphosphonate-based strategies for bone tissue engineering and orthopedic implants. Tissue Eng. Part B Rev. 2012, 18, 323–340. [Google Scholar] [CrossRef]
- Santanna, A.C.P.; Marques, M.M.; Barroso, E.C.; Passanezi, E. Cultura e caracterização de células derivadas de ligamento periodontal humano. Rev. Fac. Odontol. 2002, 10, 134–140. [Google Scholar]
- Saczko, J.; Dominiak, M.; Kulbacka, J.; Chwiłkowska, A.; Krawczykowska, H. A simple and established method of tissue culture of human gingival fibroblasts for gingival augmentation. Folia Histochem. Cytobiol. 2008, 46, 117–119. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.; Laranjo, M.; Casalta-Lopes, J.; Serra, A.; Pineiro, M.; Pina, J.; Melo, J.; Senge, M.; Botelho, M.; Martelo, M.; et al. Platinum(II) ring-fused chorins as near-infrared emitting oxygen sensors and photodynamic agents. ACS Med. Chem. Lett. 2017, 8, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Pereira, N.A.M.; Laranjo, M.; Pina, J.; Oliveira, A.S.R.; Ferreira, J.D.; Sánchez-Sánchez, C.; Casalta-Lopes, J.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Piñeiro, M.; et al. Advances on photodynamic therapy of melanoma through novel ring-fused 5,15-diphenylchlorins. Eur. J. Med. Chem. 2018, 146, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.C.; Park, A.Y.; Guan, J.L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat. Protoc. 2007, 2, 329–333. [Google Scholar] [CrossRef] [PubMed]
- Otto, S.; Pautke, C.; Opelz, C.; Westphal, I.; Drosse, I.; Schwager, J.; Bauss, F.; Ehrenfeld, M.; Schieker, M. Osteonecrosis of the jaw: Effect of bisphosphonate type, local concentration, and acidic milieu on the pathomechanism. J. Oral Maxillofac. Surg. 2010, 68, 2837–2845. [Google Scholar] [CrossRef]
- Santos, K.; Laranjo, M.; Abrantes, A.M.; Brito, A.F.; Gonçalves, C.; Sarmento Ribeiro, A.B.; Botelho, M.F.; Soares, M.I.L.; Oliveira, A.S.R.; Pinhoe Melo, T.M.V.D. Targeting triple-negative breast cancer cells with 6,7-bis(hydroxymethyl)-1H,3H-pyrrolo[1,2-c]thiazoles. Eur. J. Med. Chem. 2014, 79, 273–281. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Serra, A.C.; Gonsalves, A.M.D.A.R.; Laranjo, M.; Abrantes, A.M.; Gonçalves, A.C.; Sarmento-Ribeiro, A.B.; Botelho, M.F. Synthesis of new 2-galactosylthiazolidine-4-carboxylic acid amides. Antitumor evaluation against melanoma and breast cancer cells. Eur. J. Med. Chem. 2012, 53, 398–402. [Google Scholar] [CrossRef]
- Kim, K.H.; Sederstrom, J.M. Assaying Cell Cycle Status Using Flow Cytometry. In Current Protocols in Molecular Biology; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015. [Google Scholar]
- Rieger, A.M.; Nelson, K.L.; Konowalchuk, J.D.; Barreda, D.R. Modified Annexin V/Propidium Iodide Apoptosis Assay For Accurate Assessment of Cell Death. J. Vis. Exp. 2011, 50, e2597. [Google Scholar] [CrossRef]
- Laranjo, M.; Serra, A.C.; Abrantes, M.; Piñeiro, M.; Gonçalves, A.C.; Casalta-Lopes, J.; Carvalho, L.; Sarmento-Ribeiro, A.B.; Rocha-Gonsalves, A.; Botelho, F. 2-Bromo-5-hydroxyphenylporphyrins for photodynamic therapy: Photosensitization efficiency, subcellular localization and in vivo studies. Photodiagnosis Photodyn. Ther. 2013, 10, 51–61. [Google Scholar] [CrossRef] [PubMed]
- J Roelofs, A.; Thompson, K.; Ebetino, F.H.; Rogers, M.J.; Coxon, F.P. Bisphosphonates: Molecular mechanisms of action and effects on bone cells, monocytes and macrophages. Curr. Pharm. Des. 2010, 16, 2950–2960. [Google Scholar] [CrossRef]
- Açil, Y.; Möller, B.; Niehoff, P.; Rachko, K.; Gassling, V.; Wiltfang, J.; Simon, M.J.K. The cytotoxic effects of three different bisphosphonates in-vitro on human gingival fibroblasts, osteoblasts and osteogenic sarcoma cells. J. Craniomaxillofac. Surg. 2012, 40, e229–e235. [Google Scholar] [CrossRef] [PubMed]
- Reinholz, G.G.; Getz, B.; Pederson, L.; Sanders, E.S.; Subramaniam, M.; Ingle, J.N.; Spelsberg, T.C. Bisphosphonates directly regulate cell proliferation, differentiation, and gene expression in human osteoblasts. Cancer Res. 2000, 60, 6001–6007. [Google Scholar] [PubMed]
- Kimachi, K.; Kajiya, H.; Nakayama, S.; Ikebe, T.; Okabe, K. Zoledronic acid inhibits RANK expression and migration of osteoclast precursors during osteoclastogenesis. Naunyn. Schmiedebergs. Arch. Pharmacol. 2011, 383, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Walter, C.; Klein, M.O.; Pabst, A.; Al-Nawas, B.; Duschner, H.; Ziebart, T. Influence of bisphosphonates on endothelial cells, fibroblasts, and osteogenic cells. Clin. Oral Investig. 2010, 14, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Landesberg, R.; Cozin, M.; Cremers, S.; Woo, V.; Kousteni, S.; Sinha, S.; Garrett-Sinha, L.; Raghavan, S. Inhibition of oral mucosal cell wound healing by bisphosphonates. J. Oral Maxillofac. Surg. 2008, 66, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Kyrgidis, A.; Triaridis, S.; Antoniades, K. Effects of bisphosphonates on keratinocytes and fibroblasts having a role in the development of osteonecrosis of the jaw. Biosci. Hypotheses 2009, 2, 153–159. [Google Scholar] [CrossRef]
- Hasegawa, T.; Ri, S.; Umeda, M.; Komatsubara, H.; Kobayashi, M.; Shigeta, T.; Yoshitomi, I.; Ikeda, H.; Shibuya, Y.; Asahina, I.; et al. The observational study of delayed wound healing after tooth extraction in patients receiving oral bisphosphonate therapy. J. Craniomaxillofac. Surg. 2013, 41, 558–563. [Google Scholar] [CrossRef] [PubMed]
- George, E.L.; Lin, Y.-L.; Saunders, M.M. Bisphosphonate-related osteonecrosis of the jaw: A mechanobiology perspective. Bone Rep. 2018, 8, 104–109. [Google Scholar] [CrossRef]
- Holtmann, H.; Lommen, J.; Kübler, N.R.; Sproll, C.; Rana, M.; Karschuck, P.; Depprich, R. Pathogenesis of medication-related osteonecrosis of the jaw: A comparative study of in vivo and in vitro trials. J. Int. Med. Res. 2018, 46, 4277–4296. [Google Scholar] [CrossRef] [PubMed]
- Paulo, S.; Abrantes, A.M.; Laranjo, M.; Carvalho, L.; Serra, A.; Botelho, M.F.; Ferreira, M.M. Bisphosphonate-related osteonecrosis of the jaw: Specificities. Oncol. Rev. 2014, 8, 254. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Sinha, R.K. Bisphosphonate Related Osteonecrosis of the Jaw: An Update. J. Maxillofac. Oral Surg. 2014, 13, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Boanini, E.; Torricelli, P.; Gazzano, M.; Fini, M.; Bigi, A. The effect of zoledronate-hydroxyapatite nanocomposites on osteoclasts and osteoblast-like cells in vitro. Biomaterials 2012, 33, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Freire, E.; Vega, D.R.; Baggio, R. Zoledronate complexes. III. Two zoledronate complexes with alkaline earth metals: [Mg(C5H9N2O7P2)2(H2O)2] and [Ca(C5H8N2O7P2)(H2O)]n. Acta Crystallogr. C 2010, 66, m166–m170. [Google Scholar] [CrossRef] [PubMed]
- Daculsi, G.; Laboux, O.; Malard, O.; Weiss, P. Current state of the art of biphasic calcium phosphate bioceramics. J. Mater. Sci. Mater. Med. 2003, 14, 195–200. [Google Scholar] [CrossRef] [PubMed]
- Nery, E.B.; LeGeros, R.Z.; Lynch, K.L.; Lee, K. Tissue response to biphasic calcium phosphate ceramic with different ratios of HA/beta TCP in periodontal osseous defects. J. Periodontol. 1992, 63, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Müssig, E.; Tomakidi, P.; Steinberg, T. Gingival fibroblasts established on microstructured model surfaces: Their influence on epithelial morphogenesis and other tissue-specific cell functions in a co-cultured epithelium: An in-vitro model. J. Orofac. Orthop. 2009, 70, 351–362. [Google Scholar] [CrossRef]
- Ghatak, S.; Maytin, E.V.; Mack, J.A.; Hascall, V.C.; Atanelishvili, I.; Moreno Rodriguez, R.; Markwald, R.R.; Misra, S. Roles of Proteoglycans and Glycosaminoglycans in Wound Healing and Fibrosis. Int. J. Cell Biol. 2015, 2015, 1–20. [Google Scholar] [CrossRef]
- Oberringer, M.; Meins, C.; Bubel, M.; Pohlemann, T. In vitro wounding: Effects of hypoxia and transforming growth factorβ1 on proliferation, migration and myofibroblastic differentiation in an endothelial cell-fibroblast co-culture model. J. Mol. Histol. 2008, 39, 37–47. [Google Scholar] [CrossRef]
- Tracy, L.E.; Minasian, R.A.; Caterson, E.J. Extracellular Matrix and Dermal Fibroblast Function in the Healing Wound. Adv. Wound Care 2016, 5, 119–136. [Google Scholar] [CrossRef] [PubMed]
- Almeida-lopes, L.; Rigau, J.; Za, R.A. Comparison of the Low Level Laser Therapy Effects on Cultured Human Gingival Fibroblasts Proliferation Using Different Irradiance and Same Fluence. Lasers Surg. Med. 2001, 184, 179–184. [Google Scholar] [CrossRef] [PubMed]
- João, A.Z., Jr.; Savi, L.A.; Maria, C.; Simões, O.; Coura, G.S.; Aj, Z.J.; La, S.; Cmo, S.; Protocolo, M.R.D.S.; et al. Protocolo Preliminar de Cultura de Fibroblastos Gengivais Humanos. Rev. Bras. Implantodont. Prótese sobre Implant. 2005, 12, 190–196. [Google Scholar]
- Scheper, M.; Chaisuparat, R.; Cullen, K.; Meiller, T. A novel soft-tissue in vitro model for bisphosphonate-associated osteonecrosis. Fibrogenesis Tissue Repair 2010, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.G.; Pansani, T.N.; de Oliveira, C.F.; Turrioni, A.P.S.; Soares, D.G.; Hebling, J.; Costa, C.A.D.S. Cytotoxic Effects of Zoledronic Acid on Human Epithelial Cells and Gingival Fibroblasts. Braz. Dent. J. 2013, 24, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.G.; Soares, D.G.; Pansani, T.N.; Turrioni, A.P.S.; Scheffel, D.L.; Hebling, J.; Costa, C.A.D.S. Response of a co-culture model of epithelial cells and gingival fibroblasts to zoledronic acid. Braz. Oral Res. 2016, 30. [Google Scholar] [CrossRef]
- Kim, R.H.; Lee, R.S.; Williams, D.; Bae, S.; Woo, J.; Lieberman, M.; Oh, J.-E.; Dong, Q.; Shin, K.-H.; Kang, M.K.; et al. Bisphosphonates induce senescence in normal human oral keratinocytes. J. Dent. Res. 2011, 90, 810–816. [Google Scholar] [CrossRef]
- Pabst, A.M.; Ziebart, T.; Koch, F.P.; Taylor, K.Y.; Al-Nawas, B.; Walter, C. The influence of bisphosphonates on viability, migration, and apoptosis of human oral keratinocytes—in vitro study. Clin. Oral Investig. 2012, 16, 87–93. [Google Scholar] [CrossRef]
- Cornish, J.; Bava, U.; Callon, K.E.; Bai, J.; Naot, D.; Reid, I.R. Bone-bound bisphosphonate inhibits growth of adjacent non-bone cells. Bone 2011, 49, 710–716. [Google Scholar] [CrossRef]
- Chen, B.-R.; Cheng, H.-H.; Lin, W.-C.; Wang, K.-H.; Liou, J.-Y.; Chen, P.-F.; Wu, K.K. Quiescent fibroblasts are more active in mounting robust inflammatory responses than proliferative fibroblasts. PLoS ONE 2012, 7, e49232. [Google Scholar] [CrossRef]
- Agis, H.; Blei, J.; Watzek, G.; Gruber, R. Is zoledronate toxic to human periodontal fibroblasts? J. Dent. Res. 2010, 89, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Murray, A. Cell cycle checkpoints. Curr. Opin. Cell Biol. 1994, 6, 872–876. [Google Scholar] [CrossRef]
- Manzano-Moreno, F.J.; Ramos-Torrecillas, J.; De Luna-Bertos, E.; Ruiz, C.; García-Martínez, O. High doses of bisphosphonates reduce osteoblast-like cell proliferation by arresting the cell cycle and inducing apoptosis. J. Cranio-Maxillofacial Surg. 2015, 43, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Zafar, S.; Coates, D.E.; Cullinan, M.P.; Drummond, B.K.; Milne, T.; Seymour, G.J. Zoledronic acid and geranylgeraniol regulate cellular behaviour and angiogenic gene expression in human gingival fibroblasts. J. Oral Pathol. Med. 2014, 43, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Ohnuki, H.; Izumi, K.; Terada, M.; Saito, T.; Kato, H.; Suzuki, A.; Kawano, Y.; Nozawa-Inoue, K.; Takagi, R.; Maeda, T. Zoledronic acid induces S-phase arrest via a DNA damage response in normal human oral keratinocytes. Arch. Oral Biol. 2012, 57, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Zandi, M.; Dehghan, A.; Malekzadeh, H.; Janbaz, P.; Ghadermazi, K.; Amini, P. Introducing a protocol to create bisphosphonate-related osteonecrosis of the jaw in rat animal model. J. Craniomaxillofac. Surg. 2016, 44, 271–278. [Google Scholar] [CrossRef]
- Yanık, S.; Aras, M.H.; Erkılıç, S.; Bozdağ, Z.; Demir, T.; Çetiner, S. Histopathological features of bisphosphonates related osteonecrosis of the jaw in rats with and without vitamin d supplementation. Arch. Oral Biol. 2016, 65, 59–65. [Google Scholar] [CrossRef]
- Kaibuchi, N.; Iwata, T.; Yamato, M.; Okano, T.; Ando, T. Multipotent mesenchymal stromal cell sheet therapy for bisphosphonate-related osteonecrosis of the jaw in a rat model. Acta Biomater. 2016, 42, 400–410. [Google Scholar] [CrossRef]
- Vidal-Gutiérrez, X.; Gómez-Clavel, J.-F.; Gaitán-Cepeda, L.-A. Dental extraction following zoledronate, induces osteonecrosis in rat’s jaw. Med. Oral Patol. Oral Cir. Bucal 2017, 22, e177–e184. [Google Scholar]
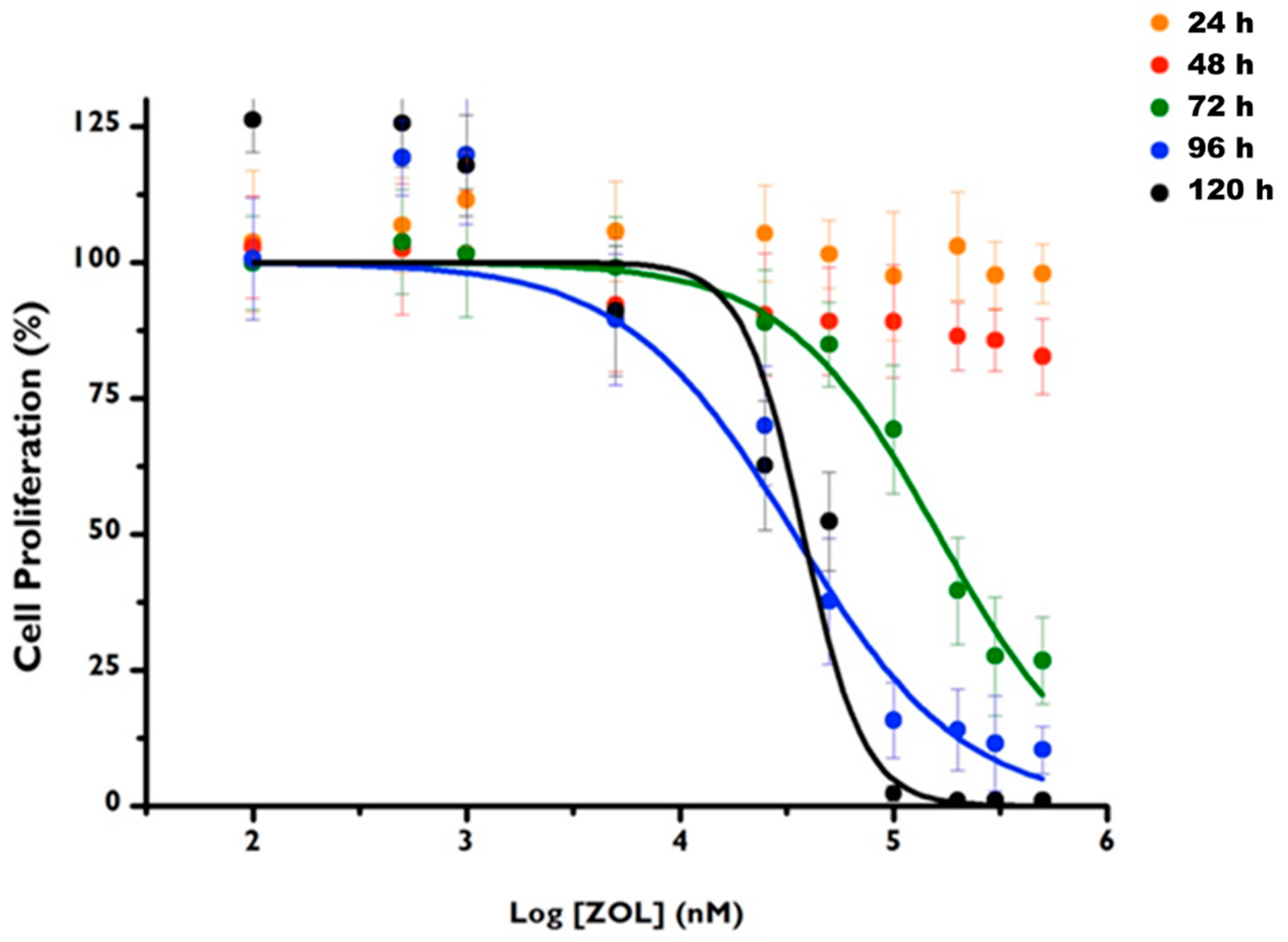
| Time | IC50 (μM) | Confidence Interval 95% | R2 |
|---|---|---|---|
| 72 h | 162.1 | [129.2; 203.3] | 0.997 |
| 96 h | 34.3 | [19.7; 59.9] | 0.943 |
| 120 h | 37.3 | [25.1; 56.8] | 0.932 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulo, S.; Laranjo, M.; Abrantes, A.M.; Casalta-Lopes, J.; Santos, K.; Gonçalves, A.C.; Paula, A.B.; Marto, C.M.; Sarmento-Ribeiro, A.B.; Carrilho, E.; et al. Synthetic Calcium Phosphate Ceramics as a Potential Treatment for Bisphosphonate-Related Osteonecrosis of the Jaw. Materials 2019, 12, 1840. https://doi.org/10.3390/ma12111840
Paulo S, Laranjo M, Abrantes AM, Casalta-Lopes J, Santos K, Gonçalves AC, Paula AB, Marto CM, Sarmento-Ribeiro AB, Carrilho E, et al. Synthetic Calcium Phosphate Ceramics as a Potential Treatment for Bisphosphonate-Related Osteonecrosis of the Jaw. Materials. 2019; 12(11):1840. https://doi.org/10.3390/ma12111840
Chicago/Turabian StylePaulo, Siri, Mafalda Laranjo, Ana M. Abrantes, João Casalta-Lopes, Kathleen Santos, Ana C. Gonçalves, Anabela Baptista Paula, Carlos Miguel Marto, Ana Bela Sarmento-Ribeiro, Eunice Carrilho, and et al. 2019. "Synthetic Calcium Phosphate Ceramics as a Potential Treatment for Bisphosphonate-Related Osteonecrosis of the Jaw" Materials 12, no. 11: 1840. https://doi.org/10.3390/ma12111840
APA StylePaulo, S., Laranjo, M., Abrantes, A. M., Casalta-Lopes, J., Santos, K., Gonçalves, A. C., Paula, A. B., Marto, C. M., Sarmento-Ribeiro, A. B., Carrilho, E., Serra, A., Botelho, M. F., & Ferreira, M. M. (2019). Synthetic Calcium Phosphate Ceramics as a Potential Treatment for Bisphosphonate-Related Osteonecrosis of the Jaw. Materials, 12(11), 1840. https://doi.org/10.3390/ma12111840










