Co-Blend Application Mode of Bulk Fill Composite Resin
Abstract
1. Introduction
2. Materials and Methods
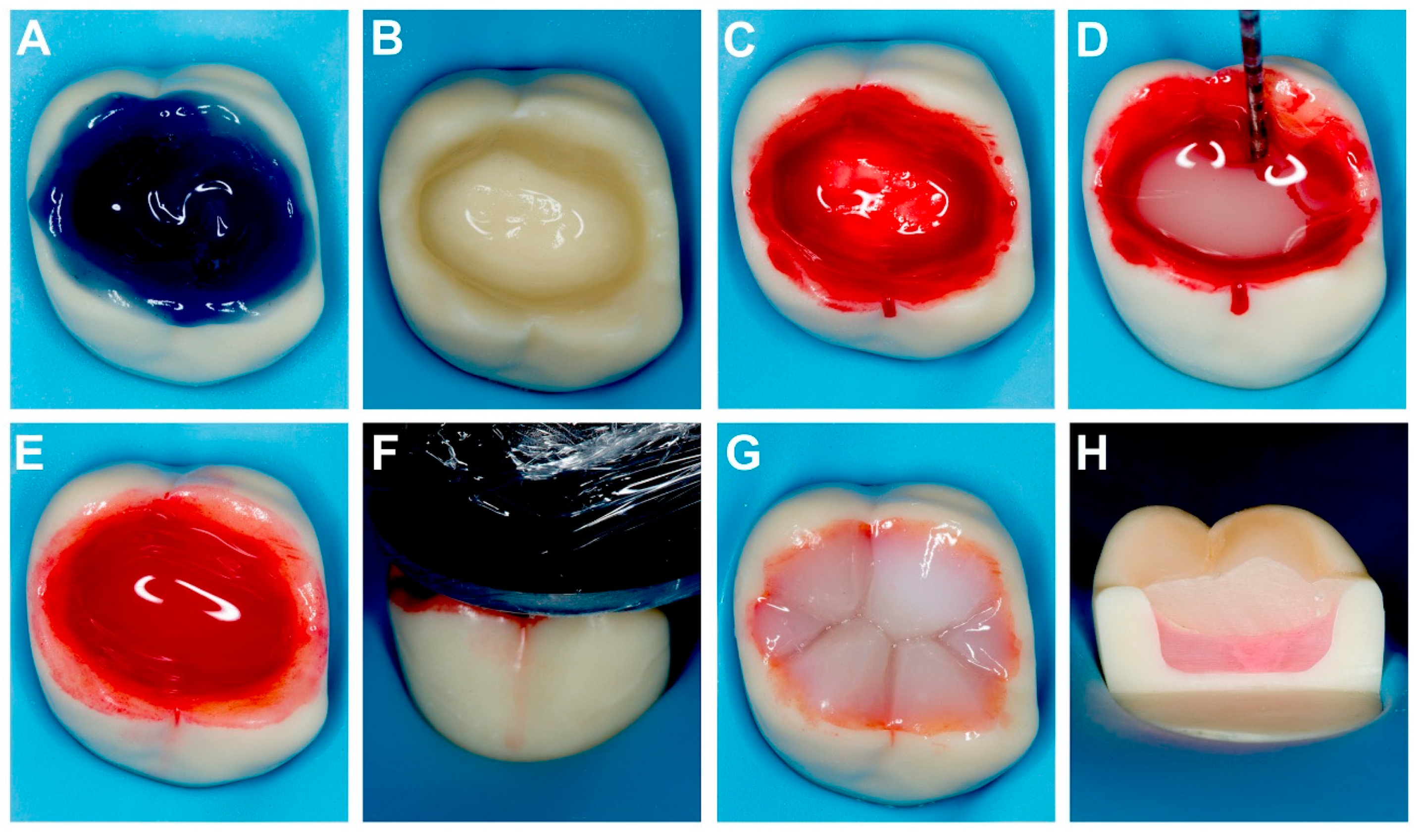
2.1. Teeth Selection and Preparation
2.2. Microtensile Bond Strength
2.3. Nanoleakage Analysis
2.4. Shrinkage and Gap Evaluation
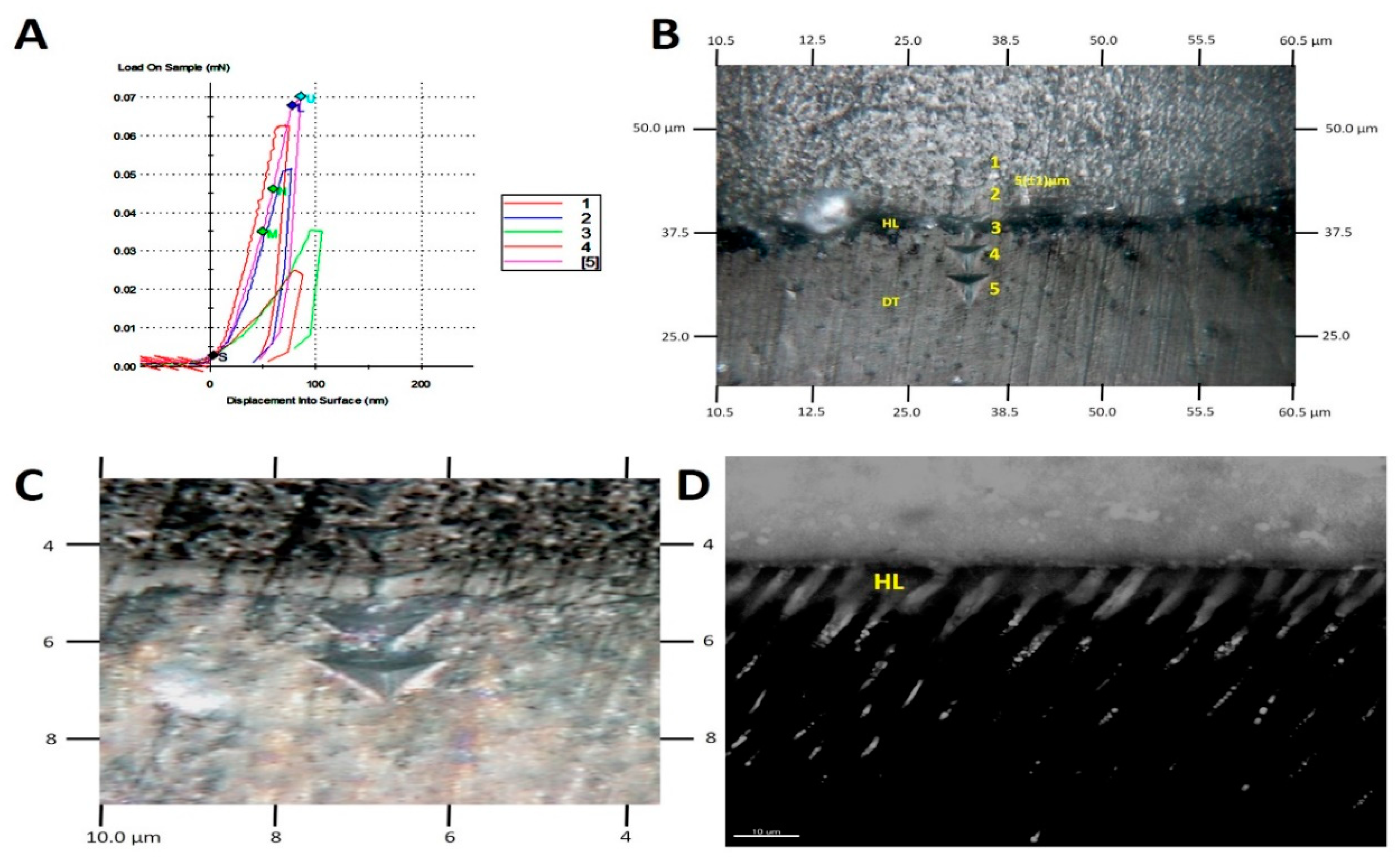
2.5. AFM Nanoindentation
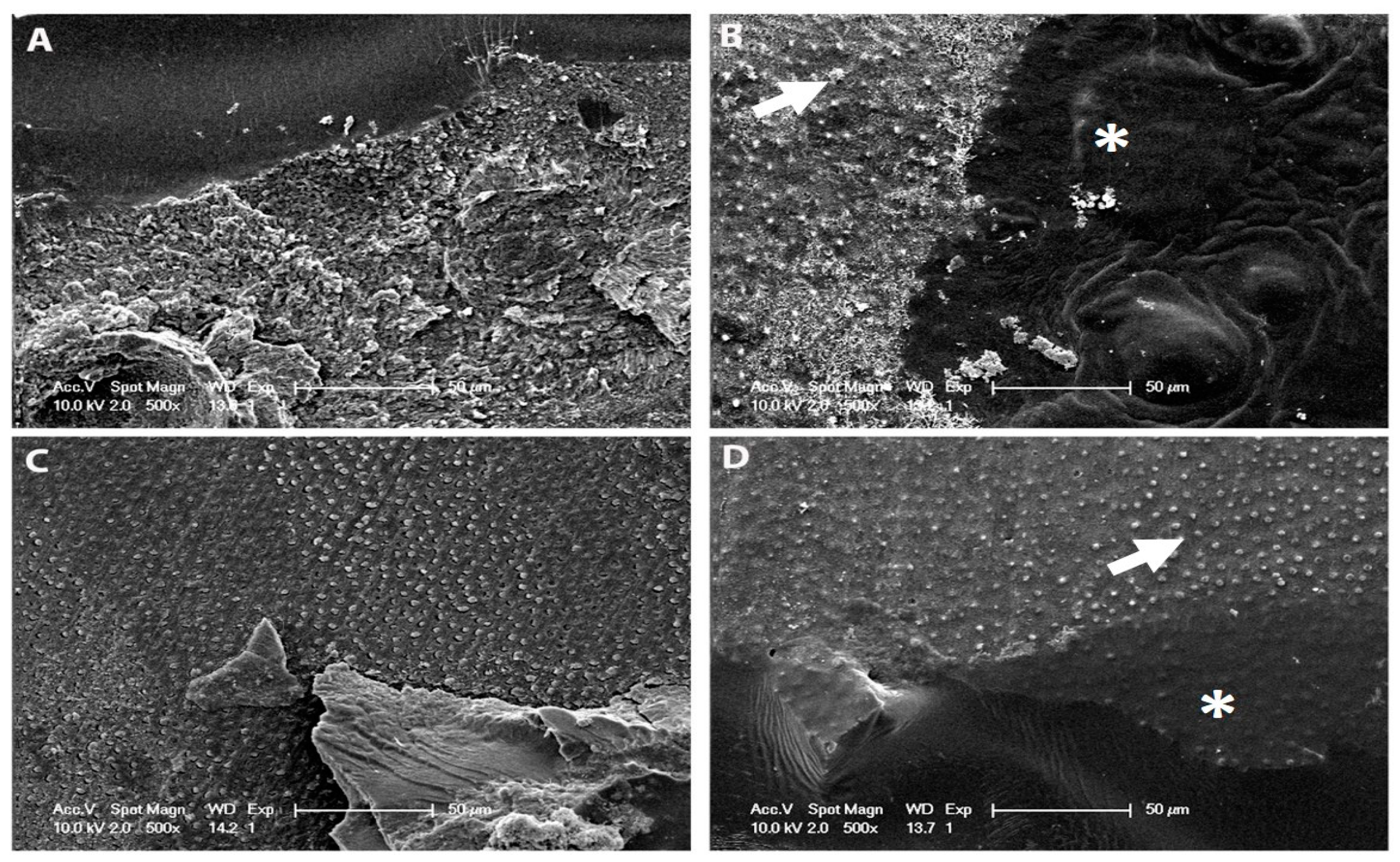
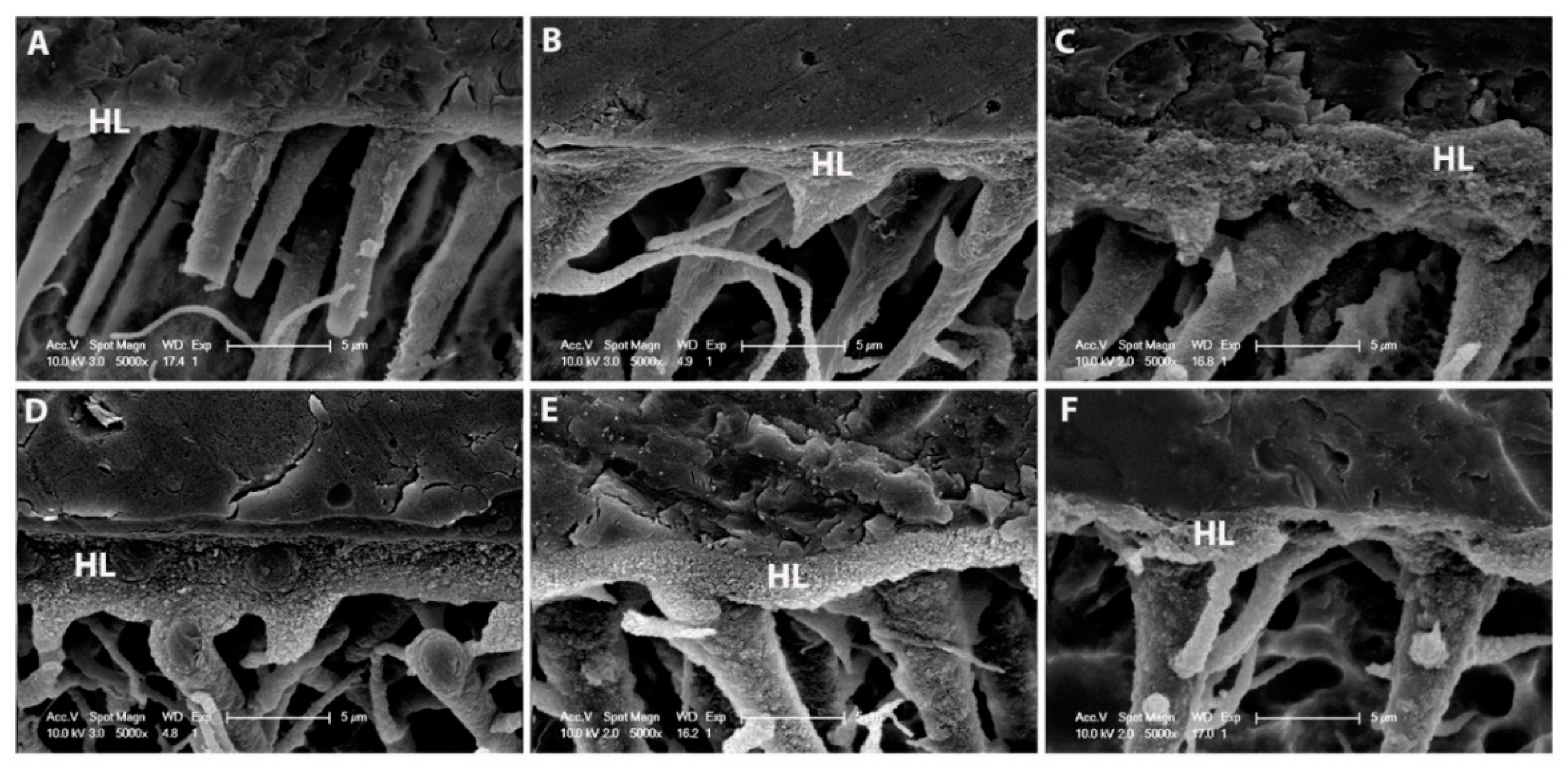
2.6. SEM Resin–Dentine Interface
2.7. Degree of Polymerization and Raman Data Acquisition/Processing
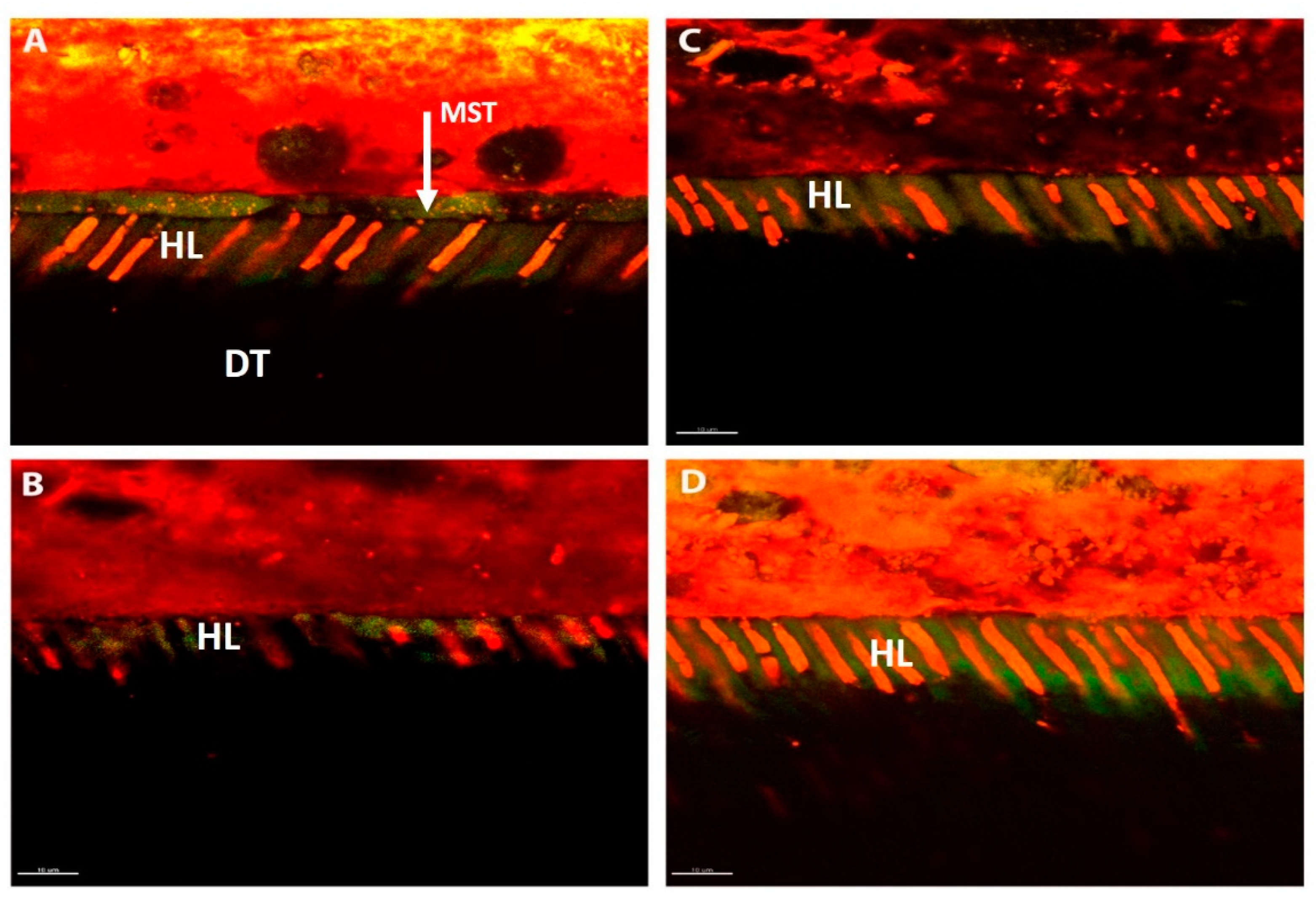
2.8. Confocal Microscopy
2.9. Statistical Analysis
3. Results
3.1. Microtensile Bond Testing
3.2. Nanoleakage Testing
3.3. Confocal Microscopy
3.4. SEM Resin–Dentine Interface
3.5. Shrinkage and Gap Evaluation
3.6. Degree of Conversion and Micro-Raman depth Penetration
3.7. Nanoindentation
4. Discussion
5. Clinical Significance
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liew, Z.; Nguyen, E.; Stella, R. Survey on the teaching and use in dental schools of resin-based materials for restoring posterior teeth. Int. Dent. J. 2011, 61, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Christensen, G.J. Should resin-based composite dominate restorative dentistry today? J. Am. Dent. Assoc. 2010, 141, 1490–1493. [Google Scholar] [CrossRef] [PubMed]
- Heintze, S.D.; Rousson, V. Clinical effectiveness of direct class II restorations—A meta-analysis. J. Adhes. Dent. 2012, 14, 407–431. [Google Scholar] [PubMed]
- Nakabayashi, N.; Kojima, K.; Masuhara, E. The promotion of adhesion by the infiltration of monomers into tooth substrates. J. Biomed. Mater. Res. 1982, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Pashley, D.H.; Tay, F.R.; Breschi, L.; Tjäderhane, L.; Carvalho, R.M.; Carrilho, M.; Tezvergil-Mutluay, A. State of the art etch-and-rinse adhesives. Dent. Mater. 2011, 27, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tjäderhane, L. Dentin bonding: Can we make it last? Oper. Dent. 2015, 40, 4–18. [Google Scholar] [CrossRef] [PubMed]
- De Munck, J.; Mine, A.; Poitevin, A.; Van Ende, A.; Cardoso, M.V.; Van Landuyt, K.L. Meta-analytical review of parameters involved in dentin bonding. J. Dent. Res. 2012, 91, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J. New Developments in dental adhesion. Dent. Clin. N. Am. 2007, 51, 333–357. [Google Scholar] [CrossRef]
- Buonocore, M.G. A simple method of increasing the adhesion of acrylic filling materials to enamel surfaces. J. Dent. Res. 1955, 34, 849–853. [Google Scholar] [CrossRef]
- Buonocore, M.G.; Wileman, W.; Brudevold, F. A report on a resin composition capable of bonding to human dentin surfaces. J. Dent. Res. 1956, 35, 846–851. [Google Scholar] [CrossRef]
- Carvalho, R.M.; Tjäderhane, L.; Manso, A.P.; Carrilho, M.R.; Carvalho, C.A.R. Dentin as a bonding substrate. Endod. Top. 2012, 21, 62–88. [Google Scholar] [CrossRef]
- Pashley, D.H.; Pashley, E.L.; Carvalho, R.M.; Tay, F.R. The effects of dentin permeability on restorative dentistry. Dent. Clin. N. Am. 2002, 46, 211–245. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H. Have dentin adhesives become too hydrophilic? J. Can. Den. Assoc. 2003, 69, 726–731. [Google Scholar]
- Tay, F.R.; Pashley, D.H.; Suh, B.I.; Carvalho, R.M.; Itthagarun, A. Single-step adhesives are permeable membranes. J. Dent. 2002, 30, 371–382. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H.; Garcia-Godoy, F.; Yiu, C.K. Single-step, self-etch adhesives behave as permeable membranes after polymerisation. Part II. Silver tracer penetration evidence. Am. J. Dent. 2004, 17, 315–322. [Google Scholar]
- Tay, F.R.; Pashley, D.H.; Suh, B.; Carvalho, R.; Miller, M. Single-step, self-etch adhesives behave as permeable membranes after polymerisation. Part I. Bond strength and morphologic evidence. Am. J. Dent. 2004, 17, 271–278. [Google Scholar]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar]
- Itthagarun, A.; Tay, F.R.; Pashley, D.H.; Wefel, J.S.; Garcia-Godoy, F.; Wei, S.H. Single-step, self-etch adhesives behave as permeable membranes after polymerisation. Part III. Evidence from fluid conductance and artificial caries inhibition. Am. J. Dent. 2004, 17, 394–400. [Google Scholar]
- Sano, H.; Shono, T.; Takatsu, T.; Hosoda, H. Microporous dentin zone beneath resin-impregnated layer. Oper. Dent. 1994, 19, 59–64. [Google Scholar]
- Pashley, D.H.; Tay, F.R.; Yiu, C.; Hashimoto, M.; Breschi, L.; Carvalho, R.M.; Ito, S. Collagen degradation by host-derived enzymes during aging. J. Dent. Res. 2004, 83, 216–221. [Google Scholar] [CrossRef]
- Cadenaro, M.; Antoniolli, F.; Sauro, S.; Tay, F.R.; Di Lenarda, R.; Prati, C.; Biasotto, M.; Contardo, L.; Breschi, L. Degree of conversion and permeability of dental adhesives. Eur. J. Oral Sci. 2005, 113, 525–530. [Google Scholar] [CrossRef]
- Spencer, P.; Wang, Y. Adhesive phase separation at the dentin interface under wet bonding conditions. J. Biomed. Mater. Res. 2002, 62, 447–456. [Google Scholar] [CrossRef]
- Kuhn, E.; Farhat, P.; Paula Teitelbaum, A.; Mena-Serrano, A.; Loguercio, A.D.; Reis, A.; Pashley, D.H. Ethanol-wet bonding technique: Clinical versus laboratory findings. Dent. Mater. 2015, 31, 1030–1037. [Google Scholar] [CrossRef]
- Tay, F.R.; Pashley, D.H.; Kapur, R.R.; Carrilho, M.R.; Hur, Y.B.; Garrett, L.V.; Tay, K.C. Bonding BisGMA to dentin-a proof of concept for hydrophobic dentin bonding. J. Dent. Res. 2007, 86, 1034–1039. [Google Scholar] [CrossRef]
- Ito, S.; Tay, F.R.; Hashimoto, M.; Yoshiyama, M.; Saito, T.; Brackett, W.W.; Waller, J.L.; Pashley, D.H. Effects of multiple coatings of two all-in-one adhesives on dentin bonding. J. Adhes. Dent. 2005, 7, 133–141. [Google Scholar]
- Taschner, M.; Kümmerling, M.; Lohbauer, U.; Breschi, L.; Petschelt, A.; Frankenberger, R. Effect of double-layer application on dentin bond durability of one-step self-etch adhesives. Oper. Dent. 2014, 39, 416–426. [Google Scholar] [CrossRef]
- King, N.M.; Tay, F.R.; Pashley, D.H.; Hashimoto, M.; Ito, S.; Brackett, W.W.; García-Godoy, F.; Sunico, M. Conversion of one-step to two-step self-etch adhesives for improved efficacy and extended application. Am. J. Dent. 2005, 18, 126–134. [Google Scholar]
- Van Landuyt, K.L.; Peumans, M.; De Munck, J.; Lambrechts, P.; Van Meerbeek, B. Extension of a one-step self-etch adhesive into a multi-step adhesive. Dent. Mater. 2006, 22, 533–544. [Google Scholar] [CrossRef]
- De Vito Moraes, A.G.; Francci, C.; Carvalho, C.N.; Soares, S.P.; Braga, R.R. Microshear bond strength of self-etching systems associated with a hydrophobic resin layer. J. Adhes. Dent. 2011, 13, 341–348. [Google Scholar]
- Chapman, J.L.; Burgess, J.O.; Holst, S.; Sadan, A.; Blatz, M.B. Precuring of self-etching bonding agents and its effect on bond strength of resin composite to dentin and enamel. Quintessence Int. 2007, 38, 637–641. [Google Scholar]
- Mena-Serrano, A.; Garcia, E.J.; Loguercio, A.D.; Reis, A. Effect of sonic application mode on the resin–dentin bond strength and nanoleakage of simplified self-etch adhesive. Clin. Oral Invest 2014, 18, 729–736. [Google Scholar] [CrossRef]
- Mena-Serrano, A.; Costa, T.R.; Patzlaff, R.T.; Loguercio, A.D.; Reis, A. Effect of sonic application mode on the resin-dentin bond strength and dentin permeability of self-etching systems. J. Adhes. Dent. 2014, 16, 435–440. [Google Scholar]
- Sauro, S.; Watson, T.F.; Thompson, I.; Toledano, M.; Nucci, C.; Banerjee, A. Influence of air-abrasion executed with polyacrylic acid-Bioglass 45S5 on the bonding performance of a resin-modified glass ionomer cement. Eur. J. Oral Sci. 2012, 2, 168–177. [Google Scholar] [CrossRef]
- Saboia, V.P.; Natob, F.; Mazzonic, A.; Orsinid, G.; Putignanoe, A.; Gianninif, M.; Breschig, L. Adhesion of a Two-step Etch-and-Rinse Adhesive on Collagen-depleted Dentin. J. Adhes. Dent. 2008, 10, 419–422. [Google Scholar]
- Sauro, S.; Osorio, R.; Watson, T.F.; Toledano, M. Influence of phosphoproteins’ biomimetic analogs on remineralization of mineral-depleted resin-dentin interfaces created with ion-releasing resin-based systems. Dent. Mater. 2015, 7, 759–777. [Google Scholar] [CrossRef]
- Chesterman, J.; Jowett, A.; Gallacher, A.; Nixon, P. Bulk-fill resin-based composite restorative materials: A review. Br. Dent. J. 2017, 222, 337–344. [Google Scholar] [CrossRef]
- Ilie, N.; Hickel, R. Investigations on a methacrylate-based flowable composite based on the SDR™ technology. Dent. Mater. 2011, 27, 348–355. [Google Scholar] [CrossRef]
- Alrahlah, A.; Silikas, N.; Watts, D.C. Post-cure depth of cure of bulk fill dental resin-composites. Dent. Mater. 2014, 30, 149–154. [Google Scholar] [CrossRef]
- De Assis, F.S.; Lima, S.N.; Tonetto, M.R.; Bhandi, S.H.; Pinto, S.C.; Malaquias, P.; Loguercio, A.D.; Bandéca, M.C. Evaluation of bond strength, marginal integrity, and fracture strength of bulk- versus incrementally-filled restorations. J. Adhes. Dent. 2016, 18, 317–323. [Google Scholar]
- Koc-Vural, U.; Baltacioglu, I.; Altinci, P. Color stability of bulk-fill and incremental-fill resin-based composites polished with aluminium-oxide impregnated disks. Restor. Dent. Endod. 2017, 42, 118–124. [Google Scholar] [CrossRef]
- Van Ende, A.; de Munck, J.; Lise, D.P.; van Meerbeek, B. Bulk-fill composites: A review of the current literature. J. Adhes. Dent. 2017, 19, 95–109. [Google Scholar]
- Gonzalez, L.G.; Hiller, J.; Terrill, N.J.; Parkinson, J.; Thomas, K.; Wess, T.J. Effects of isopropanol on collagen fibrils in new parchment. Chem. Cent. J 2012, 6, 24. [Google Scholar] [CrossRef]
- Niu, Y.; Ma, X.; Fan, M.; Zhu, S. Effect of layering techniques on the micro-tensile bond strength to dentin in resin composite restorations. Dent. Mater. 2009, 25, 129–134. [Google Scholar] [CrossRef]
- Davidson, C.L.; de Gee, A.J.; Feilzer, A. The competition between the composite-dentin bond strength and the polymerization contraction stress. J. Dent. Res. 1984, 63, 1396–1399. [Google Scholar] [CrossRef]
- Davidson, C.L.; Feilzer, A.J. Polymerization shrinkage and polymerization shrinkage stress in polymer-based restoratives. J. Dent. 1997, 6, 435–440. [Google Scholar] [CrossRef]
- Fok, A.S.L. Shrinkage stress development in dental composites—An analytical treatment. Dent. Mater. 2013, 29, 1108–1115. [Google Scholar] [CrossRef]
- Schneider, L.F.; Cavalcante, L.M.; Silikas, N. Shrinkage Stresses Generated during Resin-Composite Applications: A Review. J. Dent. Biomech. 2010, 2010, 131630. [Google Scholar] [CrossRef]
- Davidson, C.L.; de Gee, A.J. Relaxation of polymerization contraction stresses by flow in dental composites. J. Dent. Res. 1984, 63, 146–148. [Google Scholar] [CrossRef]
- Savadi Oskoee, S.; Bahari, M.; Jafari Navimipour, E.; Ajami, A.A.; Ghiasvand, N.; Savadi Oskoee, A. Factors affecting marginal integrity of class II bulk-fill composite resin restorations. J. Dent. Res. Dent. Clin. Dent. Prosp. 2017, 11, 101–109. [Google Scholar] [CrossRef]
- Farahat, F.; Daneshkazemi, A.R.; Hajiahmadi, Z. The effect of bulk depth and irradiation time on the surface hardness and degree of cure of bulk-fill composites. J. Dent. Biomater. 2016, 3, 284–291. [Google Scholar]
- Lee, S.K.; Kim, T.W.; Son, S.A.; Park, J.K.; Kim, J.H.; Kim, H.I.; Kwon, Y.H. Influence of light-curing units on the polymerization of low-shrinkage composite resins. Dent. Mater. J. 2013, 32, 688–694. [Google Scholar] [CrossRef]
| Dental Bonding Agent (DBA) | ||
|---|---|---|
| Brand Name, Manufacturer | Optibond FL, Kerr | Prime & Bond Universal |
| Resin monomers | Primer: HEMA, GPDM, PAMM Bonding: TEGDMA, UDMA, GPDM, HEMA, bis-GMA | PENTA (dipentaerythritol pentacrylate phosphate), 10-MDP (10-methacryloyloxydecyl dihydrogen phosphate), Active GuardTM Technology crosslinker |
| Initiator system | CQ/tertiary amine | CQ/tertiary amine |
| Filler load | 48 w/o | Unfilled |
| Filler size | 0.6 μm | - |
| Filler type | Barium glass | - |
| Solvent type | Ethanol, water | Isopropanol, water |
| Solvent content | 20–30 w/o | 10–24.5% Isopropanol, 5–24.5% water |
| Others | Fluoride | - |
| Delivery systems | bottle | bottle |
| Composite Resin Restorative Material | ||
|---|---|---|
| Brand name, manufacturer | CERAM.X ONE, Dentsply | SureFil SDR flow+, Dentsply |
| Resin monomers | Poly-urethanemethacrylate, bis-EMA, TEGDMA | Modified urethane dimethacrylate resin, ethoxylated bisphenol-A dimethacrylate (EBPADMA), triethyleneglycol dimethacrylate (TEGDMA) |
| Initiator/accelerator system | Camphorquinone (CQ)/butylated hydroxyl toluene (BHT) | Camphorquinone (CQ)/butylated hydroxyl toluene (BHT) |
| Filler load | 77–79 w/o (59–61 v/o) | 47.3 v/o |
| Filler size | Organic fillers ≈ 15 μm, non-agglomerated inorganic fillers ≈ 0.6 μm | Inorganic filler from 0.02–10 μm |
| Filler type | Barium glass and ytterbium fluoride fillers | Silanated barium and strontium glass fillers, ytterbium fluoride fillers, Nano fillers |
| Filler shape | Spherical, spray-granulation production method | Unagglomerated (barium and strontium glass fillers), irregularly shaped |
| Adhesives | Groups | 24 h | Thermocycling 10,000 | DOC % (30 min after light activation) | Nano Hardness (Hi) GPa |
|---|---|---|---|---|---|
| Optibond FL, Kerr | MI | 32.1 ± 4.4 A α | 29.9 ± 3.3 a β | 89.22 ± 1.9 ‡‡ | 5.9 ± 0.8 A |
| MB2 | 35.5 ± 5.2 C β | 33.3 ± 7.1 b γ | 91.43 ± 3.3 ‡‡ | 9.8 ± 1.1 B | |
| MB4 | 30.1 ± 8.0 AB α | 28.7 ± 6.3 a β | 78.66 ± 1.8 ‡ | 3.9 ± 2.3 C | |
| Prime & Bond Universal | MI | 36.4 ± 5.5.5 B β | 31.7 ± 8.0 b γ | 80.66 ± 3.1 ‡ | 4.3 ± 0.9 C |
| MB2 | 33.1±6.1 A α | 29.8 ± 6.6 a β | 82.11 ± 1.5 ‡ | 6.4 ± 2.2 BC | |
| MB4 | 27.3 ± 4.9 D ∞ | 24.1.2 ± 2.3 c ∞ | 67.61 ± 2.5 † | 3.8 ± 2.1 C |
| Sub Groups | Failure Mode | Optibond FL, Kerr | Prime & Bond UniversalTM | ||
|---|---|---|---|---|---|
| 24 h | Thermocycling 10,000 | 24 h | Thermocycling 10,000 | ||
| MI | A | 29 | 39 | 14 | 23 |
| M | 45 | 33 | 44 | 51 | |
| CD | 11 | 8 | 20 | 7 | |
| CC | 15 | 20 | 22 | 19 | |
| MB2 | A | 19 | 23 | 32 | 10 |
| M | 51 | 47 | 60 | 45 | |
| CD | 15 | 8 | 4 | 18 | |
| CC | 15 | 22 | 4 | 27 | |
| MB4 | A | 21 | 31 | 29 | 27 |
| M | 40 | 39 | 38 | 45 | |
| CD | 18 | 6 | 12 | 11 | |
| CC | 21 | 24 | 4 | 17 | |
| Optibond FL, Kerr | Sub Groups | Nanoleakage Score | |||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | p-value | ||
| Baseline | MI | 12 | 15 | 17 | 2 | 6 | 0.066 |
| MB2 | 6 | 8 | 12 | 12 | 3 | 0.03 | |
| MB4 | 8 | 9 | 21 | 14 | 5 | 0.512 | |
| Thermocycled | MI | 1 | 0 | 16 | 16 | 6 | |
| MB2 | 3 | 9 | 28 | 10 | 1 | ||
| MB4 | 0 | 11 | 24 | 5 | 6 | ||
| Prime & Bond Universal | Sub Groups | Nanoleakage Score | |||||
| 0 | 1 | 2 | 3 | 4 | p-value | ||
| Baseline | M1 | 4 | 18 | 7 | 9 | 6 | 0.000 |
| MB2 | 3 | 10 | 6 | 11 | 7 | 0.001 | |
| MB4 | 0 | 0 | 16 | 10 | 11 | 0.222 | |
| Thermocycled | M1 | 0 | 0 | 12 | 9 | 16 | |
| MB2 | 5 | 0 | 9 | 17 | 5 | ||
| MB4 | 0 | 0 | 22 | 3 | 4 | ||
| Optibond FL | Sub Groups | Estimated Mean | p-value |
| MI | 4.11 ± 1.2 | 0.23 | |
| MB2 | 0.87 ± 0.09 | 0.02 | |
| MB4 | 2.12 ± 0.9 | 0.31 | |
| Prime & Bond Universal | MI | 6.11 ± 2.1 | 0.77 |
| MB2 | 2.43 ± 0.81 | 0.04 | |
| MB4 | 2.12 ± 0.6 | 0.9 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Nabulsi, M.; Daud, A.; Yiu, C.; Omar, H.; Sauro, S.; Fawzy, A.; Daood, U. Co-Blend Application Mode of Bulk Fill Composite Resin. Materials 2019, 12, 2504. https://doi.org/10.3390/ma12162504
Al-Nabulsi M, Daud A, Yiu C, Omar H, Sauro S, Fawzy A, Daood U. Co-Blend Application Mode of Bulk Fill Composite Resin. Materials. 2019; 12(16):2504. https://doi.org/10.3390/ma12162504
Chicago/Turabian StyleAl-Nabulsi, Mohammad, Alaa Daud, Cynthia Yiu, Hanan Omar, Salvatore Sauro, Amr Fawzy, and Umer Daood. 2019. "Co-Blend Application Mode of Bulk Fill Composite Resin" Materials 12, no. 16: 2504. https://doi.org/10.3390/ma12162504
APA StyleAl-Nabulsi, M., Daud, A., Yiu, C., Omar, H., Sauro, S., Fawzy, A., & Daood, U. (2019). Co-Blend Application Mode of Bulk Fill Composite Resin. Materials, 12(16), 2504. https://doi.org/10.3390/ma12162504









