Synthesis of Calcium Aluminates from Non-Saline Aluminum Dross
Abstract
1. Introduction
2. Materials and Methods
2.1. Dross
- Sample Al-1: Dross aged 3 to 7 years.
- Sample Al-2: Dross with an age of 7 to 10 years, stored outdoors.
- Sample Al-3: Recent dross, generated in the last two years.
2.2. Characterization
2.3. Aluminate Preparation
3. Results and Discussion
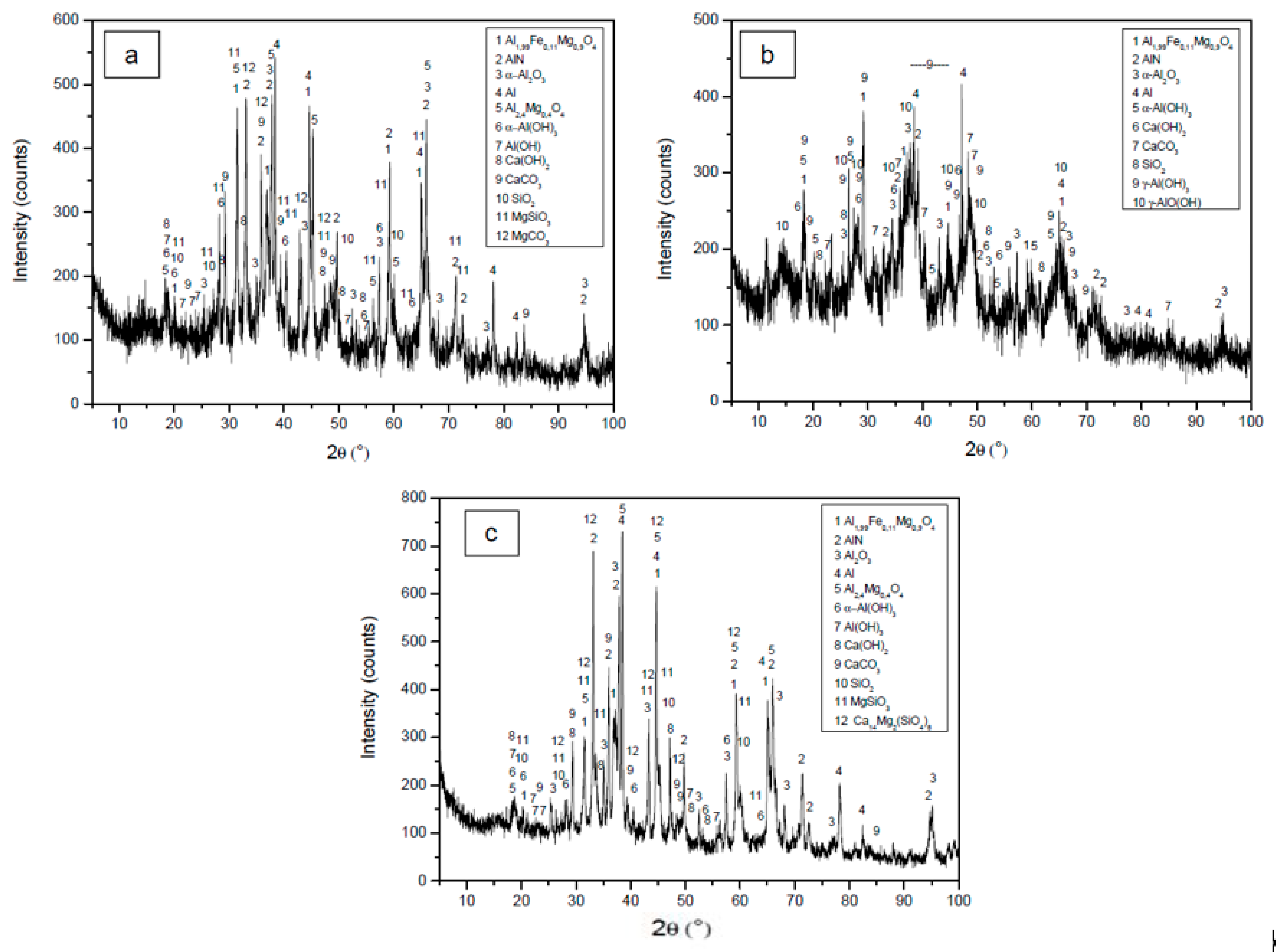
3.1. Chemical Composition
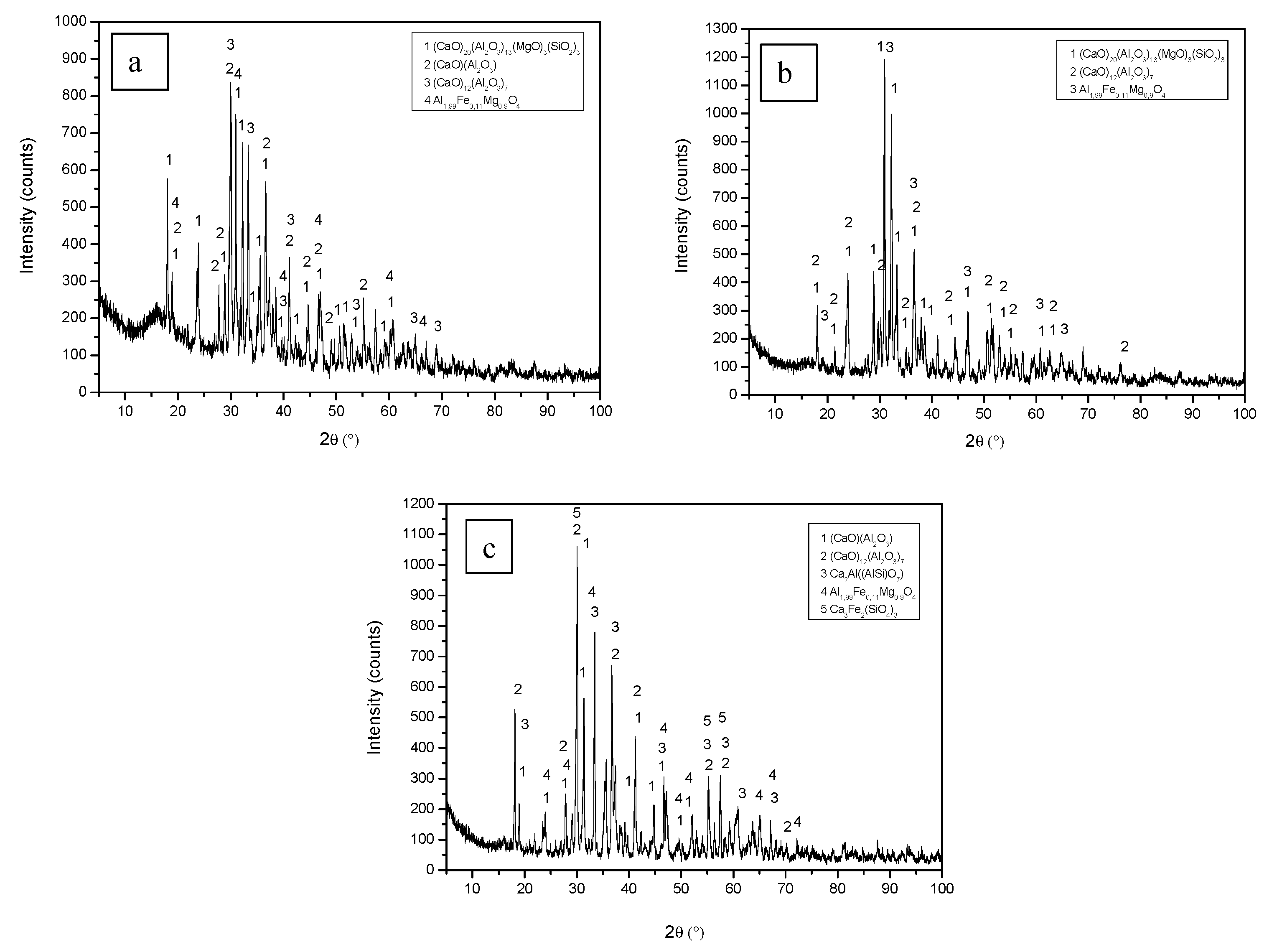
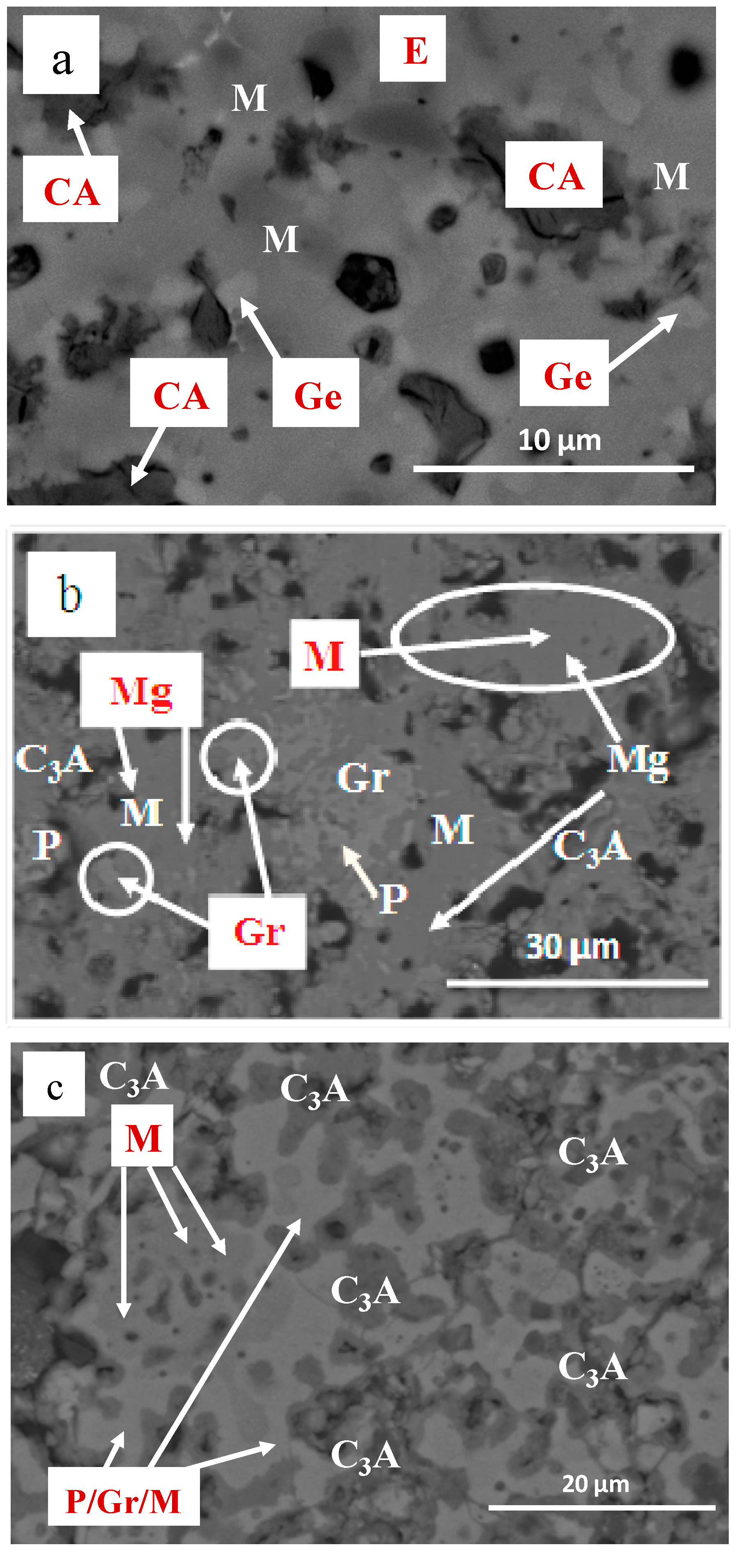
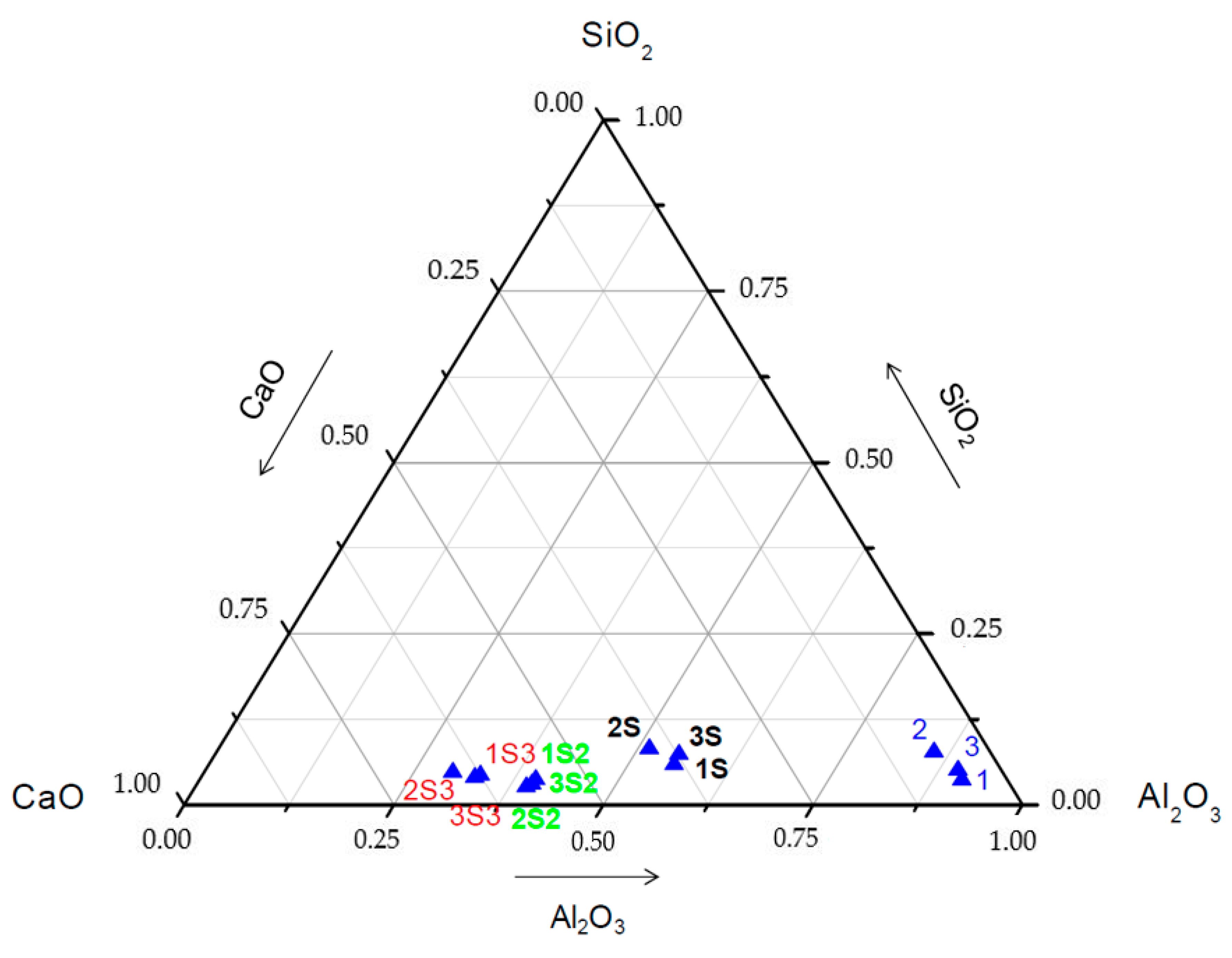
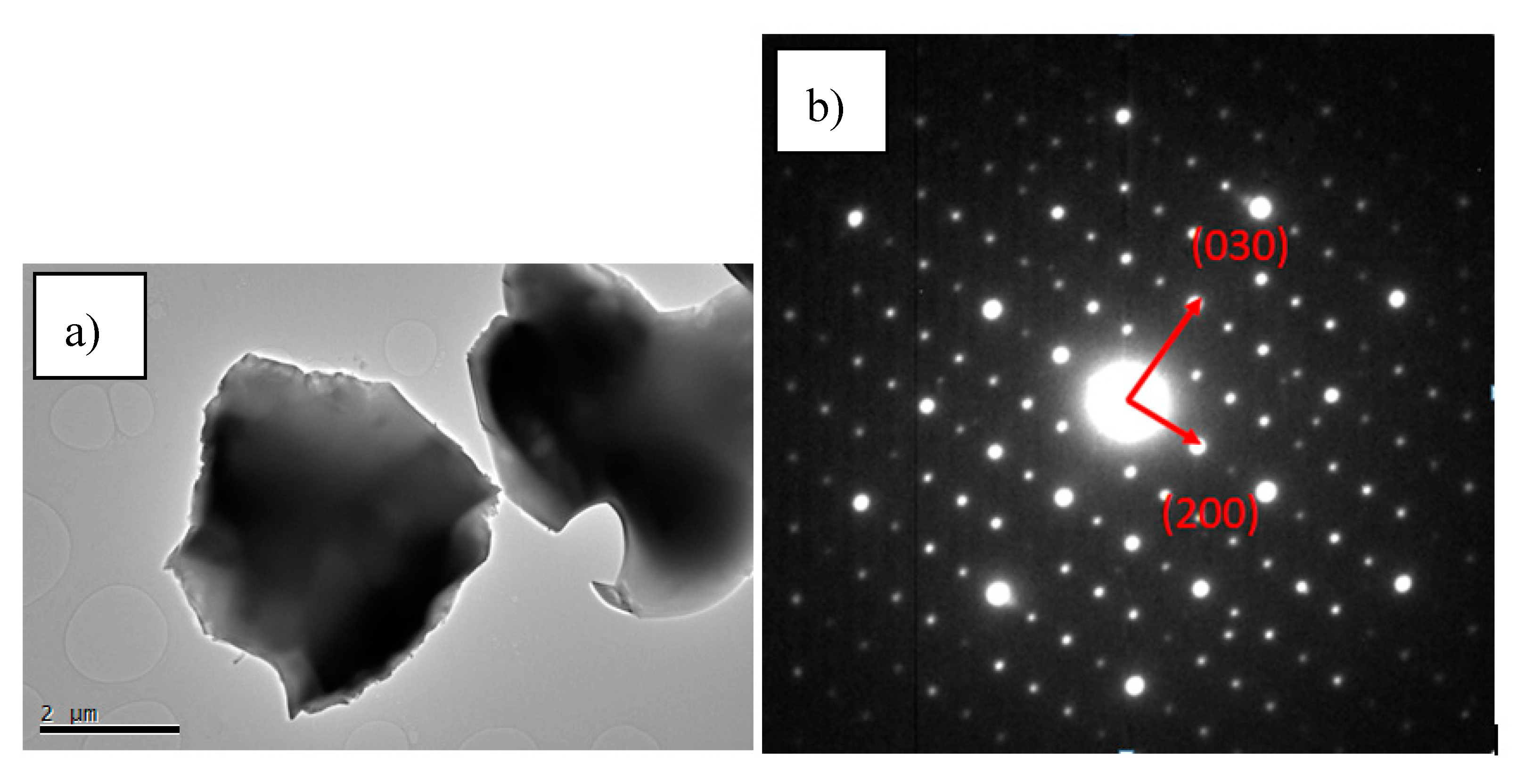
3.2. Chemical Composition and Microstructural Characterization of Sintered Materials
4. Conclusions
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hallsted, B. Assessment of the CaO- Al2O3 system. J. Am. Ceram. Soc. 1990, 73, 15–23. [Google Scholar] [CrossRef]
- Eriksson, G.; Pelton, A.D. Critical evaluation and optimization of the thermodynamic properties and phase diagrams of the CaO-Al2O3, Al2O3-SiO2, and CaO-Al2O3-SiO2 systems. Metall. Trans. B 1993, 24, 807–816. [Google Scholar] [CrossRef]
- Merlini, M.; Artioli, G.; Cerulli, T.; Cella, F.; Bravo, A. Tricalcium aluminate hydration in additivated systems. A crystallographic study by SR-XRPD. Cem. Concr. Res. 2008, 38, 477–486. [Google Scholar] [CrossRef]
- Ghoroi, C.; Suresh, A.K. Solid–solid reaction kinetics: Formation of tricalcium aluminate. AIChE J. 2007, 53, 502–513. [Google Scholar] [CrossRef]
- Iftekhar, S.; Grins, J.; Svensson, G.; Lööf, J.; Jarmar, T.; Botton, G.A.; Andrei, C.M.; Engqvist, H. Phase formation of CaAl2O4 from CaCO3–Al2O3 powder mixtures. J. Eur. Ceram. Soc. 2008, 28, 747–756. [Google Scholar] [CrossRef]
- Singh, V.K.; Ali, M.M.; Mandal, U.K. Formation Kinetics of Calcium Aluminates. J. Am. Ceram. Soc. 1990, 73, 872–876. [Google Scholar] [CrossRef]
- Kuzmenko, V.V.; Uspenskaya, I.A.; Rudnyi, E.B. Simultaneous assessment of thermodynamic functions of calcium aluminates. Bull. Des. Soc. Chim. Belg. 1997, 106, 235–242. [Google Scholar]
- Williamson, J.; Glasser, F.P. Reactions in heated lime-alumina mixtures. J. Appl. Chem. 2007, 12, 535–538. [Google Scholar] [CrossRef]
- Mohamed, B.M.; Sharp, J.H. Kinetics and mechanism of formation of tricalcium aluminate, Ca3Al2O6. Thermochim. Acta 2002, 388, 105–114. [Google Scholar] [CrossRef]
- Gaki, A.; Chrysafi, R.; Kakali, G. Chemical synthesis of hydraulic calcium aluminate compounds using the Pechini technique. J. Eur. Ceram. Soc. 2007, 27, 1781–1784. [Google Scholar] [CrossRef]
- Voicu, G.; Ghiţulicǎ, C.D.; Andronescu, E. Modified Pechini synthesis of tricalcium aluminate powder. Mater. Charact. 2012, 73, 89–95. [Google Scholar] [CrossRef]
- Zivica, V.; Palou, M.T.; Bagel, L.; Krizma, M. Low-porosity tricalcium aluminate hardened paste. Constr. Build. Mater. 2013, 38, 1191–1198. [Google Scholar] [CrossRef]
- Salimi, R.; Vaughan, J. Crystallisation of tricalcium aluminate from sodium aluminate solution using slaked lime. Powder Technol. 2016, 294, 472–483. [Google Scholar] [CrossRef]
- Stephan, D.; Wilhelm, P. Synthesis of Pure Cementitious Phases by Sol-Gel Process as Precursor. Z. Anorg. Allg. Chem. 2004, 630, 1477–1483. [Google Scholar] [CrossRef]
- Ianoş, R.; Lazǎu, I.; Pǎcurariu, C.; Barvinschi, P. Fuel mixture approach for solution combustion synthesis of Ca3Al2O6 powders. Cem. Concr. Res. 2009, 39, 566–572. [Google Scholar] [CrossRef]
- Beelen, C.M.; Van Der Knoop, W. Methods of Procesing Aluminium Dross and Aluminium Dross Residue into Calcium Aluminate. U.S. Patent 5,716,426, 10 February 1998. [Google Scholar]
- Kemeny, F.L.; Sosinsky, D.J.; Schmitt, R.J. Process for Converting Aluminum Dross to Ladle Flux for Steel Processing. U.S. Patent 5,385,601, 31 January 1995. [Google Scholar]
- Pickens, J.W.; Morris, E.L. Process for Preparing Calcium Aluminate from Aluminium Dross. U.S. Patent 6,238,633, 29 May 2001. [Google Scholar]
- Martínez Iglesias, J.; Salinas, A.; Marquínez, F. Procedimiento para Obtener Aluminato Cálcico a partir del Residuo Obtenido tras el Tratamiento de las Escorias Salinas Procedentes de la Producción de Aluminio Secundario. Patent ES2343052B, 21 July 2010. [Google Scholar]
- Ewais, E.M.; Khalil, N.M.; Amin, M.S.; Ahmed, Y.M.Z.; Barakat, M.A. Utilization of aluminum sludge and aluminum slag (dross) for the manufacture of calcium aluminate cement. Ceram. Int. 2009, 35, 3381–3388. [Google Scholar] [CrossRef]
- Li, A.; Zhang, H.; Yang, H. Evaluation of aluminum dross as raw material for high-alumina refractory. Ceram. Int. 2014, 40, 12585–12590. [Google Scholar] [CrossRef]
- López-Delgado, A.; López, F.A.; Gonzalo-Delgado, L.; López-Andrés, S.; Alguacil, F.J. Study by DTA/TG of the formation of calcium aluminate obtained from an aluminium hazardous waste. J. Therm. Anal. Calorim. 2010, 99, 999–1004. [Google Scholar] [CrossRef][Green Version]
- Fernández-González, D.; Prazuch, J.; Ruiz-Bustinza, I.; González-Gasca, C.; Piñuela-Noval, J.; Verdeja, L.F. Solar synthesis of calcium aluminates. Sol. Energy 2018, 171, 658–666. [Google Scholar] [CrossRef]
- Wesselsky, A.; Jensen, O.M. Synthesis of pure Portland cement phases. Cem. Concr. Res. 2009, 39, 973–980. [Google Scholar] [CrossRef]
- Takács, G.; Ondrejkovič, K.; Hulkó, G. A low-cost non-invasive slag detection system for continuous casting. IFAC-PapersOnLine 2017, 50, 438–445. [Google Scholar] [CrossRef]
- Fruehan, R.J. The Making, Shaping and Treating of Steel, Vol. 2: Steelmaking and Refining Volume; The AISE Steel Foundation: Pittsburgh, PA, USA, 1998; ISBN 978-0930767020. [Google Scholar]
- McCormick, P.G.; Picaro, T.; Smith, P.A.I. Mechanochemical treatment of high silica bauxite with lime. Miner. Eng. 2002, 15, 211–214. [Google Scholar] [CrossRef]
- Ou, Z.; Li, J.; Wang, Z. Application of mechanochemistry to metal recovery from second-hand resources: A technical overview. Environ. Sci. Process. Impacts 2015, 17, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Li, J. Recycling Metals from Wastes: A Novel Application of Mechanochemistry. Environ. Sci. Technol. 2015, 49, 5849–5861. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-H. Mechanical activation of calcium aluminate formation from CaCO3-Al2O3 mixtures. J. Alloys Compd. 2006, 416, 279–283. [Google Scholar] [CrossRef]
- Chen, F.; Hong, Y.; Sun, J.; Bu, J. Preparation and characterization of calcium aluminate by chemical synthesis. J. Univ. Sci. Technol. Beijing Miner. Metall. Mater. 2006, 13, 82–86. [Google Scholar] [CrossRef]
- Richarson, F.D. Physical Chemistry of Melts in Metallurgy; Academic Press: New York, NY, USA, 1974; ISBN 0125879024. [Google Scholar]

| Element | Al-1 | Al-2 | Al-3 |
|---|---|---|---|
| Al | 40.1 | 30.9 | 43.4 |
| Ca | 3.2 | 3.3 | 3.4 |
| Fe | 2.6 | 3.2 | 1.3 |
| Mg | 1.9 | 1.2 | 2.0 |
| Si | 1.4 | 2.5 | 2.1 |
| Mn | 0.19 | 0.13 | 0.2 |
| Cu | 0.11 | 0.32 | 0.08 |
| Zn | 0.032 | 2.018 | 0.043 |
| Ni | 0.020 | 0.020 | 0.010 |
| L.O.I. | 7.4 | 17.4 | 3.3 |
| Mineralogical Phase | Al-1 | Al-2 | Al-3 |
|---|---|---|---|
| Spinel, Al1.99Fe0.11Mg0.9O4 | 23.3 | 13.6 | 24.2 |
| Aluminum nitride, AlN | 13.9 | 3.1 | 12.3 |
| Corundum, α-Al2O3 | 8.3 | 6.2 | 12.0 |
| Metallic aluminum, Al | 11.4 | 3.8 | 14.4 |
| Spinel, Al2.4Mg0.4O4 | 23.3 | - | 15.8 |
| Bayerite, α-Al(OH)3 | 5.9 | 3.4 | 2.1 |
| Norstrandite, Al(OH)3 | 1.8 | - | 0.90 |
| Portlandite, Ca(OH)2 | 1.4 | 2.2 | 2.9 |
| Calcite, CaCO3 | 8.7 | 10.3 | 6.4 |
| Quartz, SiO2 | 0.80 | 1.0 | 0.40 |
| Enstatite, MgSiO3 | 0.80 | - | 4.5 |
| Magnesite, MgCO3 | 0.60 | - | - |
| Boehmite, γ-AlO(OH) | - | 50.4 | - |
| Gibbsite, γ-Al(OH)3 | - | 5.9 | - |
| Bredigite, Ca14Mg2(SiO4)8 | - | - | 3.6 |
| Element | Al1 S A:C 1:1 | Al2 S A:C 1:1 | Al3 S A:C 1:1 | Al1 2S A:C 1:2 | Al2 2S A:C 1:2 | Al3 2S A:C 1:2 | Al1 3S A:C 1:3 | Al2 3S A:C 1:3 | Al3 3S A:C 1:3 |
|---|---|---|---|---|---|---|---|---|---|
| Al | 27.4 | 26.1 | 30.0 | 22.2 | 20.2 | 20.7 | 16.9 | 15.3 | 18.4 |
| Fe | 1.9 | 1.6 | 1.51 | 1.2 | 1.75 | 0.72 | 0.95 | 1.13 | 0.61 |
| Ca | 25.6 | 28.3 | 27.3 | 42.1 | 39.9 | 39.9 | 44.2 | 45.7 | 46.7 |
| Mg | 1.2 | 0.85 | 1.1 | 0.65 | 0.85 | 0.89 | 0.72 | 0.56 | 0.80 |
| Si | 2.5 | 3.7 | 3.5 | 1.8 | 1.2 | 2.4 | 1.8 | 2.2 | 2.1 |
| Mn | 0.13 | 0.09 | 0.12 | 0.07 | 0.11 | 1.4 | 0.08 | 0.05 | 0.07 |
| Ni | 0.03 | 0.04 | 0.03 | 0.02 | 0.02 | 0.01 | 0.02 | 0.02 | 0.01 |
| Cu | 0.09 | 0.28 | 0.07 | 0.23 | 0.05 | 0.03 | 0.07 | 0.16 | 0.11 |
| Zn | 0.24 | 2.25 | 0.13 | 1.92 | 2.2 | 0.10 | 0.18 | 1.5 | 0.09 |
| Mineralogical Phase Molar Ratio Al2O3/CaO | Al1 S 1:1 | Al2 S 1:1 | Al3 S 1:1 | Al1 2S 1:2 | Al2 2S 1:2 | Al3 2S 1:2 | Al1 3S 1:3 | Al2 3S 1:3 | Al3 3S 1:3 |
|---|---|---|---|---|---|---|---|---|---|
| C3A* | - | - | - | 49.4 | 49.8 | 39.2 | 85.1 | 71.6 | 87.0 |
| C12A7* | 19.7 | 13.3 | 23.6 | 32.5 | 30.6 | 41.5 | 5.20 | 3.75 | 5.27 |
| CA* | 32.8 | - | 47.8 | - | - | - | - | - | - |
| Total Aluminates | 52.5 | 13.3 | 71.5 | 81.9 | 80.5 | 80.7 | 90.2 | 75.4 | 92.2 |
| Al1.99Fe0.11Mg0.90O4 | 5.5 | 7.0 | 10.2 | - | - | - | - | - | - |
| Ca20Mg3Al26Si3O68 | 41.5 | 79.6 | - | - | - | - | - | - | - |
| Ca3Fe2(SiO4)3 | - | - | 5.3 | - | - | - | - | - | - |
| Al2Ca2O7Si | - | - | 14.5 | - | - | - | - | - | - |
| Al1.95Fe0.49Mg2.65O12Si2.91 | - | - | - | 2.58 | 10.57 | 1.66 | 2.29 | 9.68 | 1.78 |
| Ca3Al2(SiO4)3 | - | - | - | 13.44 | - | 15.96 | 2.13 | 1.85 | 1.40 |
| Ca6(SiO4)(Si3O10) | - | - | - | - | 7.03 | - | - | 9.01 | - |
| Al0.2Fe1.8MgO4 | - | - | - | - | 1.96 | - | - | 0.97 | - |
| Silicates and Other Phases | 47.0 | 86.5 | 29.6 | 16.02 | 19.86 | 17.62 | 4.4 | 21.6 | 3.1 |
| SiO2 | - | - | - | - | - | - | - | 0.25 | - |
| CaO | - | - | - | 0.63 | - | - | 3.28 | 2.85 | 2.25 |
| MgO | - | - | - | 1.50 | - | 1.68 | 2.05 | - | 2.32 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, F.A.; Martín, M.I.; Alguacil, F.J.; Sergio Ramírez, M.; González, J.R. Synthesis of Calcium Aluminates from Non-Saline Aluminum Dross. Materials 2019, 12, 1837. https://doi.org/10.3390/ma12111837
López FA, Martín MI, Alguacil FJ, Sergio Ramírez M, González JR. Synthesis of Calcium Aluminates from Non-Saline Aluminum Dross. Materials. 2019; 12(11):1837. https://doi.org/10.3390/ma12111837
Chicago/Turabian StyleLópez, Félix Antonio, María Isabel Martín, Francisco José Alguacil, Mario Sergio Ramírez, and José Ramón González. 2019. "Synthesis of Calcium Aluminates from Non-Saline Aluminum Dross" Materials 12, no. 11: 1837. https://doi.org/10.3390/ma12111837
APA StyleLópez, F. A., Martín, M. I., Alguacil, F. J., Sergio Ramírez, M., & González, J. R. (2019). Synthesis of Calcium Aluminates from Non-Saline Aluminum Dross. Materials, 12(11), 1837. https://doi.org/10.3390/ma12111837







