Core-Shell and Hollow Particles of Carbon and SiC Prepared from Hydrochar
Abstract
1. Introduction
2. Experimental Section
2.1. Precursors
2.2. Experimental Procedure
2.2.1. Hydrothermal Carbonization
2.2.2. Pulse Current Treatment
2.2.3. Infiltration of Vaporized Silicon
2.2.4. Fabrication of Hollow Particles of SiC by Template Removal
2.3. Characterization
2.3.1. Elemental Analyses
2.3.2. Thermogravimetric Analyses (TGA)
2.3.3. N2 Adsorption and Textual Analyses
2.3.4. Argon Ion-Beam Cross-Section Polishing
2.3.5. Scanning Electron Microscopy
2.3.6. Transmission Electron Microscopy
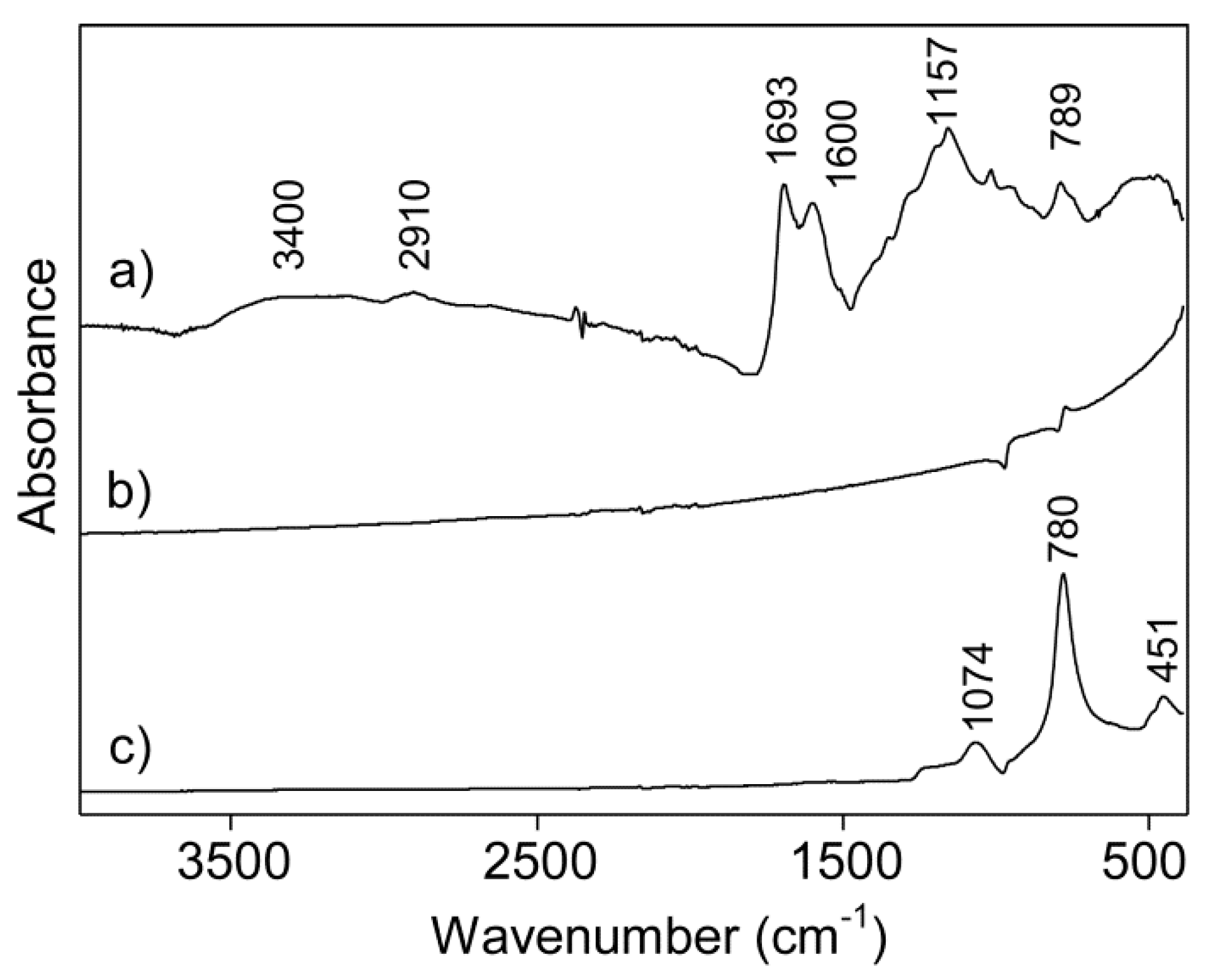
2.3.7. Infrared Spectroscopy
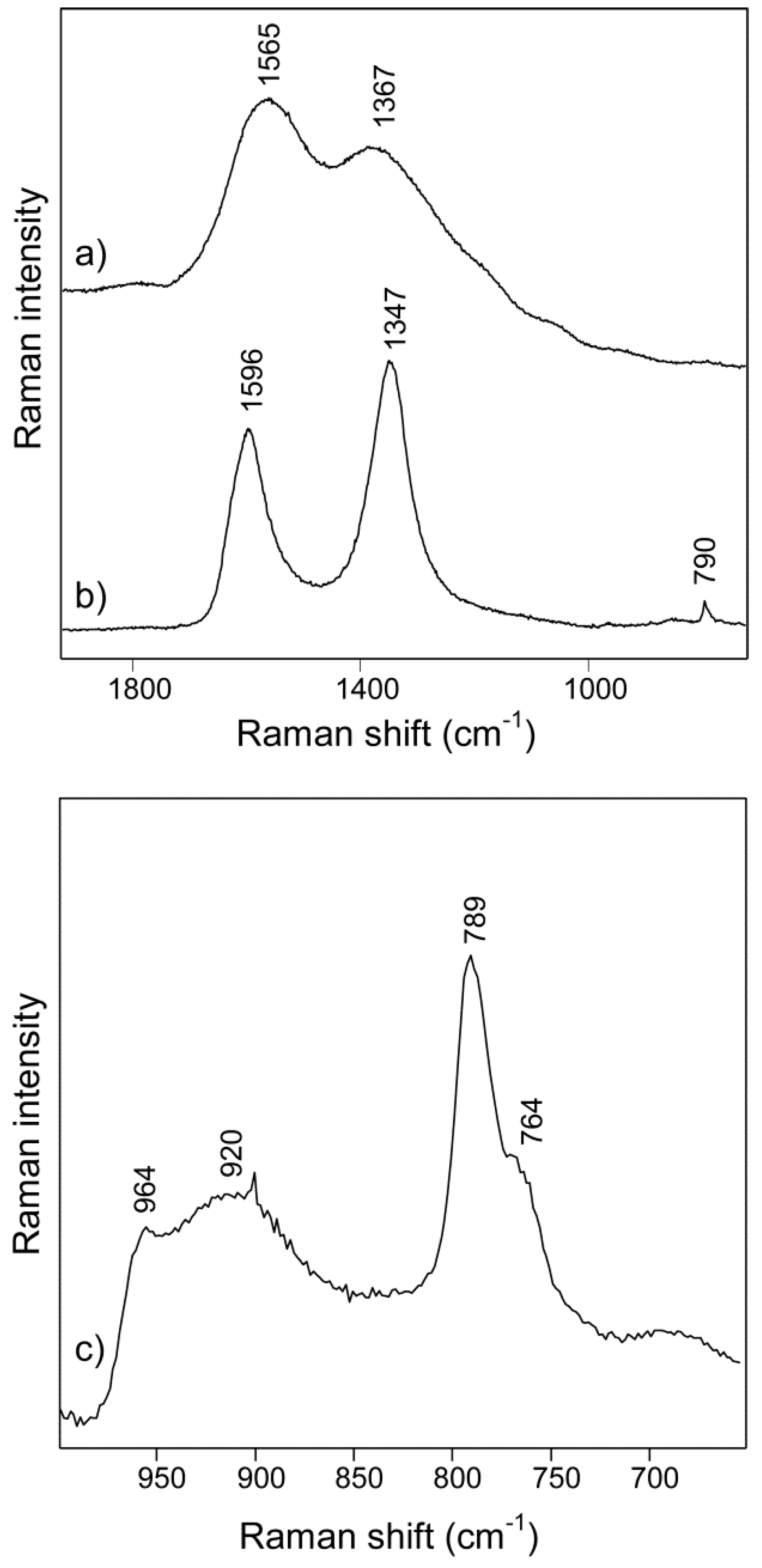
2.3.8. Raman Spectroscopy
2.3.9. X-ray Diffraction
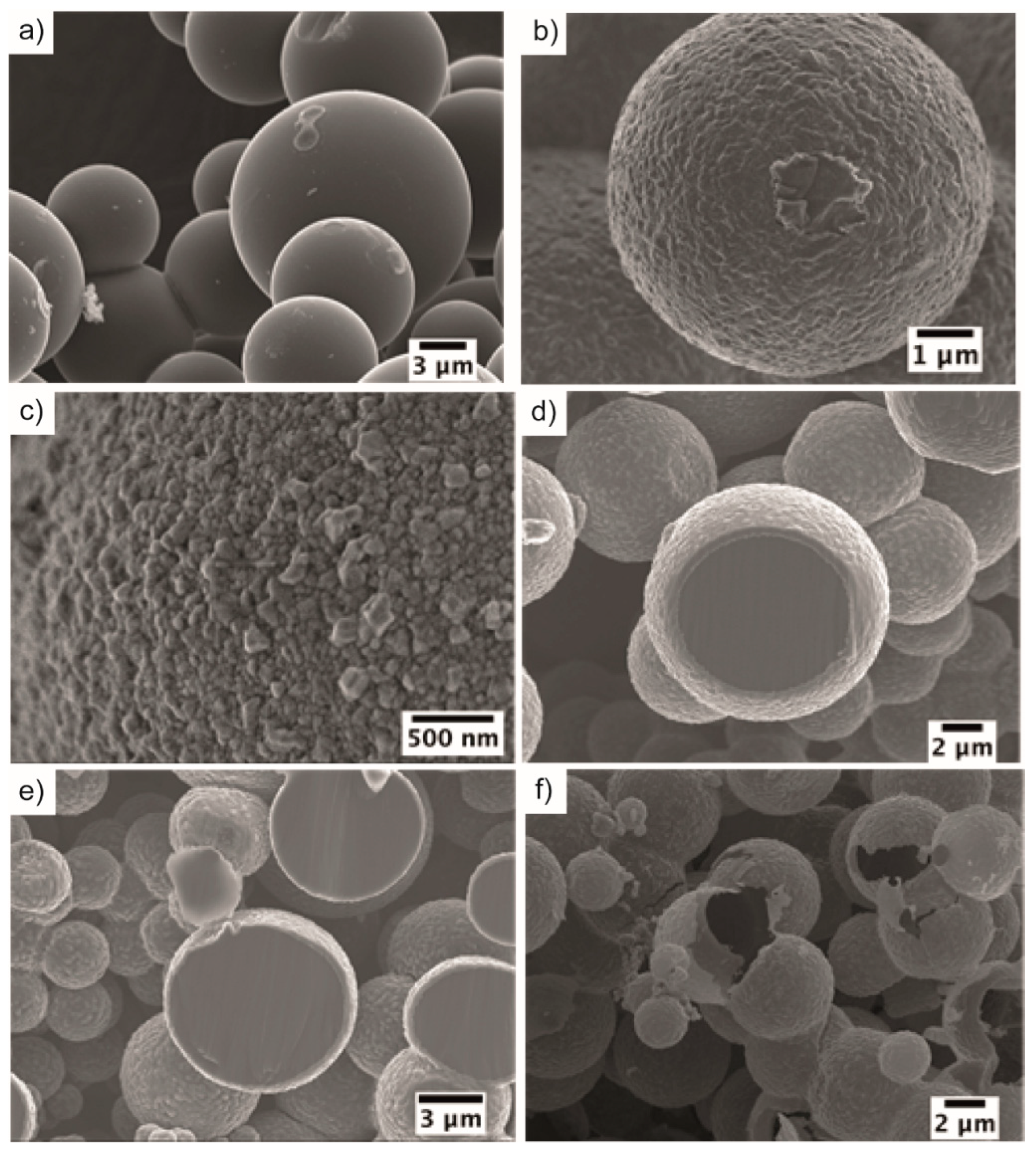
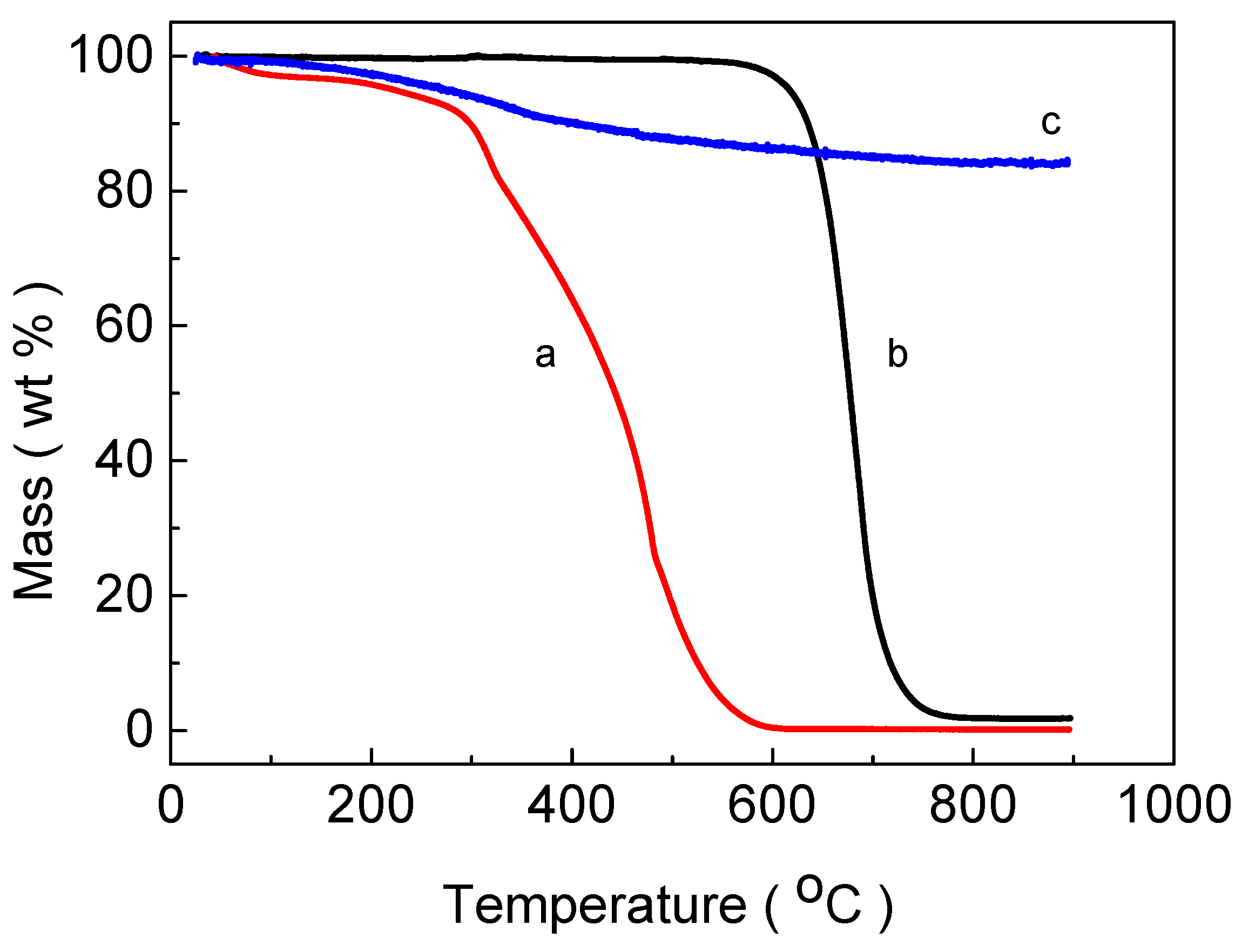
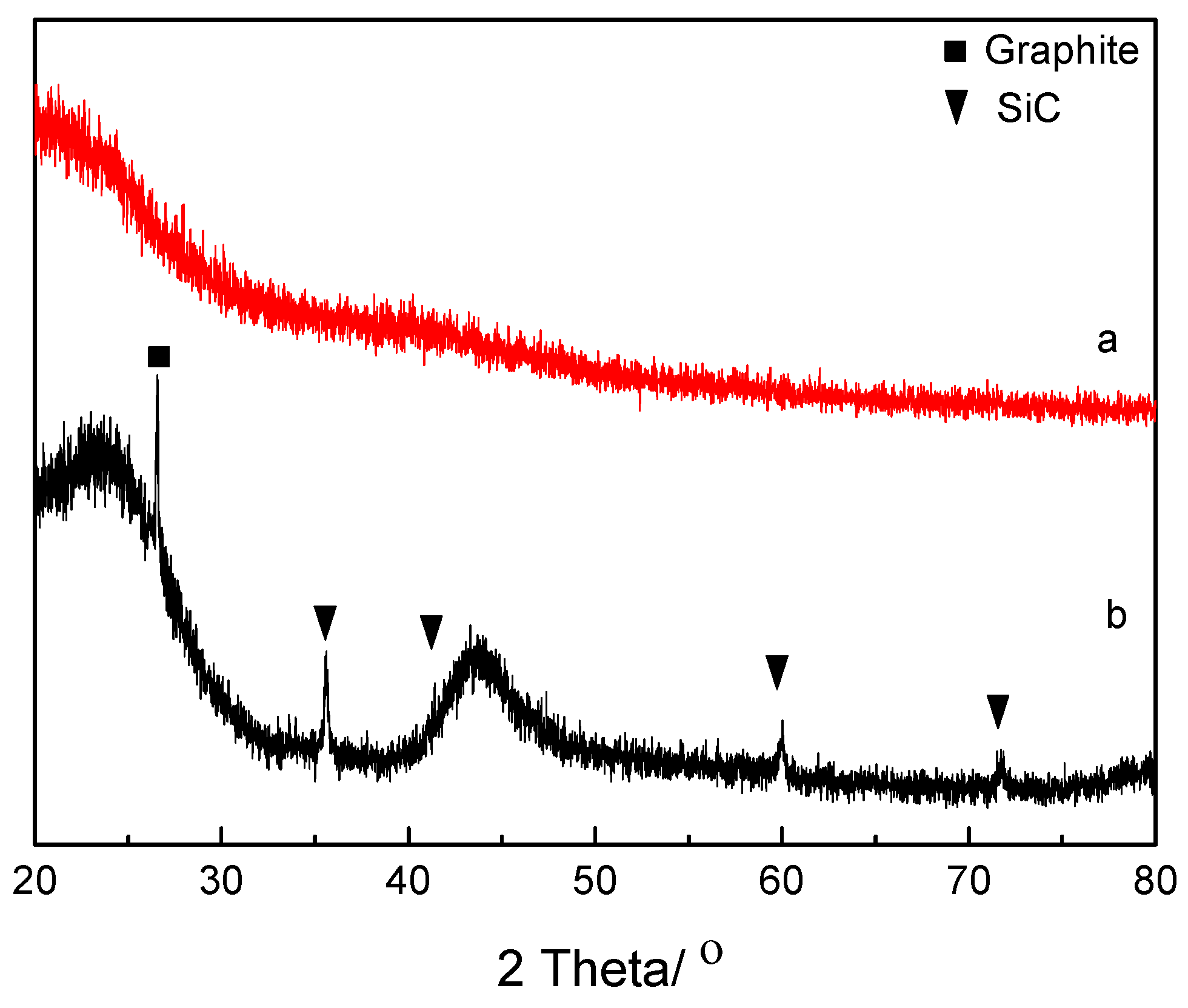
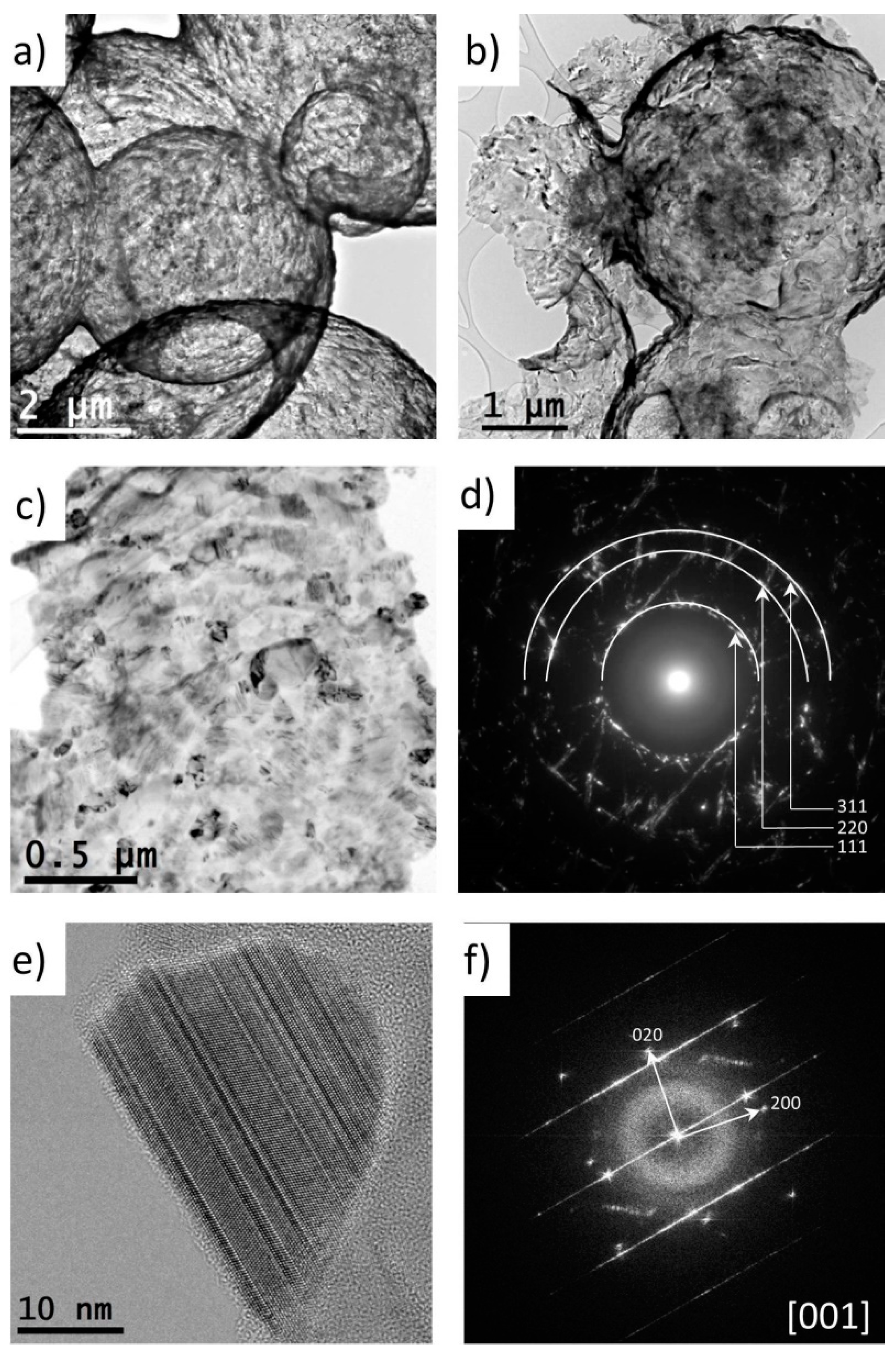
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Funke, A.; Ziegler, F. Hydrothermal carbonization of biomass: A summary and discussion of chemical mechanisms for process engineering. Biofuels Bioprod. Biorefining 2010, 4, 160–177. [Google Scholar] [CrossRef]
- Hoekman, S.K.; Broch, A.; Robbins, C. Hydrothermal Carbonization (HTC) of Lignocellulosic Biomass. Energy Fuels 2011, 25, 1802–1810. [Google Scholar] [CrossRef]
- Yao, C.; Shin, Y.; Wang, L.-Q.; Windisch, C.F., Jr.; Samuels, W.D.; Arey, B.W.; Wang, C.; Risen, W.M., Jr.; Exarhos, G.J. Hydrothermal dehydration of aqueous fructose solutions in a closed system. J. Phys. Chem. C 2007, 111, 15141–15145. [Google Scholar] [CrossRef]
- He, C.; Giannis, A.; Wang, J.-Y. Conversion of sewage sludge to clean solid fuel using hydrothermal carbonization: Hydrochar fuel characteristics and combustion behavior. Appl. Energy 2013, 111, 257–266. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B.; Solis, M.S. Chemical and Structural Properties of Carbonaceous Products Obtained by Hydrothermal Carbonization of Saccharides. Chem. A Eur. J. 2009, 15, 4195–4203. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.-M.; Thomas, A.; Antonietti, M. Back in the black: Hydrothermal carbonization of plant material as an efficient chemical process to treat the CO2 problem? New J. Chem. 2007, 31, 787. [Google Scholar] [CrossRef]
- Roman, S.; Nabais, J.V.; Ledesma, B.; González, J.F.G.; Laginhas, C.; Titirici, M.; Titirici, M. Production of low-cost adsorbents with tunable surface chemistry by conjunction of hydrothermal carbonization and activation processes. Microporous Mesoporous Mater. 2013, 165, 127–133. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B.; Mokaya, R. High density hydrogen storage in superactivated carbons from hydrothermally carbonized renewable organic materials. Energy Environ. Sci. 2011, 4, 1400. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated carbons prepared from hydrothermally carbonized waste biomass used as adsorbents for CO2. Appl. Energy 2013, 112, 526–532. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Lilliestråle, M.; Hedin, N. Activated Carbons for Water Treatment Prepared by Phosphoric Acid Activation of Hydrothermally Treated Beer Waste. Ind. Eng. Chem. Res. 2014, 53, 15389–15397. [Google Scholar] [CrossRef]
- Zhao, L.; Fan, L.-Z.; Zhou, M.-Q.; Guan, H.; Qiao, S.; Antonietti, M.; Titirici, M.-M. Nitrogen-Containing Hydrothermal Carbons with Superior Performance in Supercapacitors. Adv. Mater. 2010, 22, 5202–5206. [Google Scholar] [CrossRef] [PubMed]
- Manyà, J.J. Pyrolysis for biochar purposes: A review to establish current knowledge gaps and research needs. Environ. Sci. Tech. 2012, 46, 7939–7954. [Google Scholar] [CrossRef] [PubMed]
- Titirici, M.-M.; Antonietti, M.; Thomas, A. A Generalized Synthesis of Metal Oxide Hollow Spheres Using a Hydrothermal Approach. Chem. Mater. 2006, 18, 3808–3812. [Google Scholar] [CrossRef]
- Lai, X.; Halpert, J.E.; Wang, D. Recent advances in micro-/nano-structured hollow spheres for energy applications: From simple to complex systems. Energy Environ. Sci. 2012, 5, 5604–5618. [Google Scholar] [CrossRef]
- Wu, R.; Zhou, K.; Yue, C.Y.; Wei, J.; Pan, Y. Recent progress in synthesis, properties and potential applications of SiC nanomaterials. Prog. Mater. Sci. 2015, 72, 1–60. [Google Scholar] [CrossRef]
- Niimi, I.; Yasuhisa, K.; Noda, F.; Tsuzuki, Y. Process for Preparing Hollow Balls of Silicon Carbide and Product Formed Therby. U.S. Patent US3927181A, 16 December 1975. [Google Scholar]
- Shen, G.; Chen, D.; Tang, K.; Qian, Y.; Zhang, S. Silicon carbide hollow nanospheres, nanowires and coaxial nanowires. Chem. Phys. Lett. 2003, 375, 177–184. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, S.; Lee, W.E. Novel low temperature synthesis and characterisation of hollow silicon carbide spheres. Microporous Mesoporous Mater. 2012, 152, 25–30. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, E.-W.; Chen, Z.-Z.; Li, X.-B.; Xiao, B. Large-scale fabrication of silicon carbide hollow spheres. J. Mater. Chem. 2006, 16, 4141. [Google Scholar] [CrossRef]
- Li, P.; Xu, L.; Qian, Y. Selective Synthesis of 3C-SiC Hollow Nanospheres and Nanowires. Cryst. Growth Des. 2008, 8, 2431–2436. [Google Scholar] [CrossRef]
- Liu, Z.; Ci, L.; Jin-Phillipp, N.Y.; Rühle, M. Vapor−Solid Reaction for Silicon Carbide Hollow Spherical Nanocrystals. J. Phys. Chem. C 2007, 111, 12517–12521. [Google Scholar] [CrossRef]
- Ye, J.; Zhang, S.; Lee, W.E. Molten salt synthesis and characterization of SiC coated carbon black particles for refractory castable applications. J. Eur. Ceram. Soc. 2013, 33, 2023–2029. [Google Scholar] [CrossRef]
- Nersisyan, H.; Won, H.; Won, C.; Lee, J. Synthesis of hollow SiC microglobules by a combustion method. Microporous Mesoporous Mater. 2009, 117, 368–371. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L.; Zhang, X.; Zhang, Z.; Tong, Y.; Li, F.; Wu, J.C.-S.; Wang, X. Openmouthed β-SiC hollow-sphere with highly photocatalytic activity for reduction of CO2 with H2O. Appl. Catal. B Environ. 2017, 206, 158–167. [Google Scholar] [CrossRef]
- Cromarty, R.; Van Rooyen, G.; De Villiers, J.; Cromarty, R. Crush strength of silicon carbide coated TRISO particles: Influence of test method and process variables. J. Nucl. Mater. 2014, 445, 30–36. [Google Scholar] [CrossRef]
- Chen, F.; Yang, Y.; Shen, Q.; Zhang, L. Macro/micro structure dependence of mechanical strength of low temperature sintered silicon carbide ceramic foams. Ceram. Int. 2012, 38, 5223–5229. [Google Scholar] [CrossRef]
- Lim, K.-Y.; Kim, Y.-W.; Kim, K.J. Mechanical properties of electrically conductive silicon carbide ceramics. Ceram. Int. 2014, 40, 10577–10582. [Google Scholar] [CrossRef]
- Chen, W.; Miyamoto, Y. Fabrication of porous silicon carbide ceramics with high porosity and high strength. J. Eur. Ceram. Soc. 2014, 34, 837–840. [Google Scholar] [CrossRef]
- Winé, G.; Matta, J.; Tessonnier, J.-P.; Pham-Huu, C.; LeDoux, M.-J. Beta zeolite supported on silicon carbide for Friedel-Crafts fixed-bed reactions. Chem. Commun. 2003, 9, 530–531. [Google Scholar] [CrossRef]
- Gentile, M.; Xiao, P.; Abram, T. Palladium interaction with silicon carbide. J. Nucl. Mater. 2015, 462, 100–107. [Google Scholar] [CrossRef]
- Gentile, M.; Xiao, P.; Abram, T. XRD and TG-DSC Analysis of the Silicon Carbide-Palladium Reaction. In Proceedings of the 37th International Conference and Expo on Advanced Ceramics and Composites, Daytona Beach, FL, USA, 27 January–1 February 2013. [Google Scholar]
- Pan, Z.; Lai, H.-L.; Au, F.C.K.; Duan, X.; Zhou, W.; Shi, W.; Wang, N.; Lee, C.-S.; Wong, N.-B.; Lee, S.-T.; et al. Oriented Silicon Carbide Nanowires: Synthesis and Field Emission Properties. Adv. Mater. 2000, 12, 1186–1190. [Google Scholar] [CrossRef]
- Li, Z.J.; Gao, W.D.; Meng, A.; Geng, Z.D.; Gao, L. Large-Scale Synthesis and Raman and Photoluminescence Properties of Single Crystalline β-SiC Nanowires Periodically Wrapped by Amorphous SiO2 Nanospheres. J. Phys. Chem. C 2008, 113, 91–96. [Google Scholar] [CrossRef]
- Maglia, F.; Tredici, I.G.; Anselmi-Tamburini, U. Densification and properties of bulk nanocrystalline functional ceramics with grain size below 50nm. J. Eur. Ceram. Soc. 2013, 33, 1045–1066. [Google Scholar] [CrossRef]
- Peng, H.; Salamon, D.; Bill, J.; Rixecker, G.; Burghard, Z.; Aldinger, F.; Shen, Z. Consolidating and Deforming SiC Nanoceramics Via Dynamic Grain Sliding. Adv. Eng. Mater. 2007, 9, 303–306. [Google Scholar] [CrossRef]
- Li, M.; Li, W.; Liu, S. Hydrothermal synthesis, characterization, and KOH activation of carbon spheres from glucose. Carbohydr. Res. 2011, 346, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Keshavarzi, N.; Branger, A.; Bergström, L.; Hedin, N. Strong discs of activated carbons from hydrothermally carbonized beer waste. Carbon 2014, 78, 521–531. [Google Scholar] [CrossRef]
- Titirici, M.-M.; Antonietti, M.; Baccile, N. Hydrothermal carbon from biomass: A comparison of the local structure from poly- to monosaccharides and pentoses/hexoses. Green Chem. 2008, 10, 1204. [Google Scholar] [CrossRef]
- Baccile, N.; Laurent, G.; Babonneau, F.; Fayon, F.; Titirici, M.-M.; Antonietti, M. Structural Characterization of Hydrothermal Carbon Spheres by Advanced Solid-State MAS 13C NMR Investigations. J. Phys. Chem. C 2009, 113, 9644–9654. [Google Scholar] [CrossRef]
- Hao, W.; Björkman, E.; Yun, Y.; Lilliestråle, M.; Hedin, N. Iron Oxide Nanoparticles Embedded in Activated Carbons Prepared from Hydrothermally Treated Waste Biomass. ChemSusChem 2014, 7, 875–882. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Hoekman, S.K.; Srinivasan, M.; Balasubramanian, R.; Mp, S. Thermogravimetric investigation of hydrochar-lignite co-combustion. Bioresour. Technol. 2012, 123, 646–652. [Google Scholar] [CrossRef]
- Liu, Z.; Quek, A.; Kent Hoekman, S.; Srinivasan, M.P.; Balasubramanian, R. Erratum to “Thermogravimetric investigation of hydrochar-lignite co-combustion” [Bioresour Technol 123 (2012) 646-652]. Bioresour. Technol. 2013, 133, 639–640. [Google Scholar] [CrossRef]
- Vogli, E.; Sieber, H.; Greil, P. Biomorphic SiC-ceramic prepared by Si-vapor phase infiltration of wood. J. Eur. Ceram. Soc. 2002, 22, 2663–2668. [Google Scholar] [CrossRef]
- Carrott, P.; Nabais, J.; Carrott, M.R.; Pajares, J.; Nabais, J.M.V. Preparation of activated carbon fibres from acrylic textile fibres. Carbon 2001, 39, 1543–1555. [Google Scholar] [CrossRef]
- Misnon, I.I.; Zain, N.K.M.; Aziz, R.A.; Vidyadharan, B.; Jose, R. Electrochemical properties of carbon from oil palm kernel shell for high performance supercapacitors. Electrochim. Acta 2015, 174, 78–86. [Google Scholar] [CrossRef]
- Tempesta, G.; Capitani, G.C.; Di Pierro, S. The 6H-SiC structure model: Further refinement from SCXRD data from a terrestrial moissanite. Am. Miner. 2007, 92, 403–407. [Google Scholar]
- Titirici, M.M.; Thomas, A.; Yu, S.-H.; Müller, J.-O.; Antonietti, M.; Titirici, M. A Direct Synthesis of Mesoporous Carbons with Bicontinuous Pore Morphology from Crude Plant Material by Hydrothermal Carbonization. Chem. Mater. 2007, 19, 4205–4212. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Nakashima, S.; Harima, H. Raman Investigation of SiC Polytypes. Phys. Status Solidi 1997, 162, 39–64. [Google Scholar] [CrossRef]
- Harima, H. Raman scattering characterization on SiC. Microelectron. Eng. 2006, 83, 126–129. [Google Scholar] [CrossRef]
- Jamari, S.S.; Howse, J.R. The effect of the hydrothermal carbonization process on palm oil empty fruit bunch. Biomass Bioenergy 2012, 47, 82–90. [Google Scholar] [CrossRef]
- Falco, C.; Baccile, N.; Titirici, M.-M. Morphological and structural differences between glucose, cellulose and lignocellulosic biomass derived hydrothermal carbons. Green Chem. 2011, 13, 3273. [Google Scholar] [CrossRef]
- Yu, L.; Falco, C.; Howe, J.Y.; Titirici, M.-M.; Weber, J.; White, R.J. Carbohydrate-Derived Hydrothermal Carbons: A Thorough Characterization Study. Langmuir 2012, 28, 12373–12383. [Google Scholar] [CrossRef] [PubMed]
- Mutschke, H.; Andersen, A.C.; Clement, D.; Henning, T.; Peiter, G. Infrared properties of SiC particles. Astron. Astrophys. 1999, 345, 187–202. [Google Scholar]
| Sample | SBET (m2/g) | Ultimate (wt %) | |||||
|---|---|---|---|---|---|---|---|
| C | H | N | O a | Si | |||
| HTC | 8 | 66.2 | 4.3 | <0.10 | 29.5 | - | - |
| Core-shell-C-SiC | 10 | 98.2 | <0.10 | <0.10 | 1.47 | 0.13 b | 1.40 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, W.; Liu, Y.; Neagu, A.; Bacsik, Z.; Tai, C.-W.; Shen, Z.; Hedin, N. Core-Shell and Hollow Particles of Carbon and SiC Prepared from Hydrochar. Materials 2019, 12, 1835. https://doi.org/10.3390/ma12111835
Hao W, Liu Y, Neagu A, Bacsik Z, Tai C-W, Shen Z, Hedin N. Core-Shell and Hollow Particles of Carbon and SiC Prepared from Hydrochar. Materials. 2019; 12(11):1835. https://doi.org/10.3390/ma12111835
Chicago/Turabian StyleHao, Wenming, Yongsheng Liu, Alexandra Neagu, Zoltan Bacsik, Cheuk-Wai Tai, Zhijian Shen, and Niklas Hedin. 2019. "Core-Shell and Hollow Particles of Carbon and SiC Prepared from Hydrochar" Materials 12, no. 11: 1835. https://doi.org/10.3390/ma12111835
APA StyleHao, W., Liu, Y., Neagu, A., Bacsik, Z., Tai, C.-W., Shen, Z., & Hedin, N. (2019). Core-Shell and Hollow Particles of Carbon and SiC Prepared from Hydrochar. Materials, 12(11), 1835. https://doi.org/10.3390/ma12111835










