Polyethylene Terephthalate Textiles Enhance the Structural Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. l Polyethylene Terephthalate Textile
2.2. Textile Coating
2.3. Cell Culture and Differentiation of hiPSC-CMs
2.4. hiPS-CM Dissociation and Magnetic-Activated Cell Sorting
2.5. Calcium Imaging
2.6. Immunocytochemistry
2.7. Analysis of Cell Alignment and Sarcomere Orientation
2.8. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.9. Statistical Analysis
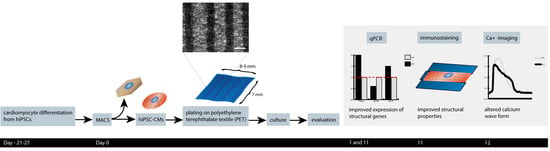
3. Results
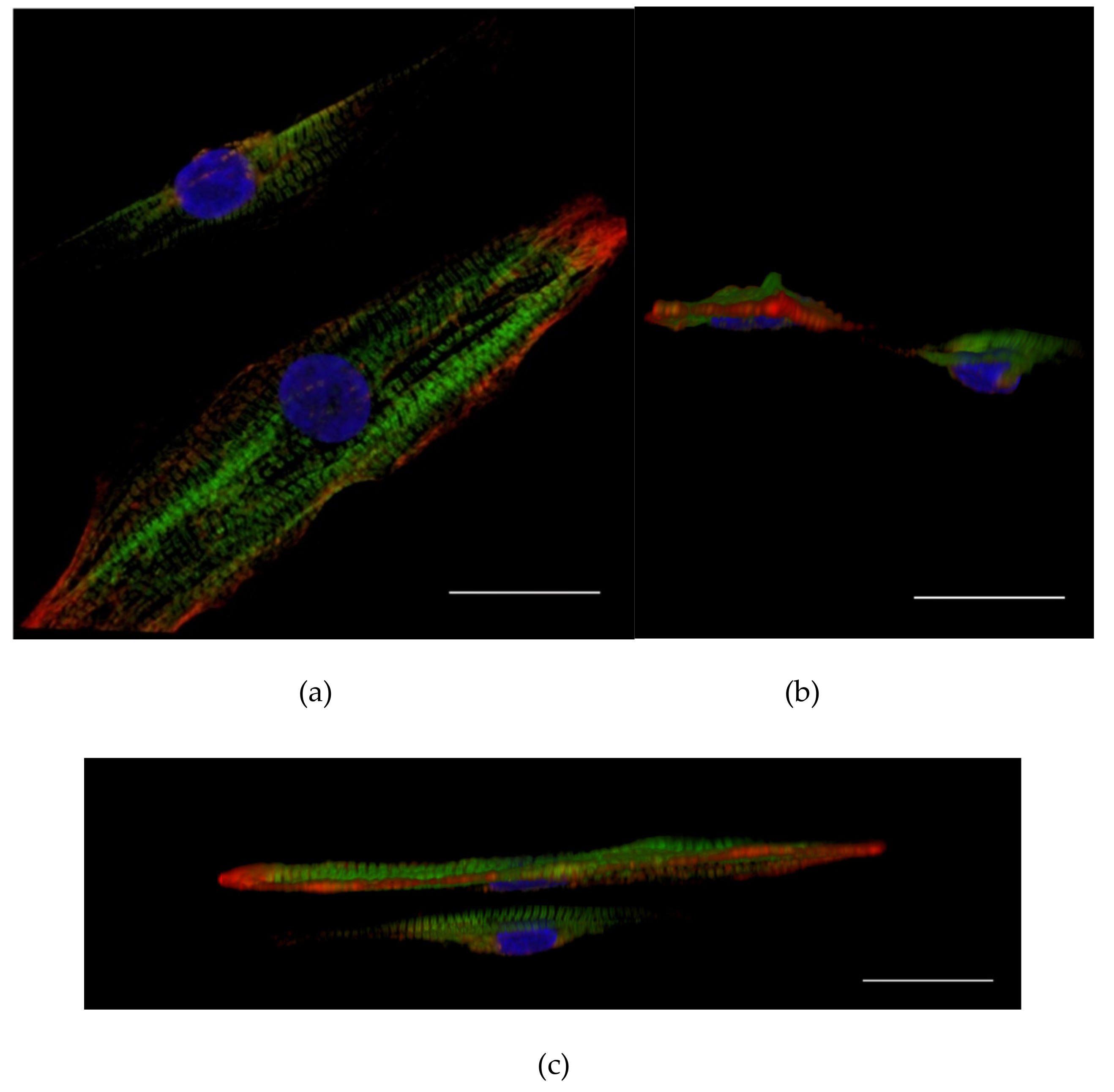
3.1. Attachment of the hiPSC-CMs to the PET Textiles
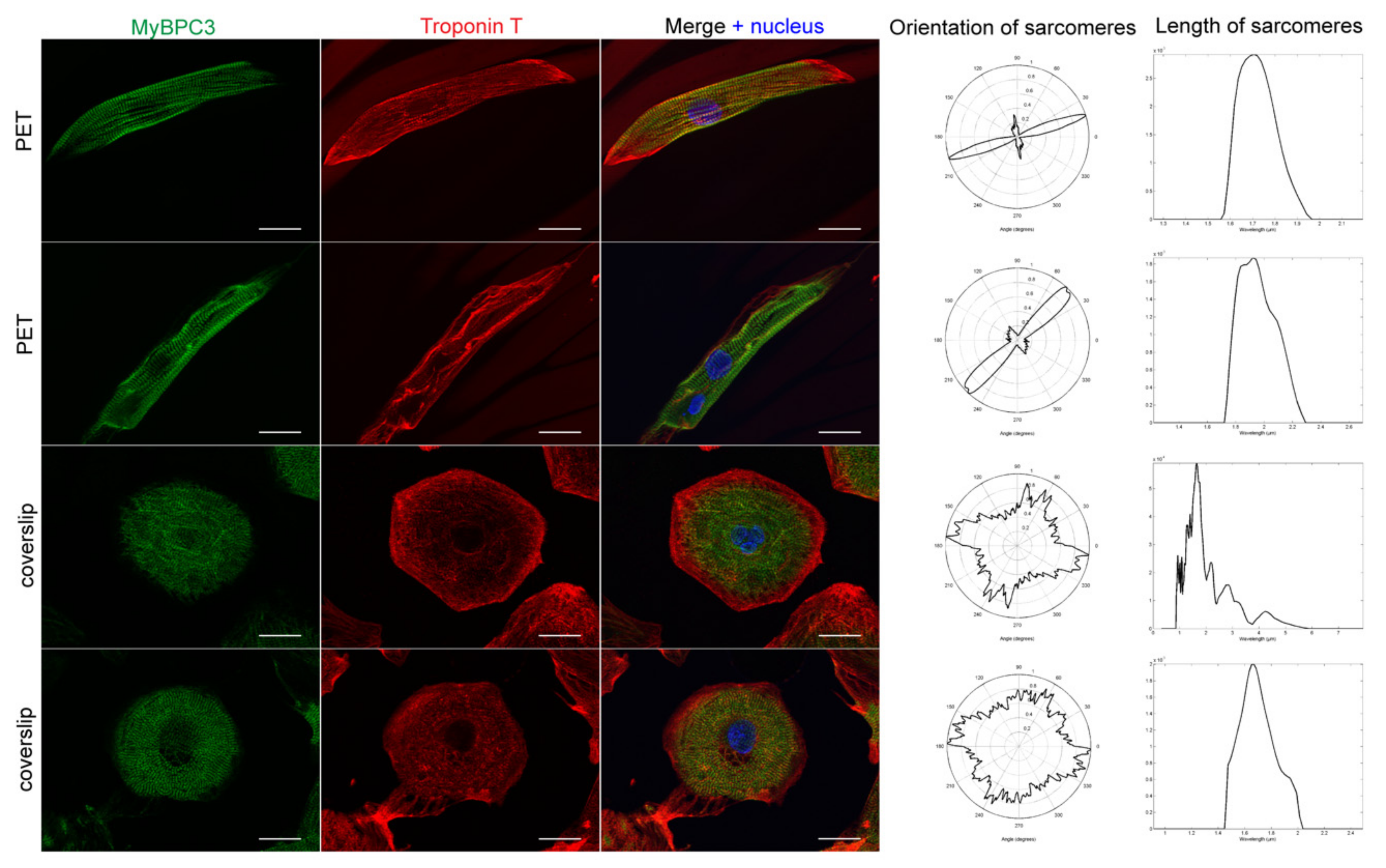
3.2. hiPSC-CM Morphology, Sarcomere Orientation, and Sarcomere Length
3.3. Calcium Handling
3.4. Expression of Cardiac-Specific Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1151–1210. [Google Scholar] [CrossRef]
- Onakpoya, I.J.; Heneghan, C.J.; Aronson, J.K. Post-marketing withdrawal of 462 medicinal products because of adverse drug reactions: A systematic review of the world literature. BMC Med. 2016, 14, 10. [Google Scholar] [CrossRef] [PubMed]
- Kannankeril, P.J.; Roden, D.M. Drug-induced long QT and torsade de pointes: Recent advances. Curr. Opin. Cardiol. 2007, 22, 39–43. [Google Scholar] [CrossRef]
- Carlsson, L. In vitro and in vivo models for testing arrhythmogenesis in drugs. J. Intern. Med. 2006, 259, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, M.B.; Matz, J.; Volders, P.G.A.; Vos, M.A. Assessing the proarrhythmic potential of drugs: Current status of models and surrogate parameters of torsades de pointes arrhythmias. Pharmacol. Ther. 2006, 112, 150–170. [Google Scholar] [CrossRef] [PubMed]
- Sala, L.; Bellin, M.; Mummery, C.L. Integrating cardiomyocytes from human pluripotent stem cells in safety pharmacology: Has the time come? Br. J. Pharmacol. 2017, 174, 3749–3765. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef]
- Di Baldassarre, A.; Cimetta, E.; Bollini, S.; Gaggi, G.; Ghinassi, B.; Di Baldassarre, A.; Cimetta, E.; Bollini, S.; Gaggi, G.; Ghinassi, B. Human-Induced Pluripotent Stem Cell Technology and Cardiomyocyte Generation: Progress and Clinical Applications. Cells 2018, 7, 48. [Google Scholar] [CrossRef]
- Smith, A.S.T.; Macadangdang, J.; Leung, W.; Laflamme, M.A.; Kim, D.H. Human iPSC-derived cardiomyocytes and tissue engineering strategies for disease modeling and drug screening. Biotechnol. Adv. 2017, 35, 77–94. [Google Scholar] [CrossRef]
- Denning, C.; Borgdorff, V.; Crutchley, J.; Firth, K.S.A.; George, V.; Kalra, S.; Kondrashov, A.; Hoang, M.D.; Mosqueira, D.; Patel, A.; et al. Cardiomyocytes from human pluripotent stem cells: From laboratory curiosity to industrial biomedical platform. Biochim. Biophys. Acta 2016, 1863, 1728–1748. [Google Scholar] [CrossRef]
- Lundy, S.D.; Zhu, W.-Z.; Regnier, M.; Laflamme, M. a Structural and functional maturation of cardiomyocytes derived from human pluripotent stem cells. Stem Cells Dev. 2013, 22, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Robertson, C.; Tran, D.D.; George, S.C. Concise Review: Maturation Phases of Human Pluripotent Stem Cell-Derived Cardiomyocytes. Stem Cells 2013, 31, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Dias, T.P.; Pinto, S.N.; Santos, J.I.; Fernandes, T.G.; Fernandes, F.; Diogo, M.M.; Prieto, M.; Cabral, J.M.S. Biophysical study of human induced Pluripotent Stem Cell-Derived cardiomyocyte structural maturation during long-term culture. Biochem. Biophys. Res. Commun. 2018, 499, 611–617. [Google Scholar] [CrossRef]
- Kuo, P.L.; Lee, H.; Bray, M.A.; Geisse, N.A.; Huang, Y.-T.; Adams, W.J.; Sheehy, S.P.; Parker, K.K. Myocyte shape regulates lateral registry of sarcomeres and contractility. Am. J. Pathol. 2012, 181, 2030–2037. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced maturation of human cardiac tissue grown from pluripotent stem cells. Nature 2018, 556, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Wu, Q.; Xia, Y.; Wagner, M.B.; Xu, C. Cell alignment induced by anisotropic electrospun fibrous scaffolds alone has limited effect on cardiomyocyte maturation. Stem Cell Res. 2016, 16, 740–750. [Google Scholar] [CrossRef] [PubMed]
- Parrag, I.C.; Zandstra, P.W.; Woodhouse, K.A. Fiber alignment and coculture with fibroblasts improves the differentiated phenotype of murine embryonic stem cell-derived cardiomyocytes for cardiac tissue engineering. Biotechnol. Bioeng. 2012, 109, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Rao, C.; Prodromakis, T.; Kolker, L.; Chaudhry, U.A.R.; Trantidou, T.; Sridhar, A.; Weekes, C.; Camelliti, P.; Harding, S.E.; Darzi, A.; et al. The effect of microgrooved culture substrates on calcium cycling of cardiac myocytes derived from human induced pluripotent stem cells. Biomaterials 2013, 34, 2399–2411. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Cuddihy, M.J.; Kotov, N.A. Three-Dimensional Cell Culture Matrices: State of the Art. Tissue Eng. Part B Rev. 2008, 14, 61–86. [Google Scholar] [CrossRef]
- Wickström, S.A.; Niessen, C.M. Cell adhesion and mechanics as drivers of tissue organization and differentiation: Local cues for large scale organization. Curr. Opin. Cell Biol. 2018, 54, 89–97. [Google Scholar] [CrossRef]
- Rodriguez, M.L.; Beussman, K.M.; Chun, K.S.; Walzer, M.S.; Yang, X.; Murry, C.E.; Sniadecki, N.J. Substrate Stiffness, Cell Anisotropy, and Cell–Cell Contact Contribute to Enhanced Structural and Calcium Handling Properties of Human Embryonic Stem Cell-Derived Cardiomyocytes. ACS Biomater. Sci. Eng. 2019. [Google Scholar] [CrossRef]
- Tulloch, N.L.; Muskheli, V.; Razumova, M.V.; Korte, F.S.; Regnier, M.; Hauch, K.D.; Pabon, L.; Reinecke, H.; Murry, C.E. Growth of engineered human myocardium with mechanical loading and vascular coculture. Circ. Res. 2011, 109, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Thavandiran, N.; Dubois, N.; Mikryukov, A.; Massé, S.; Beca, B.; Simmons, C.A.; Deshpande, V.S.; McGarry, J.P.; Chen, C.S.; Nanthakumar, K.; et al. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc. Natl. Acad. Sci. USA 2013, 110, E4698–E4707. [Google Scholar] [CrossRef]
- Conant, G.; Lai, B.F.L.; Lu, R.X.Z.; Korolj, A.; Wang, E.Y.; Radisic, M. High-Content Assessment of Cardiac Function Using Heart-on-a-Chip Devices as Drug Screening Model. Stem Cell Rev. Rep. 2017, 13, 335–346. [Google Scholar] [CrossRef]
- Vuorenpää, H.; Penttinen, K.; Heinonen, T.; Pekkanen-Mattila, M.; Sarkanen, J.-R.; Ylikomi, T.; Aalto-Setälä, K. Maturation of human pluripotent stem cell derived cardiomyocytes is improved in cardiovascular construct. Cytotechnology 2017, 69, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Vuorenpää, H.; Ikonen, L.; Kujala, K.; Huttala, O.; Sarkanen, J.-R.; Ylikomi, T.; Aalto-Setälä, K.; Heinonen, T. Novel in vitro cardiovascular constructs composed of vascular-like networks and cardiomyocytes. Vitr. Cell. Dev. Biol. Anim. 2014, 50, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Giol, E.D.; Schaubroeck, D.; Kersemans, K.; De Vos, F.; Van Vlierberghe, S.; Dubruel, P. Bio-inspired surface modification of PET for cardiovascular applications: Case study of gelatin. Colloids Surf. B Biointerfaces 2015, 134, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, V.P.; Silva-Correia, J.; Nascimento, A.I.; da Silva Morais, A.; Marques, A.P.; Ribeiro, A.S.; Silva, C.J.; Bonifácio, G.; Sousa, R.A.; Oliveira, J.M.; et al. Silk-based anisotropical 3D biotextiles for bone regeneration. Biomaterials 2017, 123, 92–106. [Google Scholar] [CrossRef]
- Ribeiro, V.P.; Almeida, L.R.; Martins, A.R.; Pashkuleva, I.; Marques, A.P.; Ribeiro, A.S.; Silva, C.J.; Bonifácio, G.; Sousa, R.A.; Oliveira, A.L.; et al. Modulating cell adhesion to polybutylene succinate biotextile constructs for tissue engineering applications. J. Tissue Eng. Regen. Med. 2017, 11, 2853–2863. [Google Scholar] [CrossRef]
- Lahti, A.L.; Kujala, V.J.; Chapman, H.; Koivisto, A.-P.; Pekkanen-Mattila, M.; Kerkelä, E.; Hyttinen, J.; Kontula, K.; Swan, H.; Conklin, B.R.; et al. Model for long QT syndrome type 2 using human iPS cells demonstrates arrhythmogenic characteristics in cell culture. Dis. Model. Mech. 2012, 5, 220–230. [Google Scholar] [CrossRef]
- Lian, X.; Hsiao, C.; Wilson, G.; Zhu, K.; Hazeltine, L.B.; Azarin, S.M.; Raval, K.K.; Zhang, J.; Kamp, T.J.; Palecek, S.P. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc. Natl. Acad. Sci. USA 2012, 109, E1848–E1857. [Google Scholar] [CrossRef] [PubMed]
- Mummery, C.; Ward-van Oostwaard, D.; Doevendans, P.; Spijker, R.; van den Brink, S.; Hassink, R.; van der Heyden, M.; Opthof, T.; Pera, M.; de la Riviere, A.B.; et al. Differentiation of human embryonic stem cells to cardiomyocytes: Role of coculture with visceral endoderm-like cells. Circulation 2003, 107, 2733–2740. [Google Scholar] [CrossRef]
- Pölönen, R.P.; Penttinen, K.; Swan, H.; Aalto-Setälä, K. Antiarrhythmic Effects of Carvedilol and Flecainide in Cardiomyocytes Derived from Catecholaminergic Polymorphic Ventricular Tachycardia Patients. Stem Cells Int. 2018, 2018, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Kartasalo, K.; Pölönen, R.-P.; Ojala, M.; Rasku, J.; Lekkala, J.; Aalto-Setälä, K.; Kallio, P. CytoSpectre: A tool for spectral analysis of oriented structures on cellular and subcellular levels. BMC Bioinform. 2015, 16, 344. [Google Scholar] [CrossRef] [PubMed]
- Ojala, M.; Prajapati, C.; Pölönen, R.-P.; Rajala, K.; Pekkanen-Mattila, M.; Rasku, J.; Larsson, K.; Aalto-Setälä, K. Mutation-Specific Phenotypes in hiPSC-Derived Cardiomyocytes Carrying Either Myosin-Binding Protein C Or α -Tropomyosin Mutation for Hypertrophic Cardiomyopathy. Stem Cells Int. 2016, 2016, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Carson, D.; Hnilova, M.; Yang, X.; Nemeth, C.L.; Tsui, J.H.; Smith, A.S.T.; Jiao, A.; Regnier, M.; Murry, C.E.; Tamerler, C.; et al. Nanotopography-Induced Structural Anisotropy and Sarcomere Development in Human Cardiomyocytes Derived from Induced Pluripotent Stem Cells. ACS Appl. Mater. Interfaces 2016, 8, 21923–21932. [Google Scholar] [CrossRef]
- Xu, C.; Wang, L.; Yu, Y.; Yin, F.; Zhang, X.; Jiang, L.; Qin, J. Bioinspired onion epithelium-like structure promotes the maturation of cardiomyocytes derived from human pluripotent stem cells. Biomater. Sci. 2017, 5, 1810–1819. [Google Scholar] [CrossRef]
- Huethorst, E.; Hortigon, M.; Zamora-Rodriguez, V.; Reynolds, P.M.; Burton, F.; Smith, G.; Gadegaard, N. Enhanced Human-Induced Pluripotent Stem Cell Derived Cardiomyocyte Maturation Using a Dual Microgradient Substrate. ACS Biomater. Sci. Eng. 2016, 2, 2231–2239. [Google Scholar] [CrossRef]
- Maitz, M.F. Applications of synthetic polymers in clinical medicine. Biosurf. Biotribol. 2015, 1, 161–176. [Google Scholar] [CrossRef]
- Wang, P.Y.; Yu, J.; Lin, J.H.; Tsai, W.B. Modulation of alignment, elongation and contraction of cardiomyocytes through a combination of nanotopography and rigidity of substrates. Acta Biomater. 2011, 7, 3285–3293. [Google Scholar] [CrossRef]
- Sarantitis, I.; Papanastasopoulos, P.; Manousi, M.; Baikoussis, N.G.; Apostolakis, E. The cytoskeleton of the cardiac muscle cell. Hell. J. Cardiol. 2012, 53, 367–379. [Google Scholar]
- Khan, M.; Xu, Y.; Hua, S.; Johnson, J.; Belevych, A.; Janssen, P.M.L.; Gyorke, S.; Guan, J.; Angelos, M.G. Evaluation of changes in morphology and function of human induced pluripotent stem cell derived cardiomyocytes (hiPSC-CMs) cultured on an aligned-nanofiber cardiac patch. PLoS ONE 2015, 10, e0126338. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.W.; Ripplinger, C.M.; Van Der Meer, P.; Sheehy, S.P.; Domian, I.; Chien, K.R.; Parker, K.K. Stem Cell Reports Repor t Functional Differences in Engineered Myocardium from Embryonic Stem Cell-Derived versus Neonatal Cardiomyocytes. Stem Cell Rep. 2013, 1, 387–396. [Google Scholar] [CrossRef] [PubMed]



| Textile Type and Details | Figure | Manufacturer | Warp/Weft | Single Filament ø (µm) |
|---|---|---|---|---|
| PET 1 Colorless, heat treated | 1A | Inka Oy, Killinkoski, Finland | Textured/textured | 24.4 ± 1.82 |
| PET 2 Colorless, heat treated | 1B | Inka Oy, Killinkoski, Finland | Straight/textured | 23.2 ± 1.43 |
| PET 3 Colorless, heat treated | 1C | Inka Oy, Killinkoski, Finland | Textured/textured | 22.9 ± 1.91 |
| PET 4 Blue, heat treated | 1D | Yarn: Finn-Nauha Oy, Haapamäki, Finland Textile: Tampere University of Technology | Straight/straight | 20.4 ± 1.53 |
| PET 5 Blue, heat treated | 1E | Yarn: Finn-Nauha Oy, Haapamäki, Finland Textile: Tampere University of Technology | Straight/straight | 22.0 ± 1.4 |
| Gene | Description | Function | TaqMan Assay ID |
|---|---|---|---|
| MYL2 | Myosin regulatory light chain 2 | Sarcomeric gene | Hs00166405_m1 |
| MYL7 | Myosin regulatory light chain 7 | Sarcomeric gene | Hs01085598_g1 |
| MYL9 | Myosin regulatory light chain 9 | Sarcomeric gene | Hs00697086_m1 |
| MYH6 | Myosin heavy chain 6 | Sarcomeric gene | Hs01101425_m1 |
| MYH7 | Myosin heavy chain 7 | Sarcomeric gene | Hs01110632_m1 |
| TNNC1 | Slow skeletal and cardiac type troponin C1 | Sarcomeric gene | Hs00896999_g1 |
| TNNT2 | Cardiac type troponin T2 | Sarcomeric gene | Hs00165960_m1 |
| ACTN2 | α-actinin 2 | Sarcomeric gene | Hs00153809_m1 |
| TTN | Titin | Sarcomeric gene | Hs00399225_m1 |
| MYBPC3 | Myosin binding protein C, cardiac | Sarcomeric gene | Hs00165232_m1 |
| TPM1 | α-tropomyosin | Sarcomeric gene | Hs00165966_m1 |
| KCNH2 | Potassium voltage-gated channel subfamily H member 2 | Potassium channel | Hs04234270_g1 |
| KCNH6 | Potassium voltage-gated channel subfamily H member 6 | Potassium channel | Hs00229215_m1 |
| KCNA10 | Potassium voltage-gated channel subfamily A member 10 | Potassium channel | Hs1563550_s1 |
| KCND3 | Potassium voltage-gated channel subfamily D member 3 | Potassium channel | Hs00542597_m1 |
| KCNQ1 | Potassium voltage-gated channel subfamily Q member 1 | Potassium channel | Hs00923522_m1 |
| HCN4 | Hyperpolarization activated cyclic nucleotide-gated potassium channel 4 | Potassium channel | Hs00975492_m1 |
| SCN5A | Voltage-gated sodium channel, V type, alpha subunit | Sodium channel | Hs00165693_m1 |
| CACNA1C | Voltage-dependent calcium channel, L type, alpha 1C subunit/CaCNA1.2 | Calcium channel | Hs00167681_m1 |
| SLC8A1 | Solute carrier family 8, member 1/NCX1 | Sodium-calcium exchanger | Hs01062258_m1 |
| PLN | Phospholamban | Protein kinase substrate | Hs01848144_s1 |
| ATP2A2 | ATPase, calcium transporting, cardiac muscle, slow twitch 2/ SERCA2a | Calcium ATPase | Hs00544877_m1 |
| EEF1A1; EE+ | Eukaryotic translation elongation factor 1 alpha 1 | Housekeeping gene | Hs00265885_g1 |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | Housekeeping gene | Hs02758991_g1 |
| TBP | TATA-box binding protein | Housekeeping gene | Hs00427620_m1 |
| Sample | Average Circular Variance (0–1) | Average Modal Sarcomere Length (μm) | Average Aspect Ratio (Length to Width) | Number of Cells Analyzed |
|---|---|---|---|---|
| PET 5 | 0.611 0.162 | 1.736 0.187 | 4.915 2.263 | 98 |
| Control | 0.882 0.069 | 1.749 0.122 | 1.567 0.455 | 174 |
| Sample | Peak Duration (ms) | Peak Amplitude (ΔF/F0) | Rise Time from 10% to 90% (ms) | Decay Time from 90% to 10% (ms) | Peak Frequency (Hz) | Cell Number |
|---|---|---|---|---|---|---|
| PET 5 | 582 ± 229 | 0.048 ± 0.037 | 112 ± 49 | 295 ± 131 | 0.93 ± 0.52 | 160 |
| Control | 590 ± 202 | 0.067 ± 0.030 | 90 ± 41 | 324 ± 96 | 0.75 ± 0.34 | 40 |
| Sample | Peak Duration (ms) | Peak Amplitude (ΔF/F0) | Rise Time from 10% to 90% (ms) | Decay Time from 90% to 10% (ms) | Peak Frequency (Hz) |
|---|---|---|---|---|---|
| Baseline | 648 ± 101 | 0.0360 ± 0.0183 | 115 ± 31 | 328 ± 73 | 0.709 ± 0.254 |
| Adrenaline | 614 ± 87 | 0.0310 ± 0.0139 | 122 ± 34 | 303 ± 63 | 0.859 ± 0.242 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pekkanen-Mattila, M.; Häkli, M.; Pölönen, R.-P.; Mansikkala, T.; Junnila, A.; Talvitie, E.; Koivisto, J.T.; Kellomäki, M.; Aalto-Setälä, K. Polyethylene Terephthalate Textiles Enhance the Structural Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Materials 2019, 12, 1805. https://doi.org/10.3390/ma12111805
Pekkanen-Mattila M, Häkli M, Pölönen R-P, Mansikkala T, Junnila A, Talvitie E, Koivisto JT, Kellomäki M, Aalto-Setälä K. Polyethylene Terephthalate Textiles Enhance the Structural Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Materials. 2019; 12(11):1805. https://doi.org/10.3390/ma12111805
Chicago/Turabian StylePekkanen-Mattila, Mari, Martta Häkli, Risto-Pekka Pölönen, Tuomas Mansikkala, Anni Junnila, Elina Talvitie, Janne T Koivisto, Minna Kellomäki, and Katriina Aalto-Setälä. 2019. "Polyethylene Terephthalate Textiles Enhance the Structural Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes" Materials 12, no. 11: 1805. https://doi.org/10.3390/ma12111805
APA StylePekkanen-Mattila, M., Häkli, M., Pölönen, R.-P., Mansikkala, T., Junnila, A., Talvitie, E., Koivisto, J. T., Kellomäki, M., & Aalto-Setälä, K. (2019). Polyethylene Terephthalate Textiles Enhance the Structural Maturation of Human Induced Pluripotent Stem Cell-Derived Cardiomyocytes. Materials, 12(11), 1805. https://doi.org/10.3390/ma12111805