Metal Ion-Chelated Tannic Acid Coating for Hemostatic Dressing
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
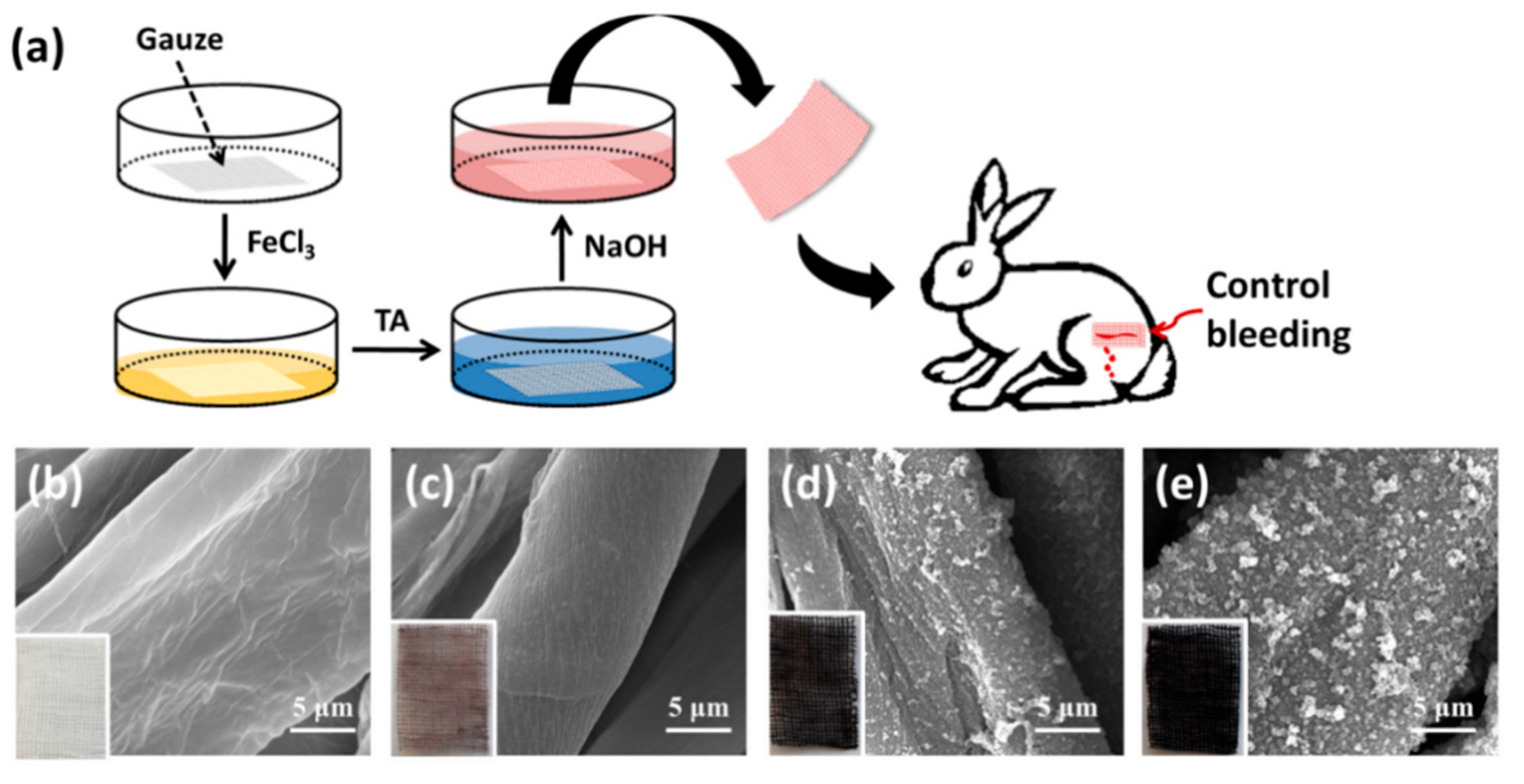
2.2. Preparation of TA Coating
2.3. Characterization of TA Coating
2.4. Adsorption and Characterization of Model Proteins on TA Coating
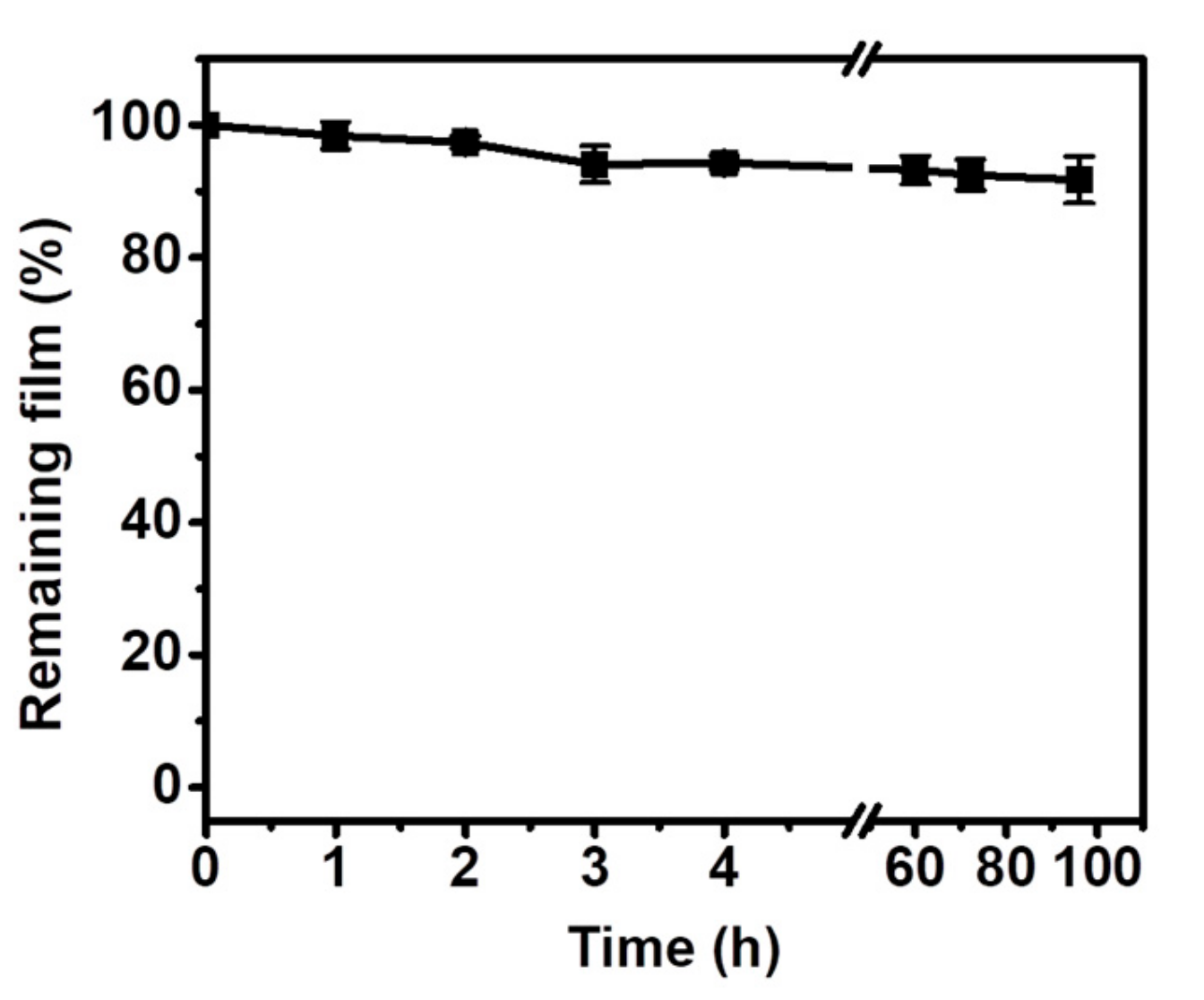
2.5. Stability of TA Coating in Physiological Environment
2.6. Characterization of Plasma Protein Adsorption on TA-Coated Gauze
2.7. Hemolysis Test of TA-Coated Gauze
2.8. Whole Blood Clotting Time of TA-Coated Gauze
2.9. Animal Wound Healing Model
2.10. Statistical Analysis
3. Results
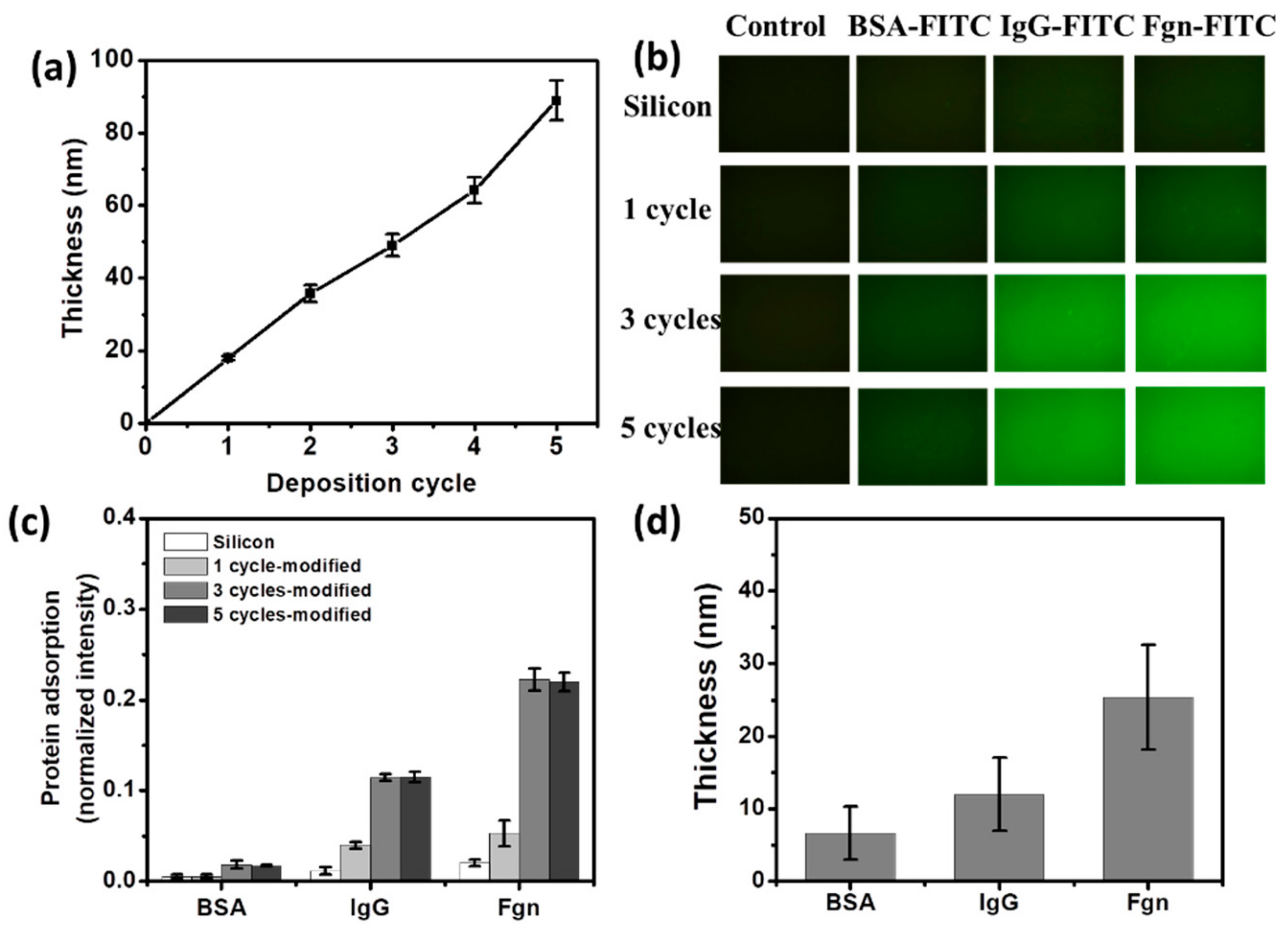
3.1. TA Coating and Model Protein Adsorption
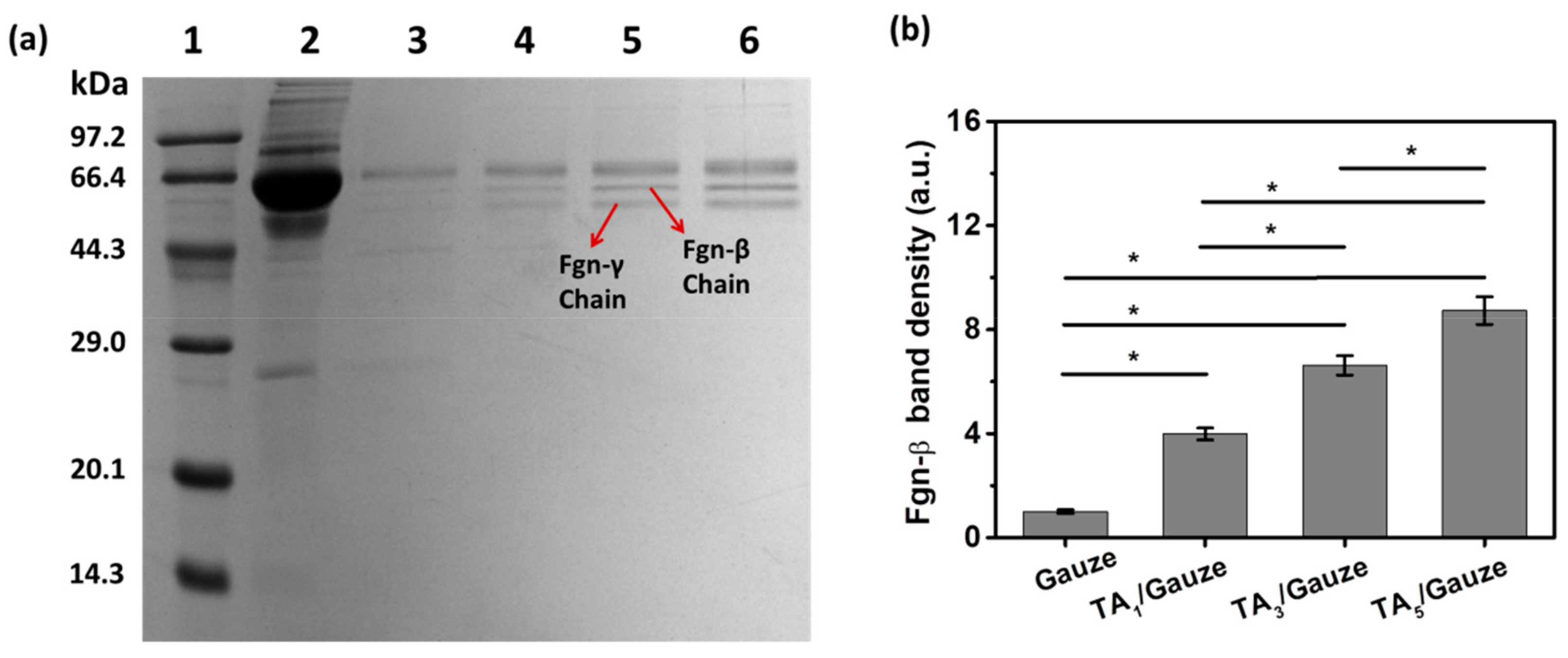
3.2. Blood Protein Adsorption onto TA-Coated Gauze
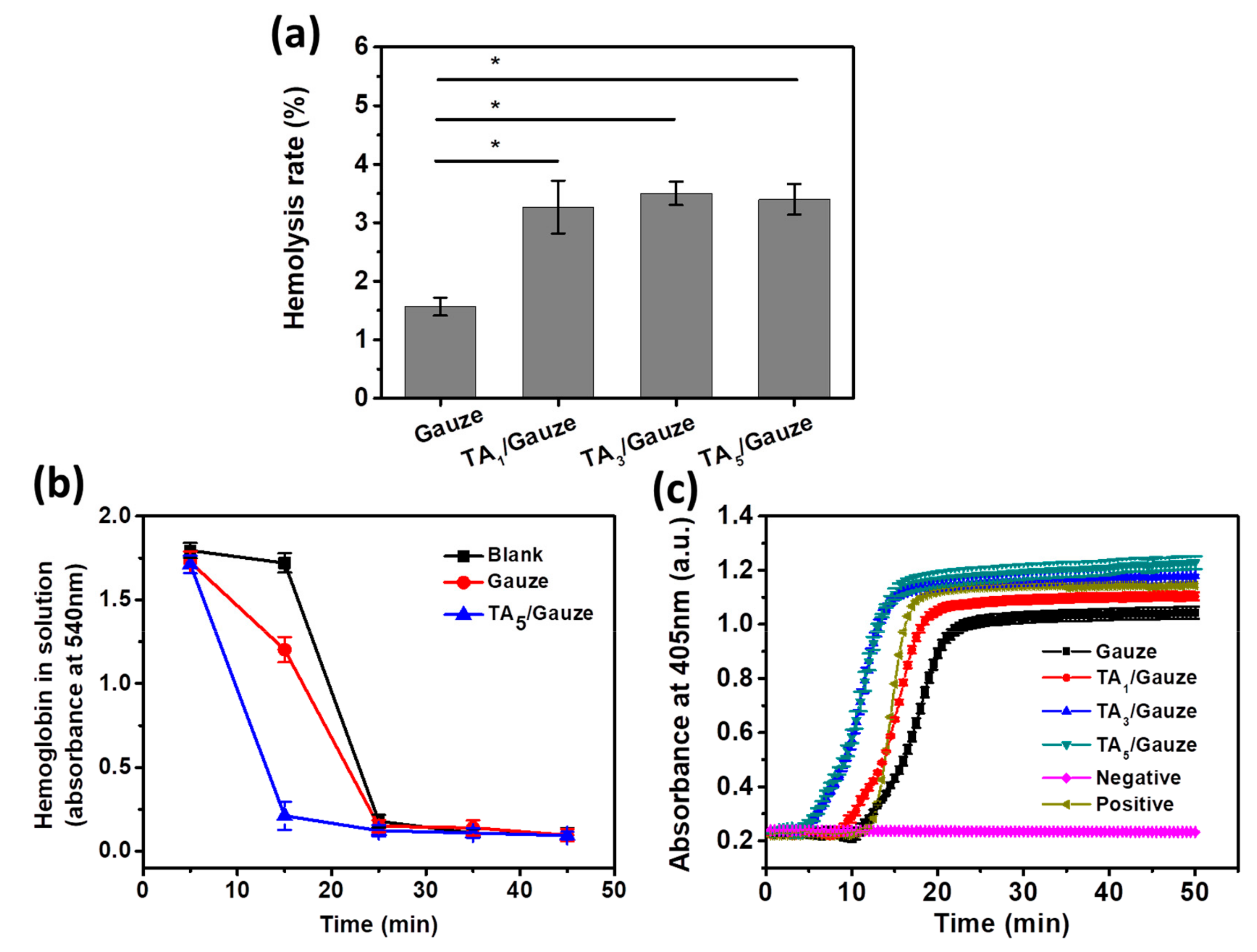
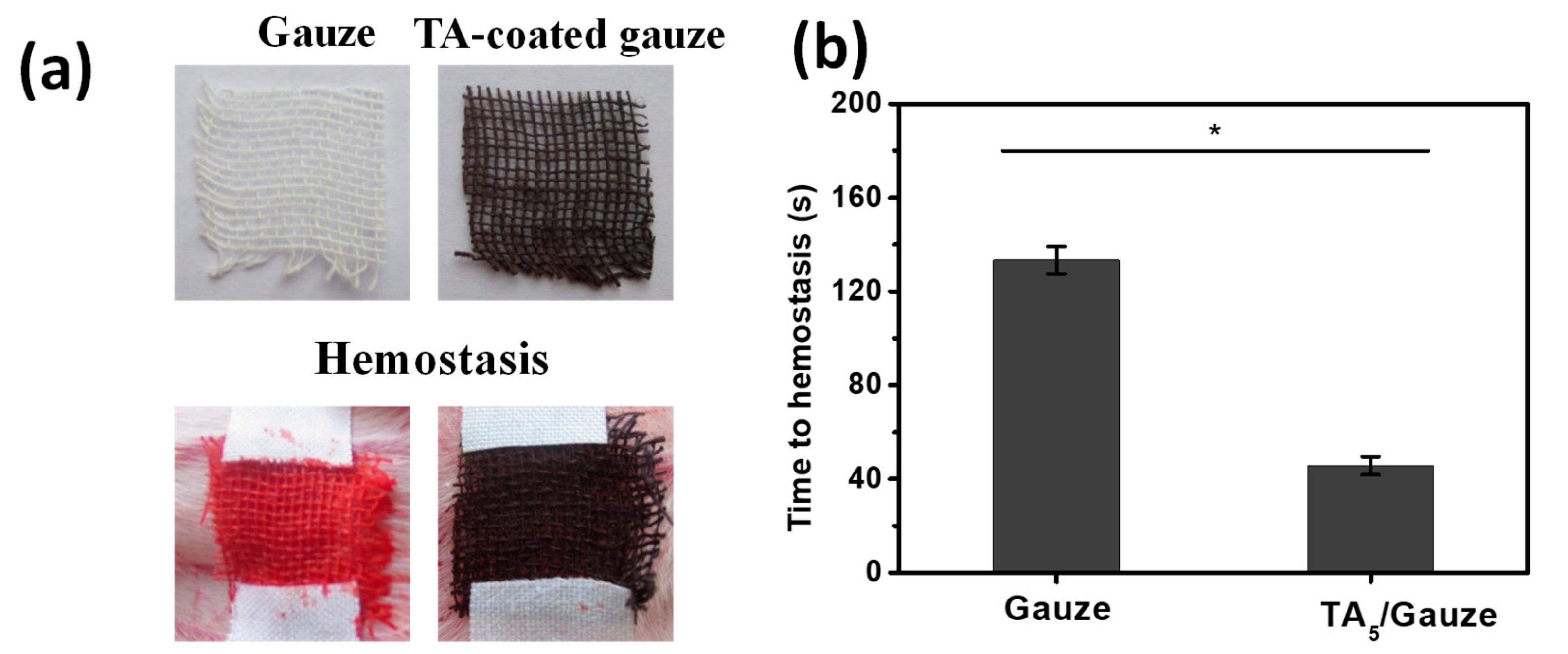
3.3. Hemostasis Performance of TA-Coated Gauze
3.4. Animal Wound Healing Model
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ejima, H.; Richardson, J.J.; Liang, K.; Best, J.P.; van Koeverden, M.P.; Such, G.K.; Cui, J.; Caruso, F. One-step assembly of coordination complexes for versatile film and particle engineering. Science 2013, 341, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, Q.; Yang, L.; Ye, T.; He, Z.; Jia, L. Facile oriented immobilization of histidine-tagged proteins on nonfouling cobalt polyphenolic self-assembly surfaces. ACS Biomater. Sci. Eng. 2017, 3, 3328–3337. [Google Scholar] [CrossRef]
- Baxter, N.J.; Lilley, T.H.; Haslam, E.; Williamson, M.P. Multiple interactions between polyphenols and a salivary proline-rich protein repeat result in complexation and precipitation. Biochem.us 1997, 36, 5566–5577. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Sousa, A.M.M.; Thomas-Gahring, A.; Fan, X.; Jin, T.; Li, X.; Tomasula, P.M.; Liu, L. Electrospun polymer nanofibers reinforced by tannic acid/Fe+++ complexes. Materials 2016, 9, 757. [Google Scholar] [CrossRef] [PubMed]
- Cass, C.A.; Burg, K.J. Tannic Acid Cross-linked Collagen Scaffolds and Their Anti-cancer Potential in a Tissue Engineered Breast Implant. J. Biomater. Sci. Polym. Ed. 2012, 23, 281–298. [Google Scholar] [CrossRef] [PubMed]
- Bridgeman, C.J.; Nguyen, T.U.; Kishore, V. Anticancer efficacy of tannic acid is dependent on the stiffness of the underlying matrix. J. Biomater. Sci. Polym. Ed. 2018, 29, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Canon, F.; Pate, F.; Cheynier, V.; Sarni-Manchado, P.; Giuliani, A.; Perez, J.; Durand, D.; Li, J.; Cabane, B. Aggregation of the salivary proline-rich protein IB5 in the presence of the tannin EgCG. Langmuir 2013, 29, 1926–1937. [Google Scholar] [CrossRef]
- Natarajan, V.; Krithica, N.; Madhan, B.; Sehgal, P.K. Preparation and properties of tannic acid cross-linked collagen scaffold and its application in wound healing. J. Biomed. Mater. Res. Part B 2013, 101B, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Franz, S.; Rammelt, S.; Scharnweber, D.; Simon, J.C. Immune responses to implants–A review of the implications for the design of immunomodulatory biomaterials. Biomaterials 2011, 32, 6692–6709. [Google Scholar] [CrossRef] [PubMed]
- Gorbet, M.B.; Sefton, M.V. Biomaterial-associated thrombosis: Roles of coagulation factors, complement, platelets and leukocytes. Biomaterials 2004, 25, 5681–5703. [Google Scholar] [CrossRef]
- Vogler, E.A.; Siedlecki, C.A. Contact activation of blood-plasma coagulation. Biomaterials 2009, 30, 1857–1869. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, D.; Yang, J.; Lui, K.Y.; Webb, A.R.; Ameer, G.A. Hernocompatibility evaluation of poly(glycerol-sebacate) in vitro for vascular tissue engineering. Biomaterials 2006, 27, 4315–4324. [Google Scholar] [CrossRef] [PubMed]
- Benesch, J.; Tengvall, P. Blood protein adsorption onto chitosan. Biomaterials 2002, 23, 2561–2568. [Google Scholar] [CrossRef]
- Yang, L.; Sun, H.; Jiang, W.; Xu, T.; Song, B.; Peng, R.; Han, L.; Jia, L. A chemical method for specific capture of circulating tumor cells using label-free polyphenol-functionalized films. Chem. Mater. 2018, 30, 4372–4382. [Google Scholar] [CrossRef]
- Yang, L.; Han, L.; Liu, Q.; Xu, X.; Jia, L. Galloyl groups-regulated fibrinogen conformation: Understanding antiplatelet adhesion on tannic acid coating. Acta Biomaterialia 2017, 64, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, L.; Jia, L. A novel platelet-repellent polyphenolic surface and its micropattern for platelet adhesion detection. ACS Appl. Mater. Interfaces 2016, 8, 26570–26577. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Han, L.; Ren, J.; Wei, H.; Jia, L. Coating process and stability of metal-polyphenol film. Colloids Surf. A Physicochem. Eng. Aspects 2015, 484, 197–205. [Google Scholar] [CrossRef]
- Imai, Y.; Nose, Y. New method for evaluation of antithrombogenicity of materials. J. Biomed. Mater. Res. 1972, 6, 165. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Mao, Z.; Han, L.; Zhao, Y.; Xi, J.; Gao, C. A density gradient of basic fibroblast growth factor guides directional migration of vascular smooth muscle cells. Colloids Surf. B Biointerfaces 2014, 117, 290–295. [Google Scholar] [CrossRef]
- Li, T.T.; Lou, C.W.; Chen, A.P.; Lee, M.C.; Ho, T.F.; Chen, Y.S.; Lin, J.H. Highly absorbent antibacterial hemostatic dressing for healing severe hemorrhagic wounds. Materials 2016, 9, 793. [Google Scholar] [CrossRef]
- Tamer, T.M.; Collins, M.N.; Valachova, K.; Hassan, M.A.; Omer, A.M.; Mohy-Eldin, M.S.; Svik, K.; Jurcik, R.; Ondruska, L.; Biro, C.; et al. MitoQ loaded chitosan-hyaluronan composite membranes for wound healing. Materials 2018, 11, 569. [Google Scholar] [CrossRef] [PubMed]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding protein adsorption phenomena at solid surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef]
- Malmsten, M. Ellipsometry studies of protein layers adsorbed at hydrophobic surfaces. J. Colloid Interface Sci. 1994, 166, 333–342. [Google Scholar] [CrossRef]
- Haslam, E.; Cai, Y. Plant polyphenols (vegetable tannins)–gallic acid metabolism. Nat. Prod. Rep. 1994, 11, 41–66. [Google Scholar] [CrossRef] [PubMed]
- Dangles, O.; Dufour, C. Flavonoid-protein binding processes and their potential impact on human health. Rec. Adv. Polyphen Res. 2008, 1, 67–87. [Google Scholar]
- Heemskerk, J.W.M.; Mattheij, N.J.A.; Cosemans, J.M.E.M. Platelet-based coagulation: Different populations, different functions. J. Thrombosis Haemostasis 2013, 11, 2–16. [Google Scholar] [CrossRef]
- Johne, J.; Blume, C.; Benz, P.M.; Pozgajova, M.; Ullrich, M.; Schuh, K.; Nieswandt, B.; Walter, U.; Renne, T. Platelets promote coagulation factor XII-mediated proteolytic cascade systems in plasma. Biolog. Chem. 2006, 387, 173–178. [Google Scholar] [CrossRef]
- Heemskerk, J.W.M.; Bevers, E.M.; Lindhout, T. Platelet activation and blood coagulation. Thrombosis Haemostasis 2002, 88, 186–193. [Google Scholar] [PubMed]
- Chung, K.T.; Wei, C.I.; Johnson, M.G. Are tannins a double-edged sword in biology and health? Trends Food Sci. Tech. 1998, 9, 168–175. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.; Yang, L.; Han, L.; Jia, L. Metal Ion-Chelated Tannic Acid Coating for Hemostatic Dressing. Materials 2019, 12, 1803. https://doi.org/10.3390/ma12111803
Song B, Yang L, Han L, Jia L. Metal Ion-Chelated Tannic Acid Coating for Hemostatic Dressing. Materials. 2019; 12(11):1803. https://doi.org/10.3390/ma12111803
Chicago/Turabian StyleSong, Bing, Liwei Yang, Lulu Han, and Lingyun Jia. 2019. "Metal Ion-Chelated Tannic Acid Coating for Hemostatic Dressing" Materials 12, no. 11: 1803. https://doi.org/10.3390/ma12111803
APA StyleSong, B., Yang, L., Han, L., & Jia, L. (2019). Metal Ion-Chelated Tannic Acid Coating for Hemostatic Dressing. Materials, 12(11), 1803. https://doi.org/10.3390/ma12111803



