Micro-Scale Surface Patterning of Titanium Dental Implants by Anodization in the Presence of Modifying Salts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Implants and Chemicals Used
2.2. Anodization
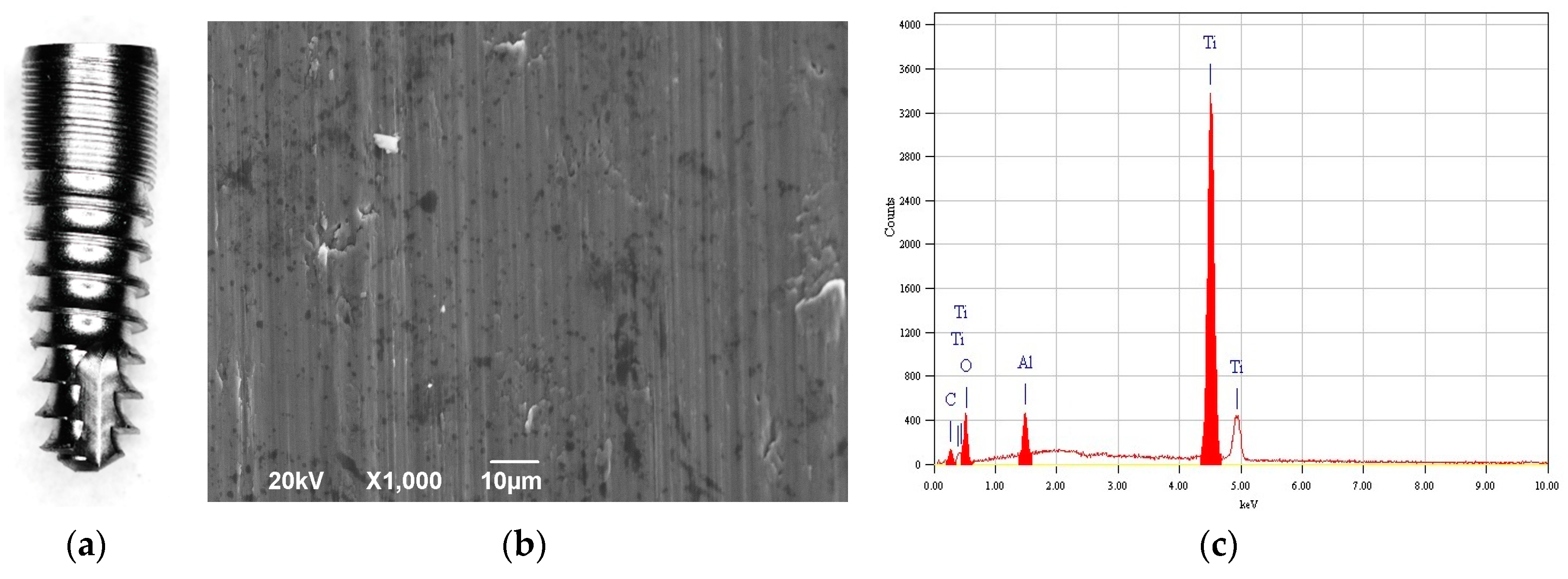
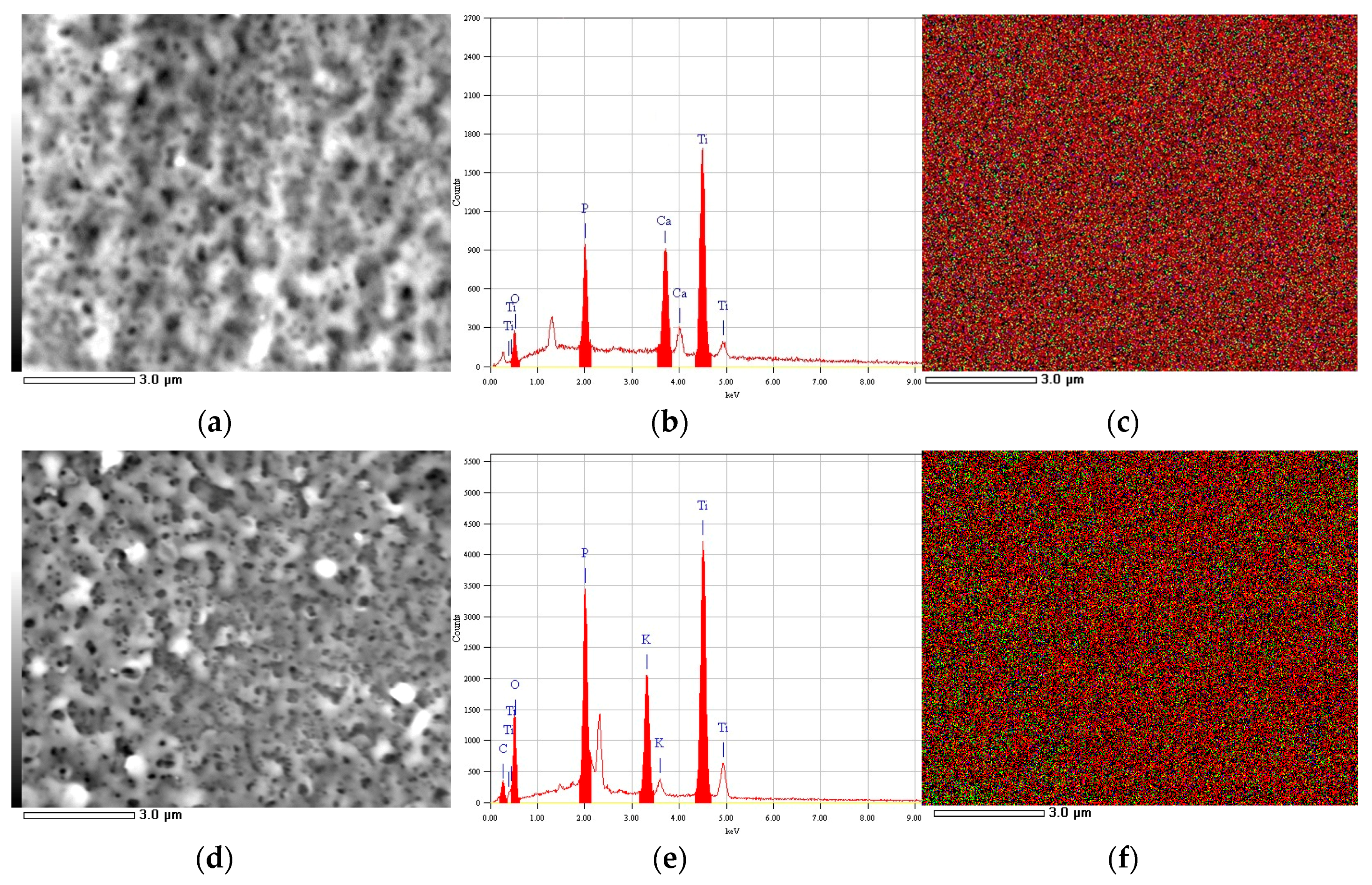
2.3. Imaging and Chemical Analysis
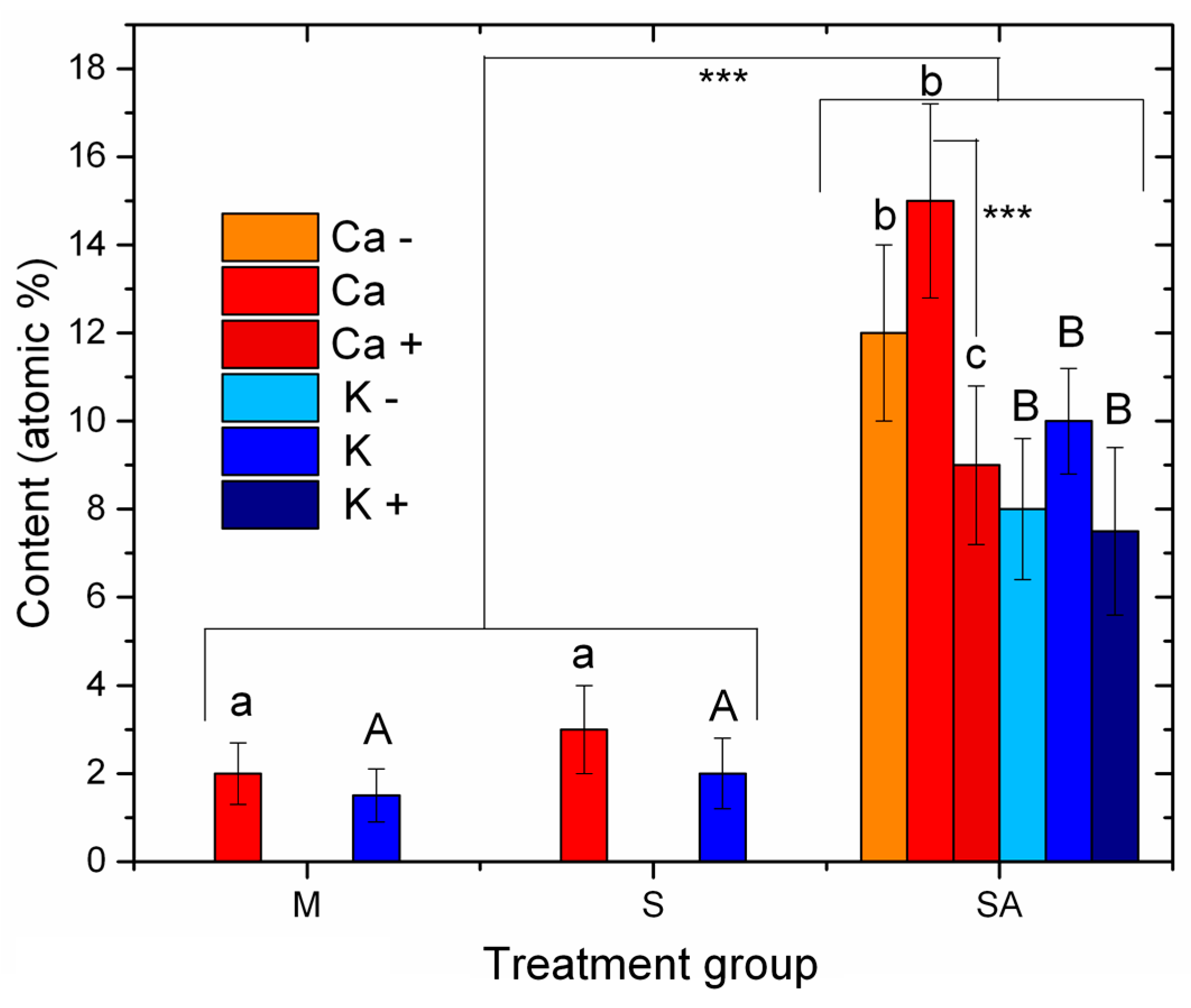
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wennerberg, A.; Albrektsson, T. Effects of titanium surface topography on bone integration: A systematic review. Clin. Oral Implants Res. 2009, 20, 172–184. [Google Scholar] [CrossRef] [PubMed]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implants 2010, 25, 63–74. [Google Scholar]
- Guehennec, L. Surface treatments of titanium dental implants for rapid osseointegration. Dent. Mater. 2007, 3, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.; Asatourian, A.; Garcia-godoy, F.; Sheibani, N. The role of angiogenesis in implant dentistry part I: Review of titanium alloys, surface characteristics and treatments. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e514–e525. [Google Scholar] [CrossRef] [PubMed]
- Saghiri, M.A.; Asatourian, A.; Garcia-Godoy, F.; Sheibani, N. The role of angiogenesis in implant dentistry part II: The effect of bone-grafting and barrier membrane materials on angiogenesis. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e526–e537. [Google Scholar] [CrossRef]
- Gittens, R.A.; Mclachlan, T.; Olivares-navarrete, R.; Cai, Y.; Berner, S.; Tannenbaum, R.; Schwartz, Z.; Sandhage, K.H.; Boyan, B.D. Biomaterials The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials 2011, 32, 3395–3403. [Google Scholar] [CrossRef]
- Coelho, P.G.; Freire, J.N.; Granato, R.; Marin, C.; Bonfante, E.A.; Gil, J.N.; Chuang, S.-K.; Suzuki, M. Bone mineral apposition rates at early implantation times around differently prepared titanium surfaces: A study in beagle dogs. Int. J. Oral Maxillofac. Implants 2011, 26, 63–69. [Google Scholar]
- Caneva, M.; Botticelli, D.; Stellini, E.; Souza, S.L.S.; Salata, L.A.; Lang, N.P. Magnesium-enriched hydroxyapatite at immediate implants: A histomorphometric study in dogs. Clin. Oral Implants Res. 2011, 22, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Alves, S.A.; Bayón, R.; de Viteri, V.S.; Garcia, M.P.; Igartua, A.; Fernandes, M.H.; Rocha, L.A. Tribocorrosion behavior of calcium- and phosphorous-enriched titanium oxide films and study of osteoblast interactions for dental implants. J. Bio- Tribo-Corr. 2015, 1, 23. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Coelho, P.G.; Kang, B.-S.; Sul, Y.-T.; Albrektsson, T. Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 2010, 28, 198–206. [Google Scholar] [CrossRef]
- Tete, S.; Mastrangelo, F.; Traini, T.; Vinci, R. A macro- and nanostructure evaluation of a novel dental implant. Implant Dent. 2008, 17, 309–320. [Google Scholar] [CrossRef]
- Ehrenfest, D.M.D.; Vazquez, L.; Park, Y.; Sammartino, G.; Bernard, J. Identification card and codification of the chemical and morphological characteristics of 14 dental implant surfaces. J. Oral Implantol. 2011, 37, 525–542. [Google Scholar] [CrossRef]
- Radtke, A.; Ehlert, M. The Morphology, Structure, Mechanical properties and biocompatibility of nanotubular titania coatings before and after autoclaving process. J. Clin. Med. 2019, 8, 272. [Google Scholar] [CrossRef]
- Shibli, J.A.; Grassi, S.; De Figueiredo, L.C.; Feres, M.; Marcantonio, E.; Iezzi, G.; Piattelli, A. Influence of implant surface topography on early osseointegration: A histological study in human jaws. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 80, 377–385. [Google Scholar] [CrossRef]
- Coelho, P.G.; Takayama, T.; Yoo, D.; Jimbo, R.; Karunagaran, S.; Tovar, N.; Janal, M.N.; Yamano, S. Nanometer-scale features on micrometer-scale surface texturing: A bone histological, gene expression, and nanomechanical study. Bone 2014, 65, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Marenzi, G.; Impero, F.; Scherillo, F.; Sammartino, C.; Squillace, A.; Spagnuolo, G. Effect of different surface treatments on titanium dental implant micro-morphology. Materials 2019, 12, 733. [Google Scholar] [CrossRef]
- Nappo, A.; Rengo, C.; Pantaleo, G.; Spagnuolo, G.; Ferrari, M. Influence of implant dimensions and position on implant stability: A prospective clinical study in maxilla using resonance frequency analysis. Appl. Sci. 2019, 9, 860. [Google Scholar] [CrossRef]
- Shayganpour, A.; Rebaudi, A.; Cortella, P.; Diaspro, A.; Salerno, M. Electrochemical coating of dental implants with anodic porous titania for enhanced osteointegration. Beilstein J. Nanotechnol. 2015, 6, 2183–2192. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Moon, H.; Li, T.; Kumar, P.T.S. Titania nanopores with dual micro-/nano-topography for selective cellular bioactivity. Mater. Sci. Eng. C 2018, 91, 624–630. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Gulati, K.; Wang, N.; Zhang, Z.; Ivanovski, S. Bridging the gap: Optimized fabrication of robust titania nanostructures on complex implant geometries towards clinical translation. J. Colloid Interface Sci. 2018, 529, 452–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor, G.K.; Varghese, O.K.; Paulose, M.; Shankar, K.; Grimes, C.A. A review on highly ordered, vertically oriented TiO2 nanotube arrays: Fabrication, material properties, and solar energy applications. Sol. Energy Mater. Sol. Cells 2006, 90, 2011–2075. [Google Scholar] [CrossRef]
- Ali, G.; Chen, C.; Yoo, S.H.; Kum, J.M.; Cho, S.O. Fabrication of complete titania nanoporous structures via electrochemical anodization of Ti. Nanoscale Res. Lett. 2011, 6, 332. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Heo, S.J.; Koak, J.Y.; Kim, S.K.; Lee, J.B.; Kim, S.H.; Lim, Y.J. Osseointegration of anodized titanium implants under different current voltages: A rabbit study. J. Oral Rehabil. 2007, 34, 517–527. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Fukushima, Y.; Nakamura, I.; Tanaki, T.; Jerkiewicz, G. Preparation and characterization of microporous layers on titanium by anodization in sulfuric acid with and without hydrogen charging. Appl. Mater. Interfaces 2013, 5, 3340–3347. [Google Scholar] [CrossRef] [PubMed]
- Uttiya, S.; Contarino, D.; Prandi, S.; Mm, C.; Gemme, G.; Mattera, L.; Rolandi, R.; Canepa, M.; Cavalleri, O. Anodic oxidation of titanium in sulphuric acid and phosphoric acid electrolytes. J. Mater. Sci. Nanotechnol. 2014, 1, S106. [Google Scholar] [CrossRef]
- Li, T.; Gulati, K.; Wang, N.; Zhang, Z. Understanding and augmenting the stability of therapeutic nanotubes on anodized titanium implants. Mater. Sci. Eng. C 2018, 88, 182–195. [Google Scholar] [CrossRef] [Green Version]
- Gulati, K.; Ivanovski, S. Dental implants modified with drug releasing titania nanotubes: Therapeutic potential and developmental challenges. Expert Opin. Drug Deliv. 2017, 14, 1009–1024. [Google Scholar] [CrossRef]
- Gulati, K.; Losic, D. Titania nanotubes for orchestrating osteogenesis at the bone—Implant interface. Nanomedicine 2016, 11, 1847–1864. [Google Scholar] [CrossRef]
- Gulati, K.; Hamlet, S.M. Tailoring the immuno-responsiveness of anodized nano-engineered titanium implants. J. Mater. Chem. B 2018, 6, 2677–2689. [Google Scholar] [CrossRef]
- Sul, Y.T.; Johansson, C.B.; Jeong, Y.; Albrektsson, T. The electrochemical oxide growth behaviour on titanium in acid and alkaline electrolytes. Med. Eng. Phys. 2001, 23, 329–346. [Google Scholar] [CrossRef]
- Yao, C.; Webster, T. Prolonged antibiotic delivery from anodized nanotubular titanium using a co-precipitation drug loading method. J. Biomed. Mater. Res. B Appl. Biomater. 2009, 91, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Sulka, G.D. Highly Ordered Anodic Porous Alumina Formation by Self-organized Anodizing. In Nanostructured Materials in Electrochemistry; Eftekhari, A., Ed.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; pp. 1–96. ISBN 9783527318766. [Google Scholar]
- Shin, E.; Yong, I.; Baek, S.; Ohtsuki, C. Hydroxyapatite formation on titania-based materials in a solution mimicking body fluid: Effects of manganese and iron addition in anatase. Mater. Sci. Eng. C 2015, 48, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Kimb, H.-M.; Kawashita, M.; Wanga, L.; Xiong, T.; Kokubo, T.; Nakamura, T. Preparation of bioactive titania films on titanium metal via anodic oxidation. Dent. Mater. 2008, 5, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Kogawa, M.; Prideaux, M.; Findlay, D.M.; Atkins, G.J.; Losic, D. Drug-releasing nano-engineered titanium implants: therapeutic efficacy in 3D cell culture model, controlled release and stability. Mater. Sci. Eng. C 2016, 69, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Aw, M.S.; Losic, D. Drug-eluting Ti wires with titania nanotube arrays for bone fixation and reduced bone infection. Nanoscale Res. Lett. 2011, 6, 571. [Google Scholar] [CrossRef]
- Reisch, M.S. Essential Minerals. In Medical Biochemistry; Blanco, G., Blanco, A., Eds.; Academic Press: London, UK, 2017; pp. 715–743. ISBN 978-0-12-803550-4. [Google Scholar]
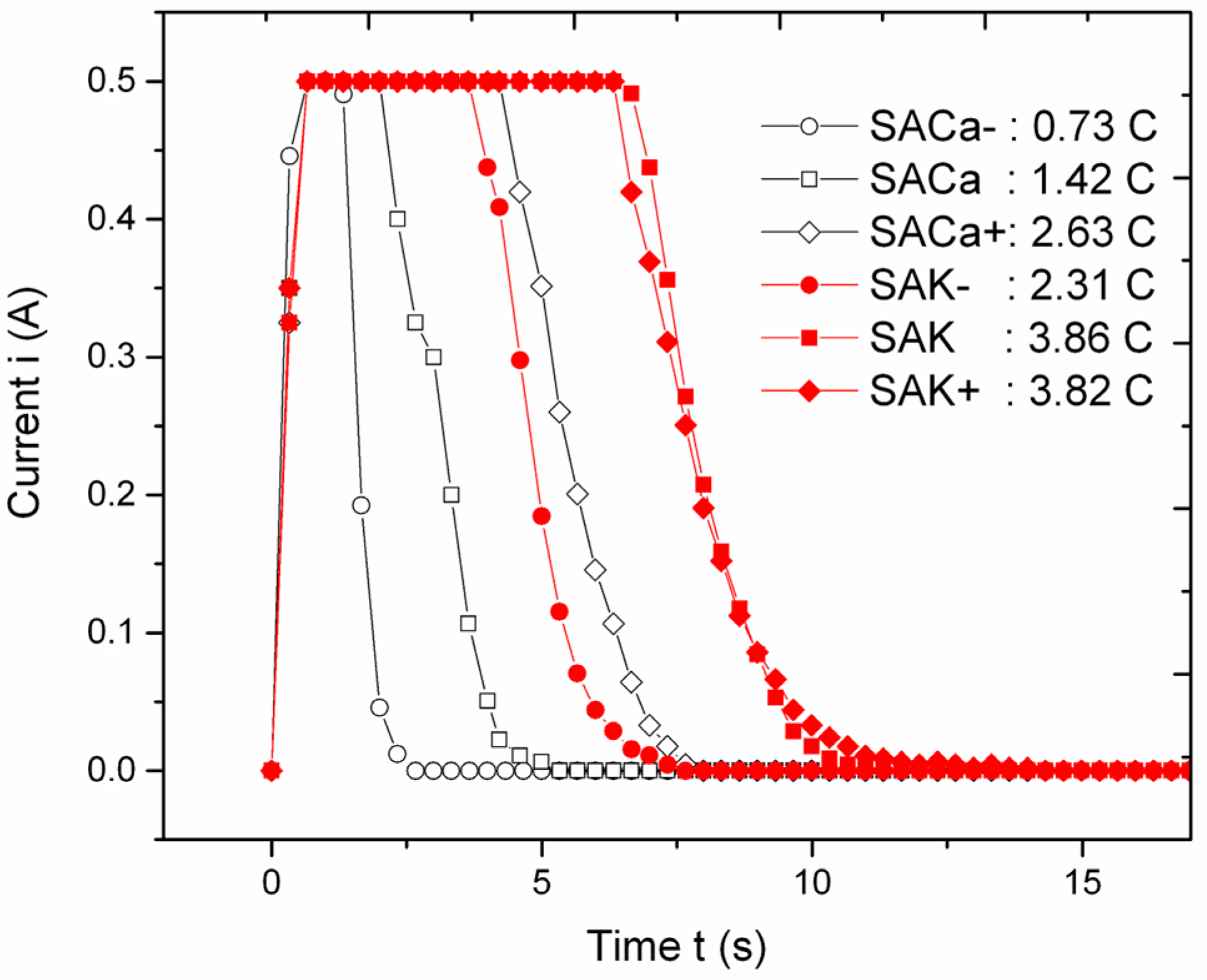
| Treatment | Metal of the Added Salt | Salt Concentration (M) | Implant ID |
|---|---|---|---|
| Machined only (M) | Ca | 1 | MCa |
| K | MK | ||
| Sandblasted (S) | Ca | SCa | |
| K | SK | ||
| Sandblasted + Anodized (SA) | Ca | 0.5 | SACa− |
| 1 | SACa | ||
| 1.5 | SACa+ | ||
| K | 0.5 | SAK− | |
| 1 | SAK | ||
| 1.5 | SAK+ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marenzi, G.; Spagnuolo, G.; Sammartino, J.C.; Gasparro, R.; Rebaudi, A.; Salerno, M. Micro-Scale Surface Patterning of Titanium Dental Implants by Anodization in the Presence of Modifying Salts. Materials 2019, 12, 1753. https://doi.org/10.3390/ma12111753
Marenzi G, Spagnuolo G, Sammartino JC, Gasparro R, Rebaudi A, Salerno M. Micro-Scale Surface Patterning of Titanium Dental Implants by Anodization in the Presence of Modifying Salts. Materials. 2019; 12(11):1753. https://doi.org/10.3390/ma12111753
Chicago/Turabian StyleMarenzi, Gaetano, Gianrico Spagnuolo, Josè Camilla Sammartino, Roberta Gasparro, Alberto Rebaudi, and Marco Salerno. 2019. "Micro-Scale Surface Patterning of Titanium Dental Implants by Anodization in the Presence of Modifying Salts" Materials 12, no. 11: 1753. https://doi.org/10.3390/ma12111753
APA StyleMarenzi, G., Spagnuolo, G., Sammartino, J. C., Gasparro, R., Rebaudi, A., & Salerno, M. (2019). Micro-Scale Surface Patterning of Titanium Dental Implants by Anodization in the Presence of Modifying Salts. Materials, 12(11), 1753. https://doi.org/10.3390/ma12111753









