Enhancement of Complex Permittivity and Attenuation Properties of Recycled Hematite (α-Fe2O3) Using Nanoparticles Prepared via Ball Milling Technique
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of α-Fe2O3 Nanoparticles from Mill Scale
2.2. Microstructural Characterization
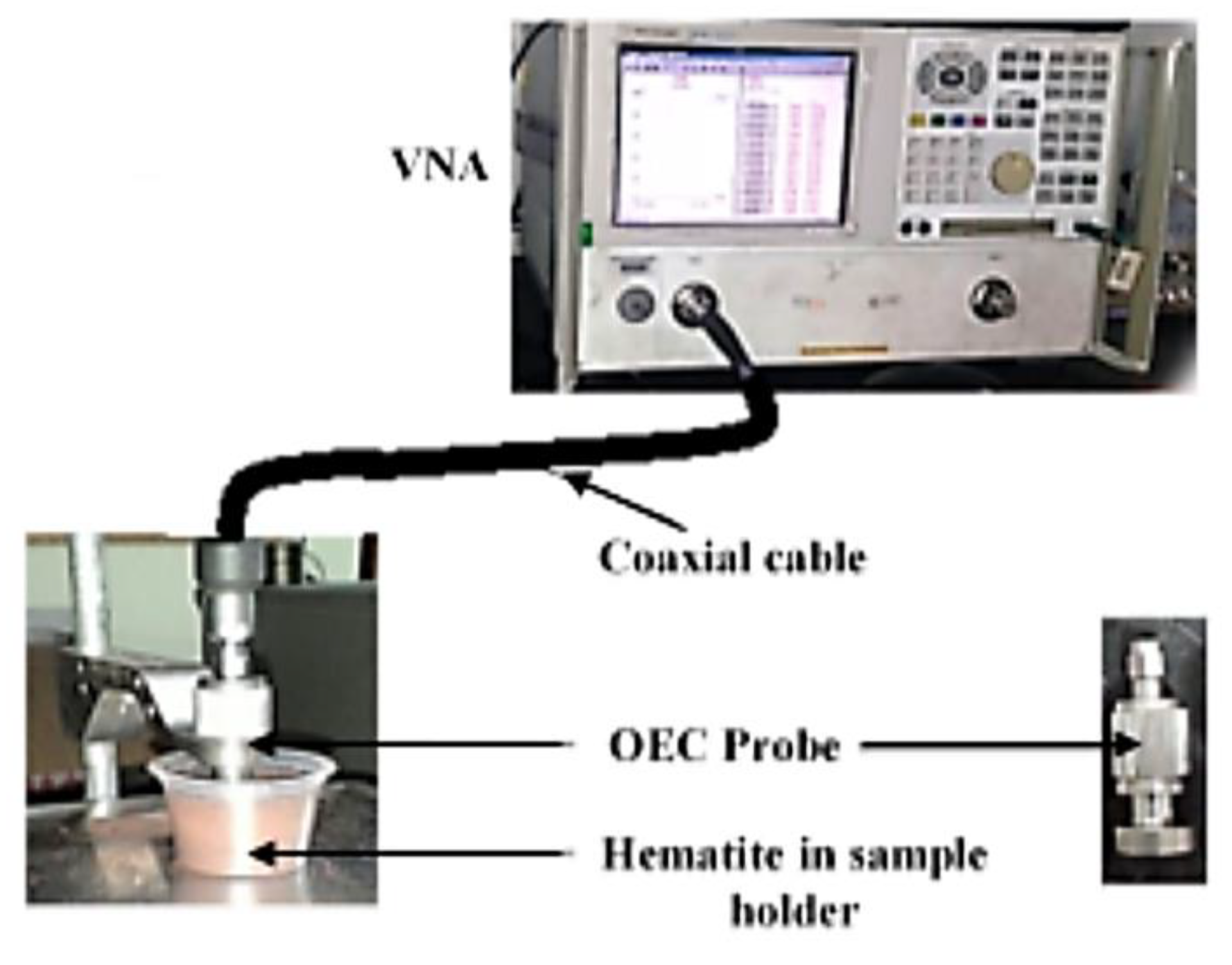
2.3. Measurement of Complex Permittivity
2.4. Measurement of Complex Permeability
2.5. Material Absorption Properties Based On FEM
3. Results and Discussion
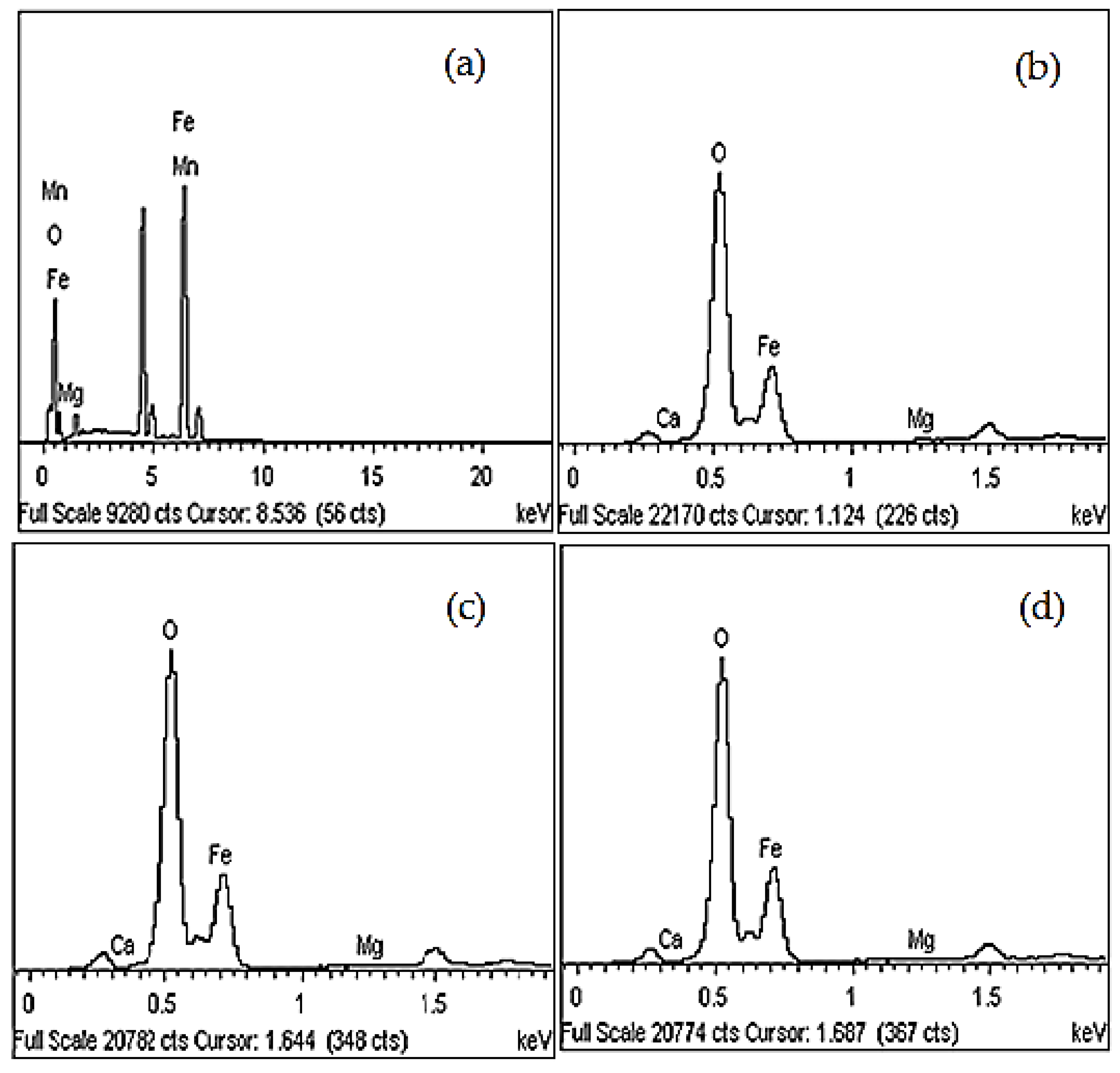
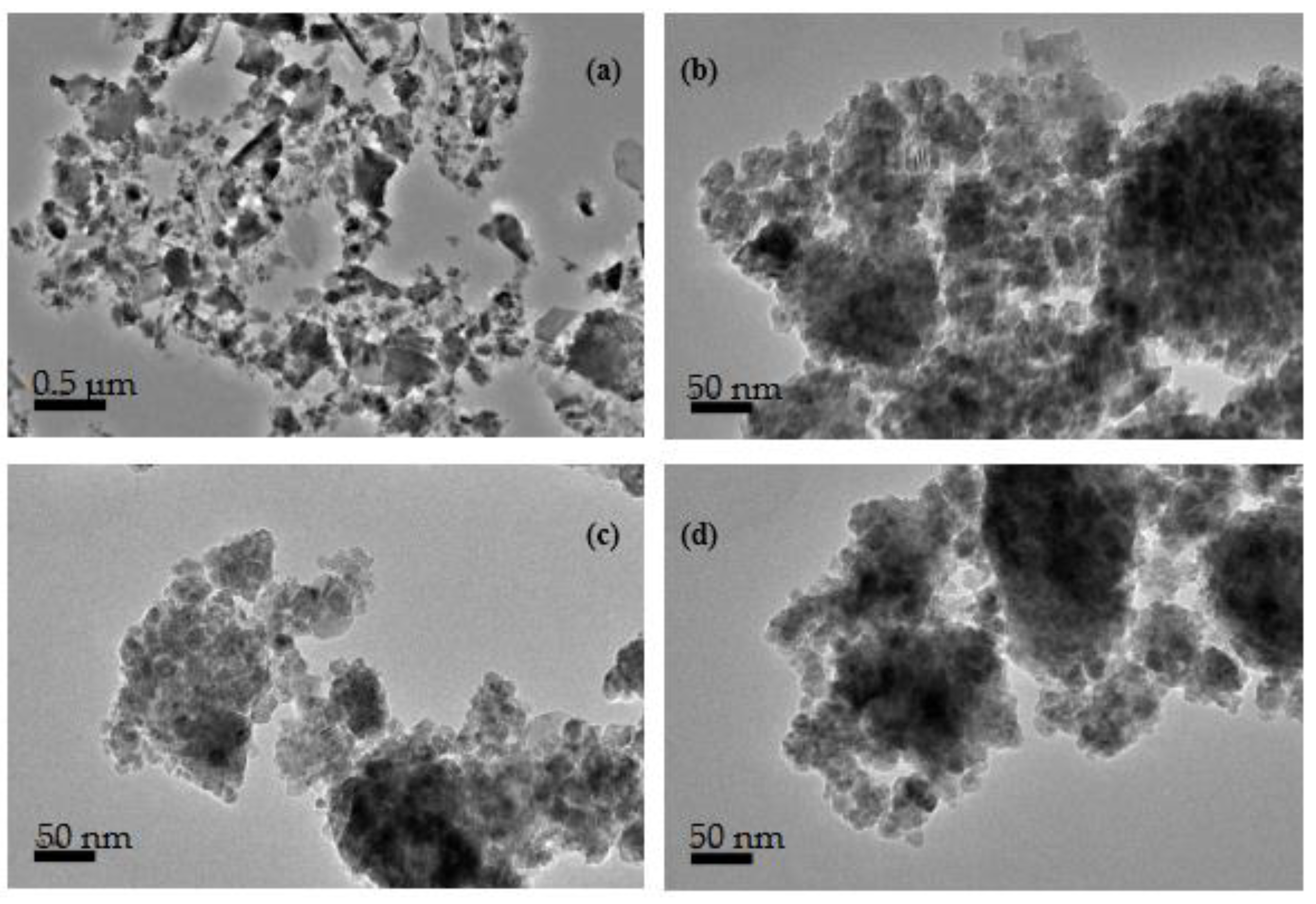
3.1. Microstructural Characterization
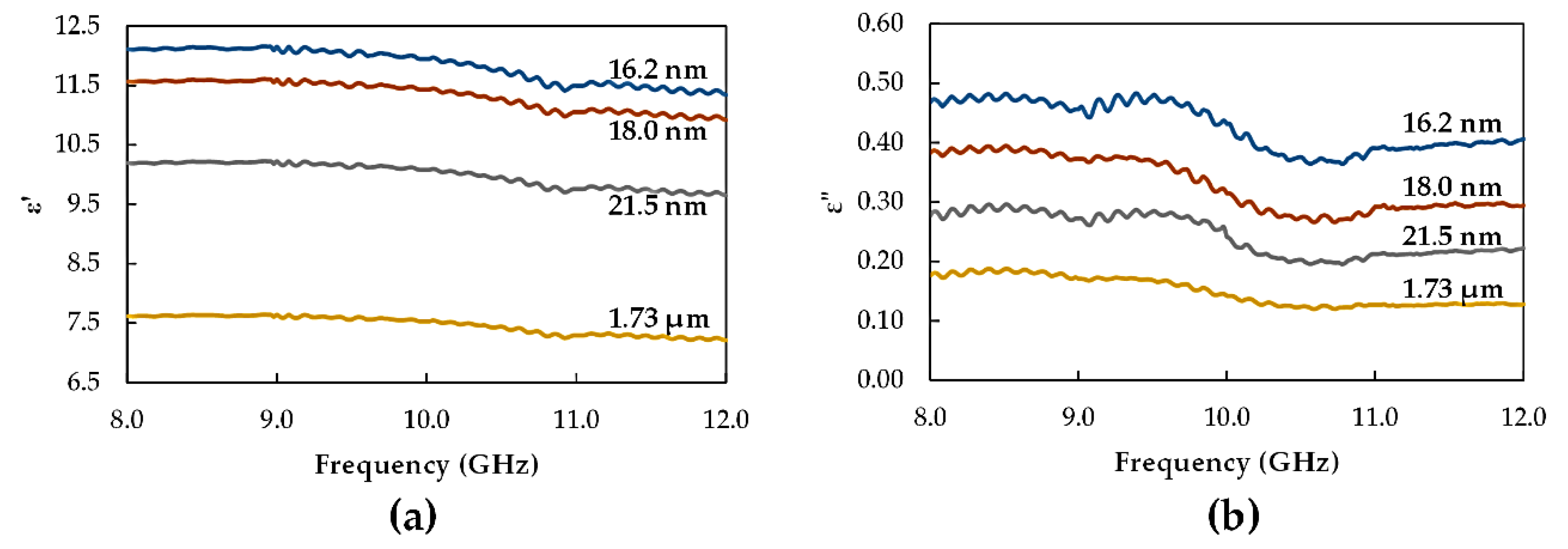
3.2. Dielectric Characterization
3.3. Complex Permeability
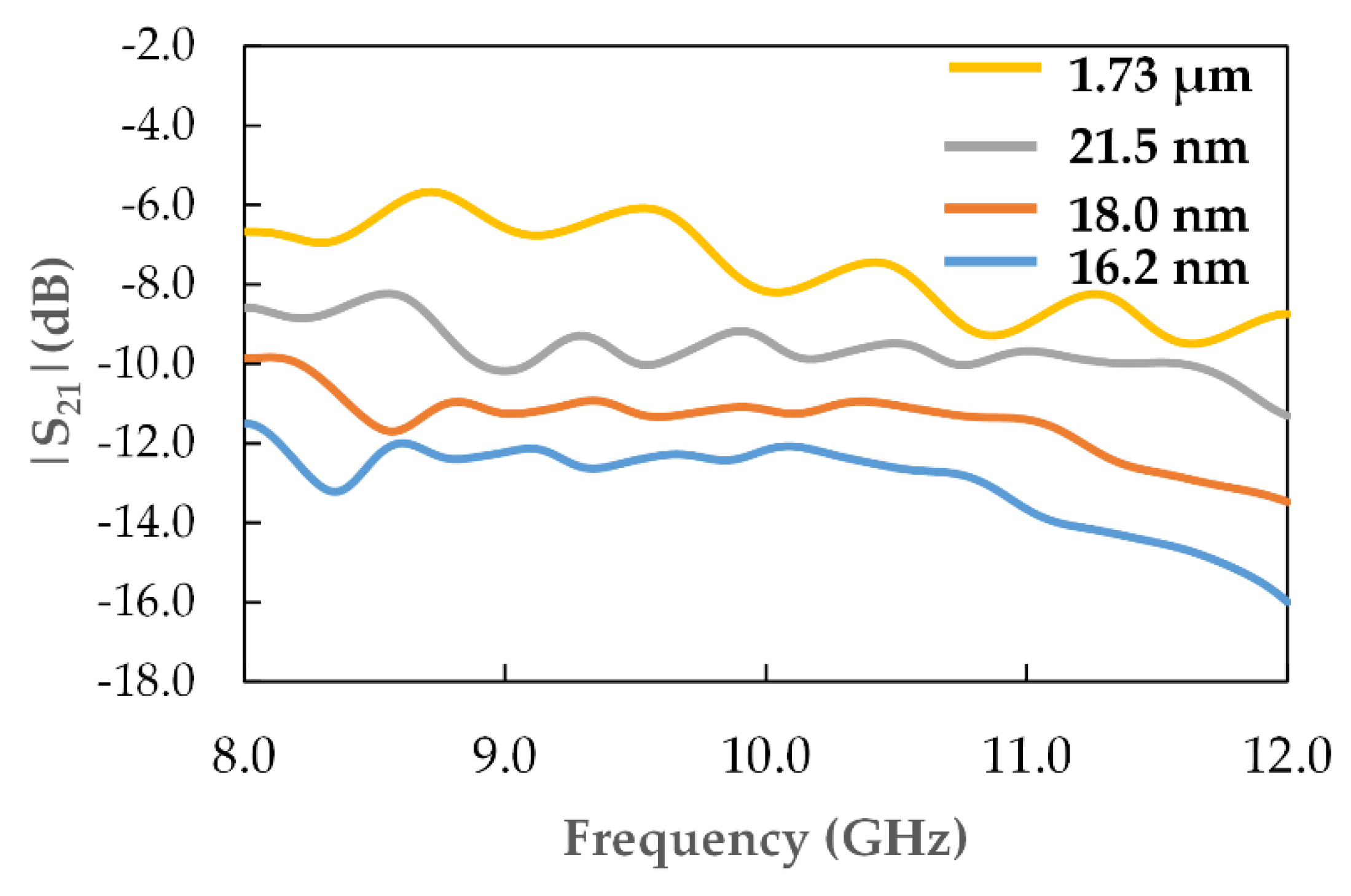
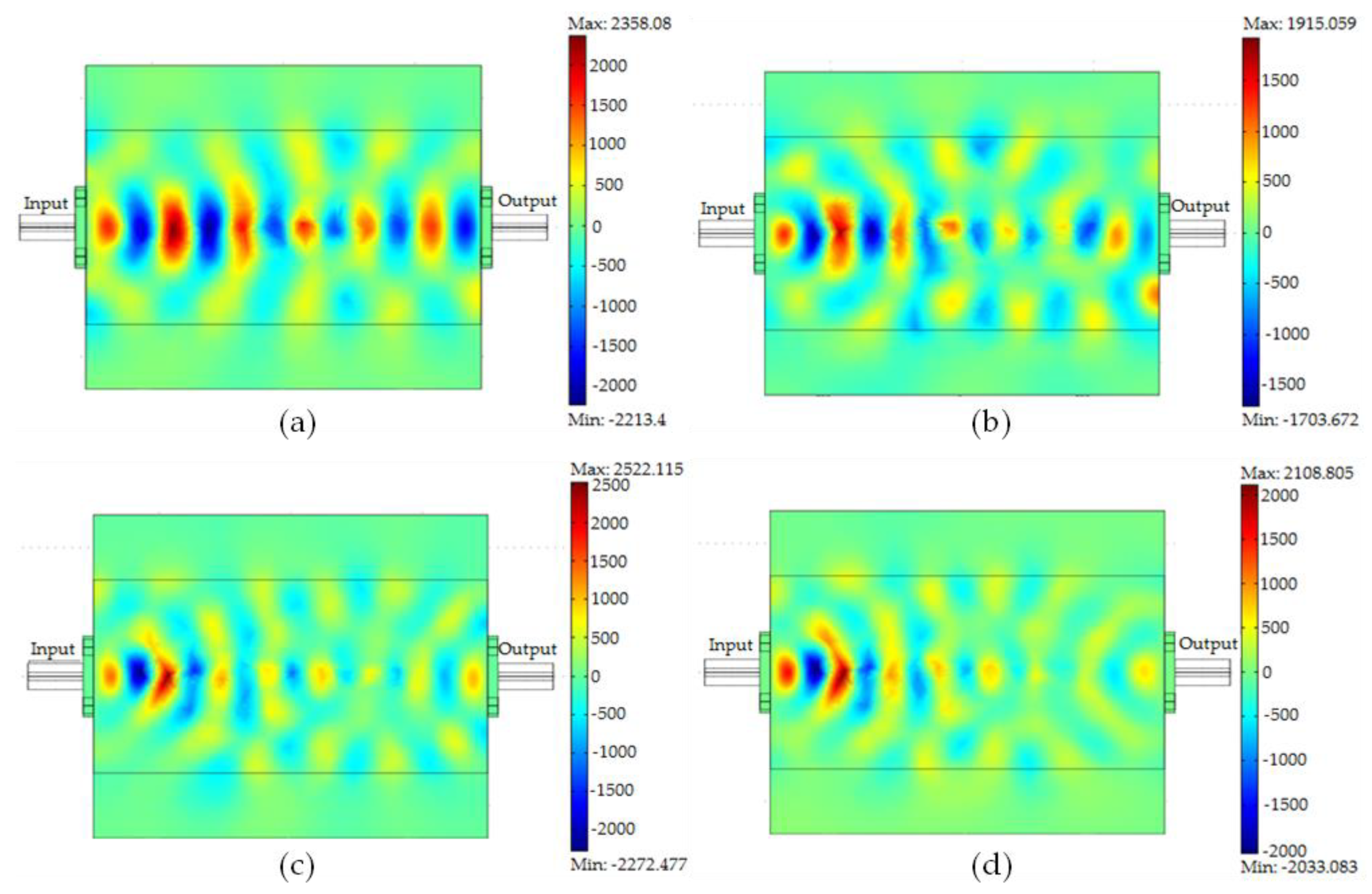
3.4. Material Absorption
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, H.; Ye, T.; Lin, Y.; Zhu, J.; Wang, F. Microwave absorbing properties of the ferrite composites based on graphene. J. Alloys Compd. 2016, 683, 567–574. [Google Scholar] [CrossRef]
- Li, L.; Liu, S.; Lu, L. Synthesis and significantly enhanced microwave absorption properties of cobalt ferrite hollow microspheres with protrusions/polythiophene composites. J. Alloys Compd. 2017, 722, 158–165. [Google Scholar] [CrossRef]
- Zhang, K.; Gao, X.; Zhang, Q.; Chen, H.; Chen, X. Fe3O4 nanoparticles decorated MWCNTs @ C ferrite nanocomposites and their enhanced microwave absorption properties. J. Magn. Magn. Mater. 2018, 452, 55–63. [Google Scholar] [CrossRef]
- Rostami, M.; Hossein, M.; Ara, M. The dielectric, magnetic and microwave absorption properties of Cu—substituted Mg–Ni spinel ferrite—MWCNT nanocomposites. Ceram. Int. 2019, 46, 7606–7613. [Google Scholar] [CrossRef]
- Daud, N.; Azis, R.A.S.; Hashim, M.; Matori, K.A.; Jumiah, H.; Saiden, N.M.; Mariana, N. Preparation and characterization of Sr1−xNdxFe12O19 derived from steel-waste product via mechanical alloying. Mater. Sci. Forum 2016, 846, 403–409. [Google Scholar] [CrossRef]
- Esa, F.; Abbas, Z.; Idris, F.M.; Hashim, M. Characterization of Nix Zn1−x Fe2O4 and permittivity of solid material of NiO, ZnO, Fe2O3, and NixZn1−x Fe2O4 at microwave frequency using open ended coaxial probe. Int. J. Microw. Sci. Technol. 2015, 2015, 219195. [Google Scholar] [CrossRef]
- Abdalhadi, D.M.; Abbas, Z.; Ahmad, A.F.; Ibrahim, N.A. Determining the complex permittivity of oil palm empty fruit bunch fibre material by open-ended coaxial probe technique for microwave applications. BioResources 2017, 12, 3976–3991. [Google Scholar] [CrossRef][Green Version]
- Shahrani, M.M.; Azis, R.S.; Hashim, M.; Hassan, J. Effect of variation sintering temperature on magnetic permeability and grain sizes of Y3Fe5O12 via mechanical alloying technique. Mater. Sci. Forum 2016, 846, 395–402. [Google Scholar] [CrossRef]
- Zdujić, Č.; Jovalekić, Č.; Karanović, L.; Mitrić, M. The ball milling induced transformation of α-Fe2O3 powder in air and oxygen atmosphere. Mater. Sci. Eng. A 1999, 262, 204–213. [Google Scholar] [CrossRef]
- Azis, R.S.; Hashim, M.; Saiden, N.M.; Daud, N.; Shahrani, N.M.M. Study the iron environments of the steel waste product and its possible potential applications in ferrites. Adv. Mater. Res. 2015, 1109, 295–299. [Google Scholar] [CrossRef]
- Guerrero-Suarez, S.; Martín-Hernández, F. Magnetic anisotropy of hematite natural crystals: Increasing low-field strength experiments. Int. J. Earth Sci. 2012, 101, 625–636. [Google Scholar] [CrossRef]
- Shenoy, D.; Joy, P.A.; Anantharaman, M.R. Effect of mechanical milling on the structural, magnetic and dielectric properties of coprecipitated ultrafine zinc ferrite. J. Magn. Magn. Mater. 2004, 269, 217–226. [Google Scholar] [CrossRef]
- Ungár, T. Microstructural parameters from X-ray diffraction peak broadening. Scr. Mater. 2004, 51, 777–778. [Google Scholar] [CrossRef]
- Bhowmik, R.N.; Saravanan, A. Surface magnetism, morin transition, and magnetic dynamics in antiferromagnetic α-Fe2O3(hematite) nanograins. J. Appl. Phys. 2010, 107, 053916. [Google Scholar] [CrossRef]
- Fukuhara, M. Lattice expansion of nanoscale compound particles. Phys. Lett. A 2003, 313, 427–430. [Google Scholar] [CrossRef]
- Kalita, A.; Kalita, M.P.C. Size dependence of lattice parameters in ZnO nanocrystals. Appl. Phys. A 2015, 121, 521–524. [Google Scholar] [CrossRef]
- André-Filho, J.; León-Félix, L.; Coaquira, J.A.H.; Garg, V.K.; Oliveira, A.C. Size dependence of the magnetic and hyperfine properties of nanostructured hematite (α-Fe2O3) perpared by the ball milling technique. Hyperfine Interact. 2014, 224, 189–196. [Google Scholar] [CrossRef]
- Muda, N.N.C.; Azis, R.S.; Shaari, A.H.; Hassan, J.; Sulaiman, S. Elemental analysis and IR band characteristics of α-Fe2O3 and BaFe12O19 steel waste product base. J. Sol. Stat. Sci. Tech. 2016, 24, 45–51. [Google Scholar]
- Kendall, K.; Kendall, M.; Rehfeldt, F. Adhesion of Cells, Viruses and Nanoparticles; Springer Science & Business Media: Berlin, Germany, 2011; pp. 1–282. [Google Scholar]
- Kumar, E.R.; Kamzin, A.S.; Prakash, T. Effect of particle size on structural, magnetic and dielectric properties of manganese substituted nickel ferrite nanoparticles. J. Magn. Magn. Mater. 2015, 378, 389–396. [Google Scholar] [CrossRef]
- Bhat, B.H.; Want, B. Magnetic, dielectric and complex impedance properties of lanthanum and magnesium substituted strontium hexaferrite. J. Mater. Sci. Mater. Electron. 2016, 27, 12582–12590. [Google Scholar] [CrossRef]
- Sadiq, I.; Naseem, S.; Naeem Ashiq, M.; Khan, M.A.; Niaz, S.; Rana, M.U. Structural and dielectric properties of doped ferrite nanomaterials suitable for microwave and biomedical applications. Prog. Nat. Sci. Mater. Int. 2015, 25, 419–424. [Google Scholar] [CrossRef]
- Maleknejad, Z.; Gheisari, K.; Raouf, A.H. Structure, microstructure, magnetic, electromagnetic, and dielectric properties of nanostructured Mn–Zn ferrite synthesized by microwave-induced urea-nitrate process. J. Supercond. Nov. Magn. 2016, 29, 2523–2534. [Google Scholar] [CrossRef]
- Micheli, D.; Pastore, R.; Vricella, A.; Marchetti, M. Matter’s electromagnetic signature reproduction by graded-dielectric multilayer assembly. IEEE Trans. Microw. Theory Tech. 2017, 65, 2801–2809. [Google Scholar] [CrossRef]


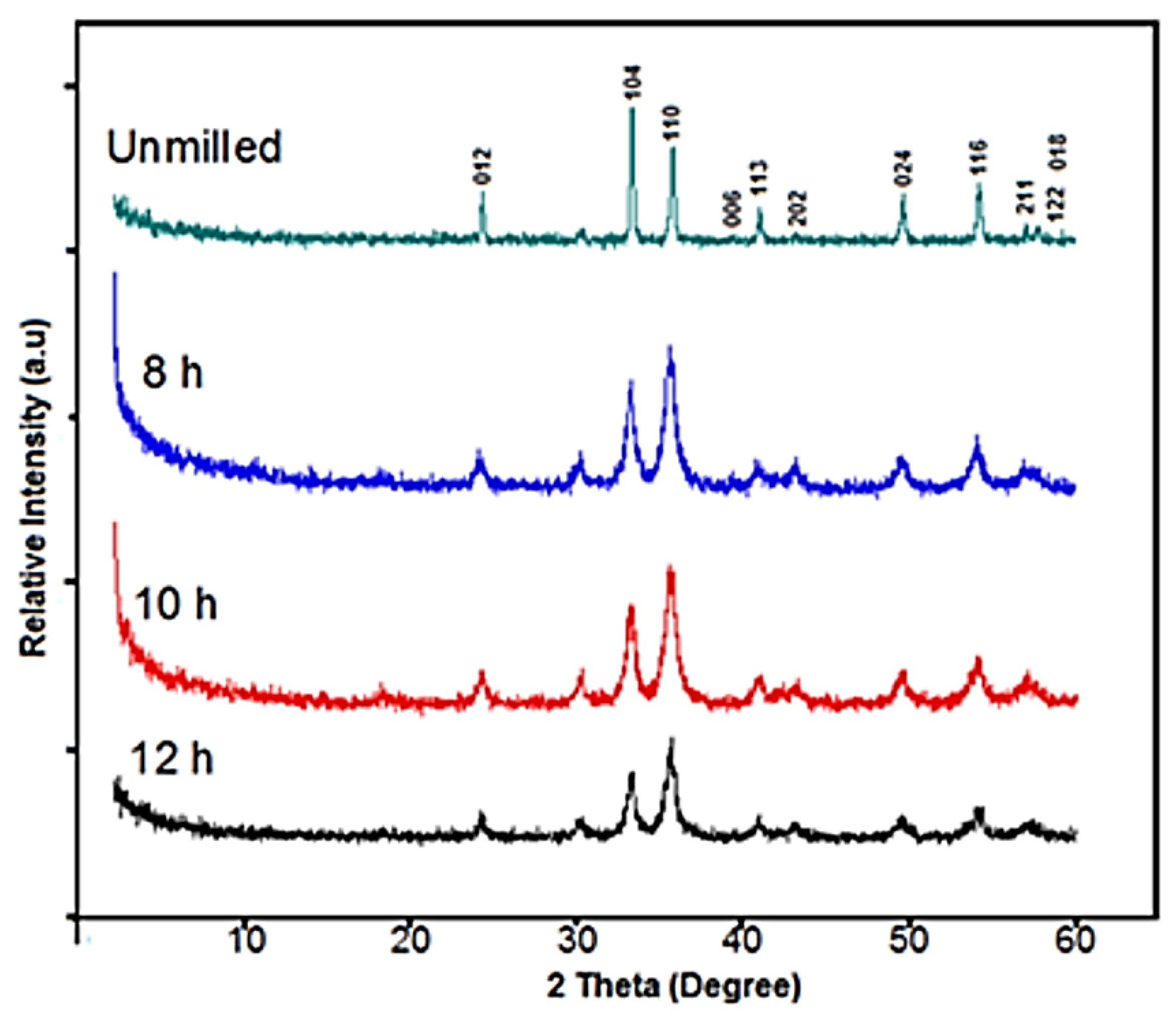



| Condition | a = b (Å) | c (Å) | D (nm) |
|---|---|---|---|
| Unmilled | 5.0290 | 13.7360 | 106.2 |
| 8 h of milling | 5.0340 | 13.7480 | 12.3 |
| 10 h of milling | 5.0350 | 13.7500 | 11.8 |
| 12 h of milling | 5.0380 | 13.7390 | 11.1 |
| Sample | Element (%) | |||||
|---|---|---|---|---|---|---|
| Fe | O | Mn | Mg | Ca | Total | |
| Unmilled | 78.07 | 20.69 | 0.69 | 0.55 | - | 100.0 |
| 8 h of milling | 58.87 | 40.92 | - | 0.11 | 0.10 | 100.0 |
| 10 h of milling | 56.70 | 43.13 | - | 0.12 | 0.05 | 100.0 |
| 12 h of milling | 57.22 | 42.63 | - | 0.09 | 0.06 | 100.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mensah, E.E.; Abbas, Z.; Azis, R.S.; Khamis, A.M. Enhancement of Complex Permittivity and Attenuation Properties of Recycled Hematite (α-Fe2O3) Using Nanoparticles Prepared via Ball Milling Technique. Materials 2019, 12, 1696. https://doi.org/10.3390/ma12101696
Mensah EE, Abbas Z, Azis RS, Khamis AM. Enhancement of Complex Permittivity and Attenuation Properties of Recycled Hematite (α-Fe2O3) Using Nanoparticles Prepared via Ball Milling Technique. Materials. 2019; 12(10):1696. https://doi.org/10.3390/ma12101696
Chicago/Turabian StyleMensah, Ebenezer Ekow, Zulkifly Abbas, Raba’ah Syahidah Azis, and Ahmad Mamoun Khamis. 2019. "Enhancement of Complex Permittivity and Attenuation Properties of Recycled Hematite (α-Fe2O3) Using Nanoparticles Prepared via Ball Milling Technique" Materials 12, no. 10: 1696. https://doi.org/10.3390/ma12101696
APA StyleMensah, E. E., Abbas, Z., Azis, R. S., & Khamis, A. M. (2019). Enhancement of Complex Permittivity and Attenuation Properties of Recycled Hematite (α-Fe2O3) Using Nanoparticles Prepared via Ball Milling Technique. Materials, 12(10), 1696. https://doi.org/10.3390/ma12101696





