Dual Oxygen Defects in Layered La1.2Sr0.8−xBaxInO4+δ (x = 0.2, 0.3) Oxide-Ion Conductors: A Neutron Diffraction Study
Abstract
1. Introduction
2. Experimental Section
2.1. Synthesis
2.2. Structural Characterization
2.3. Conductivity Measurements
3. Results and Discussion
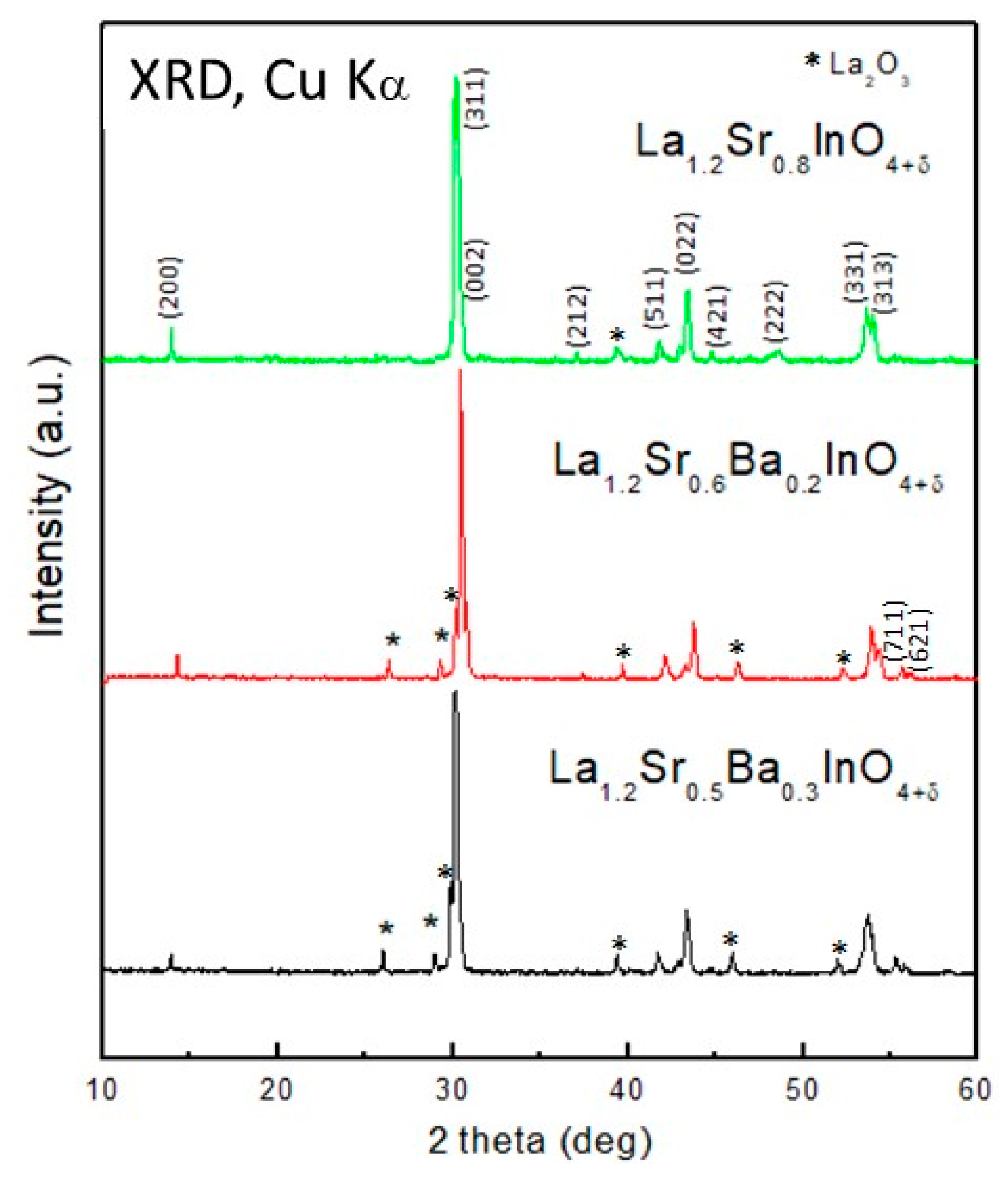
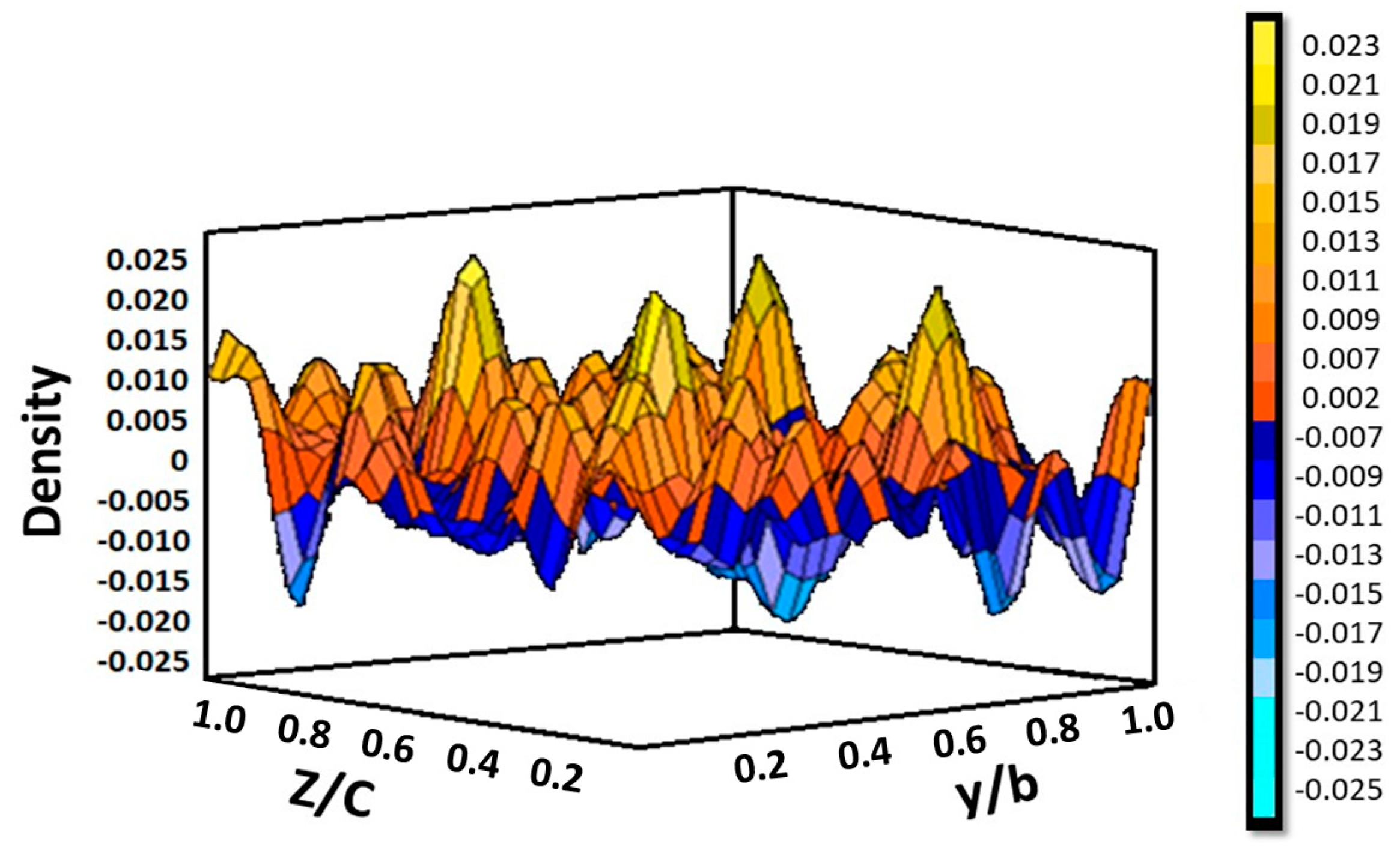
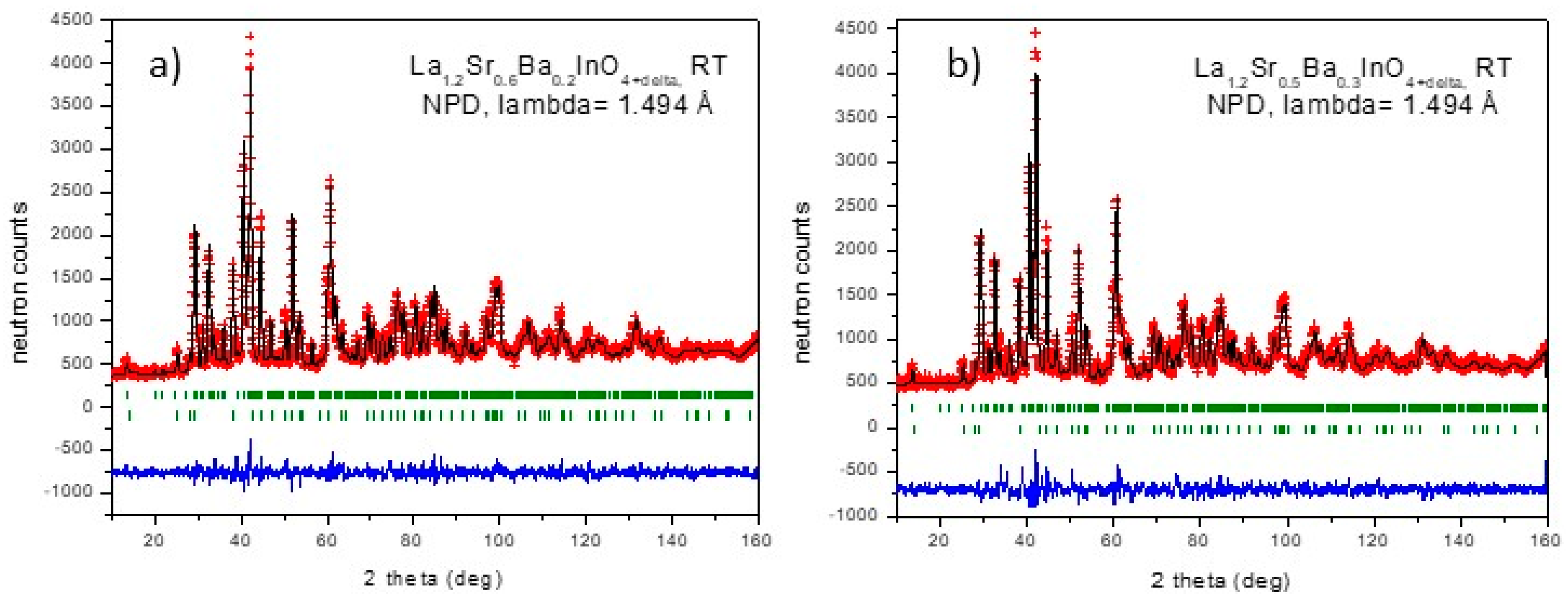
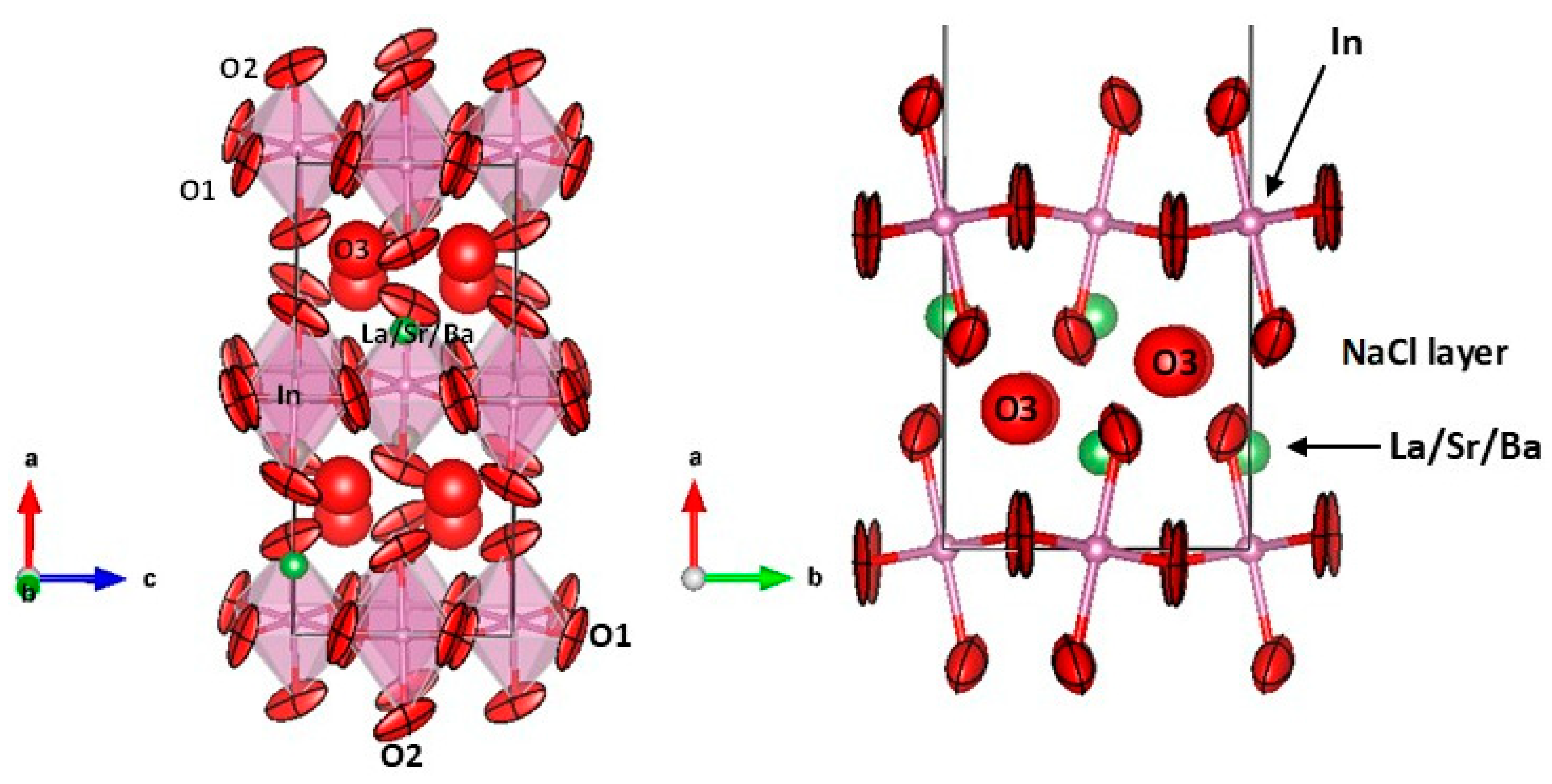
3.1. Crystallographic Characterization
3.2. Electrical Conductivity Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lutz, A.E.; Larson, R.S.; Keller, J.O. Thermodynamic comparison of fuel cells to the Carnot cycle. Int. J. Hydrogen Energy 2002, 27, 1103–1111. [Google Scholar] [CrossRef]
- Ramadhani, F.; Hussain, M.A.; Mokhlis, H.; Hajimolana, S. Optimization strategies for Solid Oxide Fuel Cell (SOFC) application: A literature survey. Renew. Sust. Energ Rev. 2017, 76, 460–484. [Google Scholar] [CrossRef]
- Choudhary, T.; Kumar Sahu, M. CFD Modeling of SOFC Cogeneration System for Building Application. Energy Procedia 2017, 109, 361–368. [Google Scholar] [CrossRef]
- Wilberforce, T.; Alaswad, A.; Palumbo, A.; Dassisti, M.; Olabi, A.G. Advances in stationary and portable fuel cell applications. Int. J. Hydrogen Energy 2016, 41, 16509–16522. [Google Scholar] [CrossRef]
- Badwal, S.P.S. Zirconia-based solid electrolytes: microstructure, stability and ionic conductivity. Solid State Ionics 1992, 52, 23–32. [Google Scholar] [CrossRef]
- Steele, B.C. Appraisal of Ce1−yGdyO2−y/2 electrolytes for IT-SOFC operation at 500° C. Solid State Ionics 2000, 129, 95–110. [Google Scholar] [CrossRef]
- Huang, K.; Tichy, R.S.; Goodenough, J.B. Superior perovskite oxide-ion conductor; strontium-and magnesium-doped LaGaO3: I, phase relationships and electrical properties. J. Am. Ceram. Soc. 1998, 81, 2565–2575. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, X.; Xu, Z.; Deng, H.; Dong, W.; Wang, B.; Wang, H. Semiconductor-Ionic Nanocomposite La0.1Sr0.9MnO3−δ-Ce0.8Sm0.2O2−δ Functional Layer for High Performance Low Temperature SOFC. Materials 2018, 11, 1549. [Google Scholar] [CrossRef]
- Benes, A.; Molinari, A.; Witte, R.; Kruk, R.; Brötz, J.; Chellali, R.; Clemens, O. Proton Conduction in Grain-Boundary-Free Oxygen-Deficient BaFeO2.5+δ Thin Films. Materials 2017, 11, 52. [Google Scholar] [CrossRef]
- Accardo, G.; Dell’Agli, G.; Mascolo, M.C.; Spiridigliozzi, L.; Yoon, S.P. Controlled Coprecipitation of Amorphous Cerium-Based Carbonates with Suitable Morphology as Precursors of Ceramic Electrolytes for IT-SOFCs. Materials 2019, 12, 702. [Google Scholar] [CrossRef]
- Lai, Y.W.; Wei, W.C. Synthesis and Study on Ionic Conductive (Bi1−x, Vx) O1.5−δ Materials with a Dual-Phase Microstructure. Materials 2016, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Perry, N.; Ishihara, T. Roles of bulk and surface chemistry in the oxygen exchange kinetics and related properties of mixed conducting perovskite oxide electrodes. Materials 2016, 9, 858. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.H.; Grilo, J.P.; Loureiro, F.J.; Fagg, D.P.; Fonseca, F.C.; Macedo, D.A. Structure, densification and electrical properties of Gd3+ and Cu2+ co-doped ceria solid electrolytes for SOFC applications: Effects of Gd2O3 content. Ceram. Int. 2018, 44, 2745–2751. [Google Scholar] [CrossRef]
- Bi, L.; Da’as, E.H.; Shafi, S.P. Proton-conducting solid oxide fuel cell (SOFC) with Y-doped BaZrO3 electrolyte. Electrochem. Commun. 2017, 80, 20–23. [Google Scholar] [CrossRef]
- Béchade, E.; Masson, O.; Iwata, T.; Julien, I.; Fukuda, K.; Thomas, P.; Champion, E. Diffusion path and conduction mechanism of oxide ions in apatite-type lanthanum silicates. Chem. Mater. 2009, 21, 2508–2517. [Google Scholar] [CrossRef]
- Bhosale, D.R.; Yusuf, S.M.; Kumar, A.; Mukadam, M.D.; Patil, S.I. High oxide ion conductivity below 500 °C in the garnets LaxY3−xFe5O12+δ. Phys. Rev. Mater. 2017, 1, 015001. [Google Scholar] [CrossRef]
- Berger, C.; Bucher, E.; Egger, A.; Strasser, A.T.; Schrödl, N.; Gspan, C.; Sitte, W. Synthesis and characterization of the novel K2NiF4-type oxide Pr2Ni0.9Co0.1O4+δ. Solid State Ionics 2018, 316, 93–101. [Google Scholar] [CrossRef]
- Yang, G.; Su, C.; Ran, R.; Tade, M.O.; Shao, Z. Advanced symmetric solid oxide fuel cell with an infiltrated K2NiF4-type La2NiO4 electrode. Energy Fuels 2014, 28, 356–362. [Google Scholar] [CrossRef]
- Sayers, R.; Liu, J.; Rustumji, B.; Skinner, S.J. Novel K2NiF4-type materials for solid oxide fuel cells: Compatibility with electrolytes in the intermediate temperature range. Fuel Cells 2008, 8, 338–343. [Google Scholar] [CrossRef]
- Lee, D.; Lee, H. Controlling oxygen mobility in Ruddlesden–Popper oxides. Materials 2017, 10, 368. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen ion conductors. Mater. Today 2003, 6, 30–37. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Fernández-Díaz, M.T.; Aguadero, A. Introduction of interstitial oxygen atoms in the layered perovskite LaSrIn1−xBxO4+δ system (B = Zr, Ti). Solid State Ionics 2015, 282, 82–87. [Google Scholar] [CrossRef]
- Troncoso, L.; Alonso, J.A.; Aguadero, A. Low activation energies for interstitial oxygen conduction in the layered perovskites La1+xSr1−xInO4+δ. J. Mater. Chem. A 2015, 3, 17797–17803. [Google Scholar] [CrossRef]
- Rietveld, H. A profile refinement method for nuclear and magnetic structures. J. Appl. Crystallogr. 1969, 2, 65–71. [Google Scholar] [CrossRef]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Phys. B Condensed Matter 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Caneiro, A.; Bavdaz, P.; Fouletier, J.; Abriata, J.P. Adaptation of an electrochemical system for measurement and regulation of oxygen partial pressure to a symmetrical thermogravimetric analysis system developed using a Cahn 1000 electrobalance. Rev. Sci. Instrum. 1982, 53, 1072–1075. [Google Scholar] [CrossRef]
- Yu, A.; Titov, N.M.; Belyavina, V.; Ya Markiv, M.S.; Slobodyanik, Y.; Krayevska, A. Synthesis and crystal structure of BaLaInO4 and SrLnInO4 (Ln—La, Pr). Dopov. Nats. Akad. Nauk. Ukr. 2009, 160, 166. [Google Scholar]

| Reticular Parameters | 0.0 * | 0.2 | 0.3 | 0.4 |
|---|---|---|---|---|
| a (Å) | 12.6085(6) | 12.6267(9) | 12.640(1) | 12.635(2) |
| b (Å) | 5.8789(3) | 5.9000(4) | 5.9073(5) | 5.9016(8) |
| c (Å) | 5.8338(3) | 5.8421(4) | 5.8546(6) | 5.863(1) |
| V (Å3) | 432.42(4) | 435.23(5) | 437.15(7) | 437.22(11) |
| Atoms | x = 0.0 * | x = 0.2 | x = 0.3 | |
|---|---|---|---|---|
| La/Sr/Ba 8c (x,y,z) | x | 0.1458(8) | 0.1469(8) | 0.1463(1) |
| y | −0.0155(2) | −0.0146(3) | −0.0124(4) | |
| z | 0.9726(2) | 0.9928(6) | 1.0019(9) | |
| B(Å2) | 1.14(5) | 1.52(1) | 1.51(7) | |
| focc | 0.59(1)/0.41(1) | 0.6/0.3/0.1 | 0.6/0.25/0.15 | |
| In 4b (1/2 0 0) | B(Å2) | 0.69(11) | 0.60(1) | 0.68(13) |
| focc | 1.0 | 1.0 | 1.0 | |
| O1 8c (x,y,z) | x | 0.0269(1) | 0.0219(2) | 0.0199(3) |
| y | 0.2161(3) | 0.2322(5) | 0.2407(1) | |
| z | 0.2120(5) | 0.2224(4) | 0.2267(6) | |
| focc | 1.0 | 1.0 | 1.0 | |
| O2 8c (x,y,z) | x | 0.3268(1) | 0.3236(2) | 0.3251(3) |
| y | 0.0807(3) | 0.0752(4) | 0.0672(6) | |
| z | 0.0301(4) | 0.0146(9) | −0.0142(5) | |
| focc | 1.0 | 0.897(2) | 0.906(3) | |
| O3 8c (x,y,z) | x | 0.216(2) | 0.2135(8) | 0.2009(2) |
| y | 0.249(5) | 0.2393(4) | 0.2217(1) | |
| z | 0.267(5) | 0.1901(4) | 0.2196(9) | |
| B(Å2) | 3.88(1) | 3.4(3) | 6.6(1) | |
| focc | 0.054(6) | 0.103(2) | 0.144(3) | |
| Discrepancy Factors | χ2 | 2.80 | 2.36 | 2.72 |
| Rp (%) | 4.44 | 4.39 | 4.38 | |
| Rwp (%) | 5.96 | 5.61 | 5.71 | |
| RBragg (%) | 5.98 | 5.97 | 6.93 | |
| Atom | x | 0.0 * | 0.2 | 0.3 |
|---|---|---|---|---|
| O1 8c (x,y,z) | β11 | 26(3) | 71(5) | 109(8) |
| β22 | 61(10) | 14(11) | 81(16) | |
| β33 | 126(13) | 111(14) | 122(19) | |
| β12 | −27(5) | 5(9) | 16(22) | |
| β13 | 5(5) | −5(8) | 72(17) | |
| β23 | 44(10) | 30(0) | 70(0) | |
| O2 8c (x,y,z) | β11 | 16(2) | 35(3) | 49(5) |
| β22 | 57(9) | 79(11) | 129(18) | |
| β33 | 262(15) | 279(22) | 478(42) | |
| β12 | 13(4) | 17(5) | −14(9) | |
| β13 | 16(6) | −36(15) | −85(22) | |
| β23 | 20(11) | −44(22) | 175(37) |
| x | 0.0 * | 0.2 | 0.3 |
|---|---|---|---|
| La/Sr/Ba–O1 | 2.468(2) | 2.532(3) | 2.553(6) |
| La/Sr/Ba–O1 | 2.695(2) | 3.089(3) | 3.011(6) |
| La/Sr/Ba–O1 | 2.767(2) | 2.788(3) | 2.832(5) |
| La/Sr/Ba–O1 | 2.786(4) | 2.779(6) | |
| La/Sr/Ba–O2 | 2.366(2) | 2.297(3) | 2.311(4) |
| La/Sr/Ba–O2 | 2.636(3) | 2.841(6) | 3.060(10) |
| La/Sr/Ba–O2 | 2.425(2) | 3.092(6) | 2.874(10) |
| La/Sr/Ba–O2 | 2.452(3) | 2.511(4) | |
| La/Sr/Ba–O3 | 2.621(5) | 2.067(12) | 2.01(5) |
| La/Sr/Ba–O3 | 2.694(4) | 2.827(11) | 2.83(4) |
| La/Sr/Ba–O3 | 2.81(2) | 2.559(11) | 2.80(4) |
| La/Sr/Ba–O3 | 2.15(2) | 2.546(12) | 2.48(5) |
| In–O1 (x2) | 2.115(2) | 2.064(3) | 2.042(5) |
| In–O1 (x2) | 2.122(2) | 2.141(3) | 2.155(5) |
| In–O2 (x2) | 2.249(2) | 2.273(3) | 2.248(4) |
| In–O1–In | 155.55(8) | 161.68(10) | 164.34(19) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Troncoso, L.; Mariño, C.; Arce, M.D.; Alonso, J.A. Dual Oxygen Defects in Layered La1.2Sr0.8−xBaxInO4+δ (x = 0.2, 0.3) Oxide-Ion Conductors: A Neutron Diffraction Study. Materials 2019, 12, 1624. https://doi.org/10.3390/ma12101624
Troncoso L, Mariño C, Arce MD, Alonso JA. Dual Oxygen Defects in Layered La1.2Sr0.8−xBaxInO4+δ (x = 0.2, 0.3) Oxide-Ion Conductors: A Neutron Diffraction Study. Materials. 2019; 12(10):1624. https://doi.org/10.3390/ma12101624
Chicago/Turabian StyleTroncoso, Loreto, Carlos Mariño, Mauricio D. Arce, and José Antonio Alonso. 2019. "Dual Oxygen Defects in Layered La1.2Sr0.8−xBaxInO4+δ (x = 0.2, 0.3) Oxide-Ion Conductors: A Neutron Diffraction Study" Materials 12, no. 10: 1624. https://doi.org/10.3390/ma12101624
APA StyleTroncoso, L., Mariño, C., Arce, M. D., & Alonso, J. A. (2019). Dual Oxygen Defects in Layered La1.2Sr0.8−xBaxInO4+δ (x = 0.2, 0.3) Oxide-Ion Conductors: A Neutron Diffraction Study. Materials, 12(10), 1624. https://doi.org/10.3390/ma12101624







