Study on the Product Characteristics of Pyrolysis Lignin with Calcium Salt Additives
Abstract
:1. Introduction
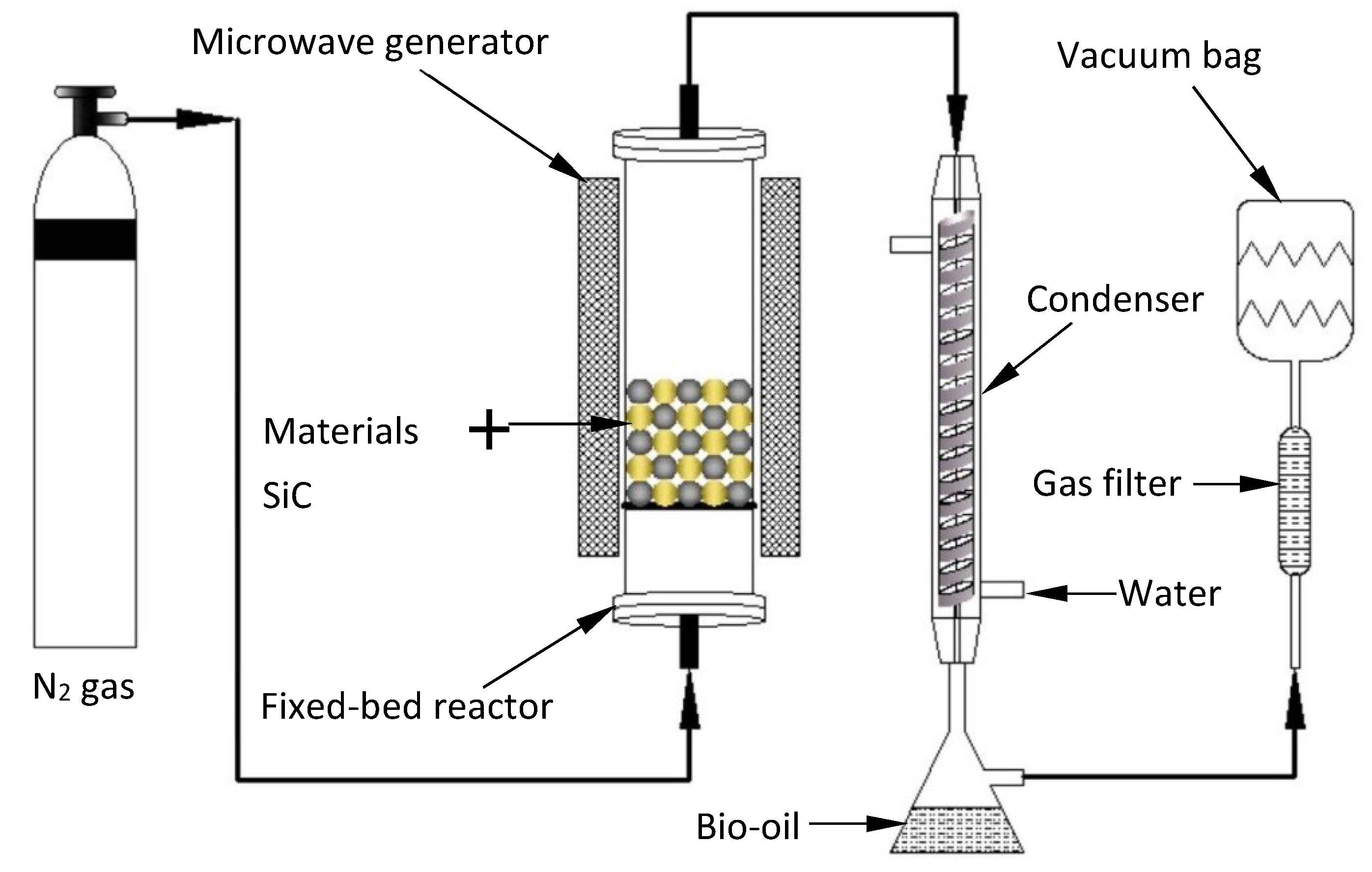
2. Material and Methods
2.1. Material Preparation
2.2. Experimental Methods
3. Results and Discussion
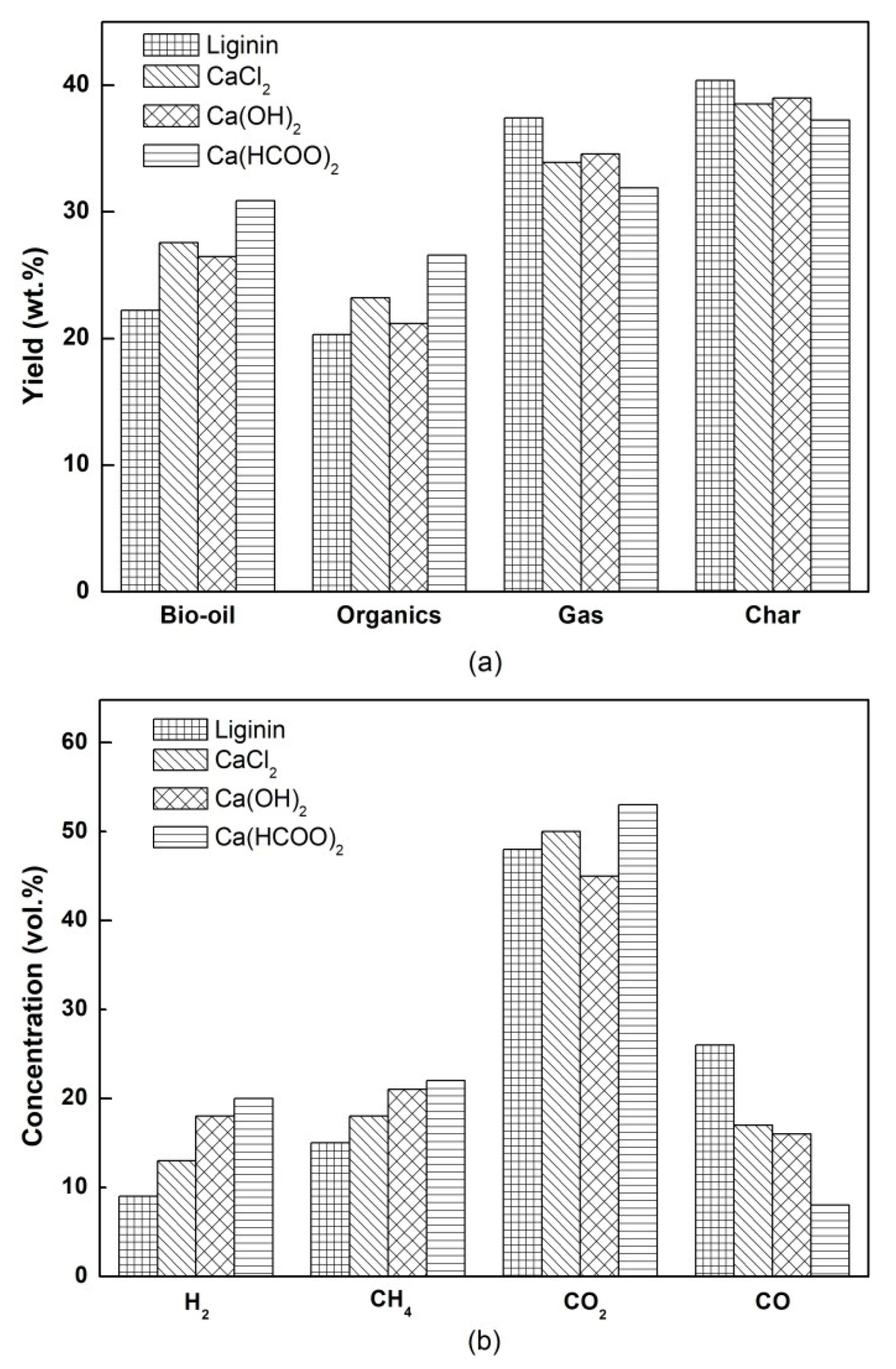
3.1. The Yields of Pyrolysis Products
3.2. The Chemical Composition of Bio-Oil
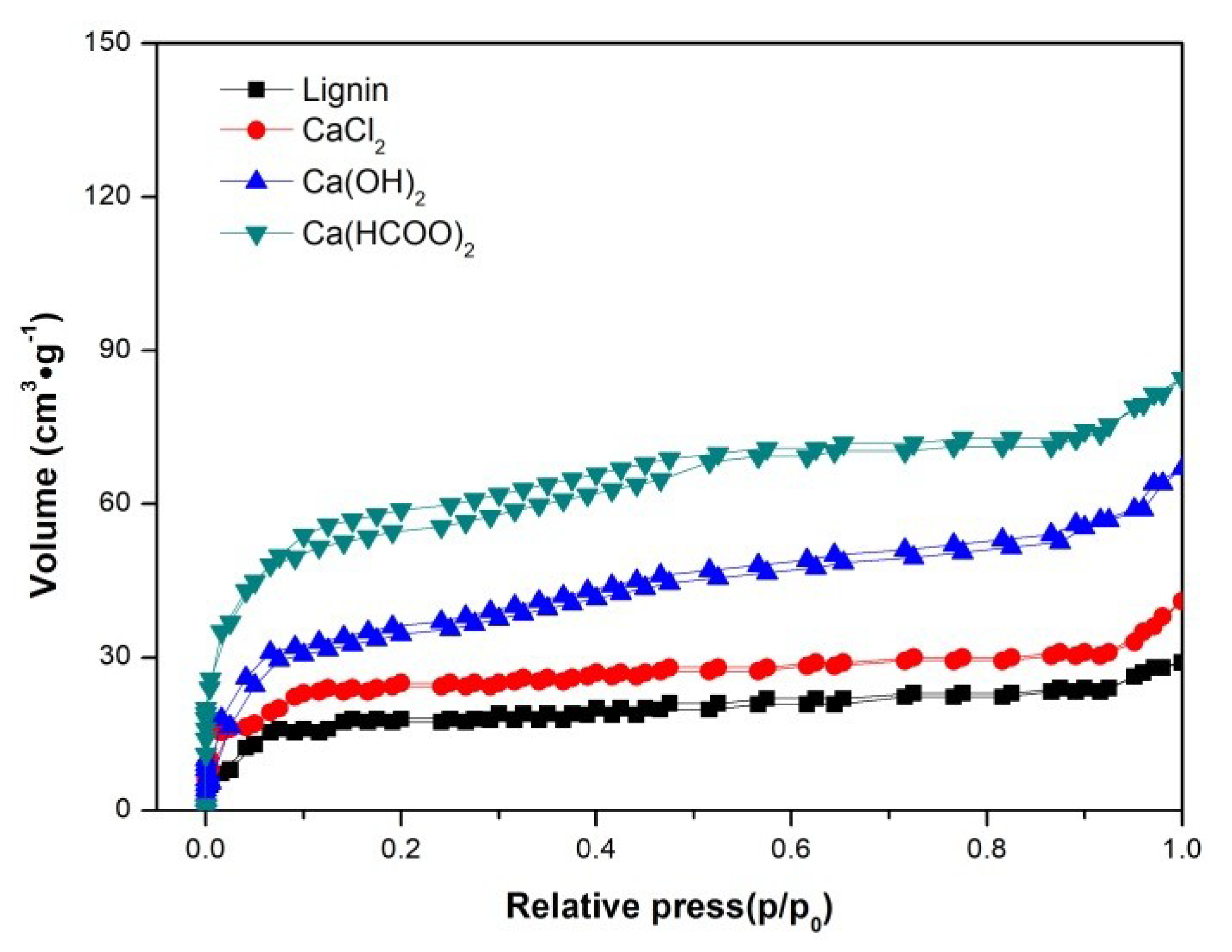
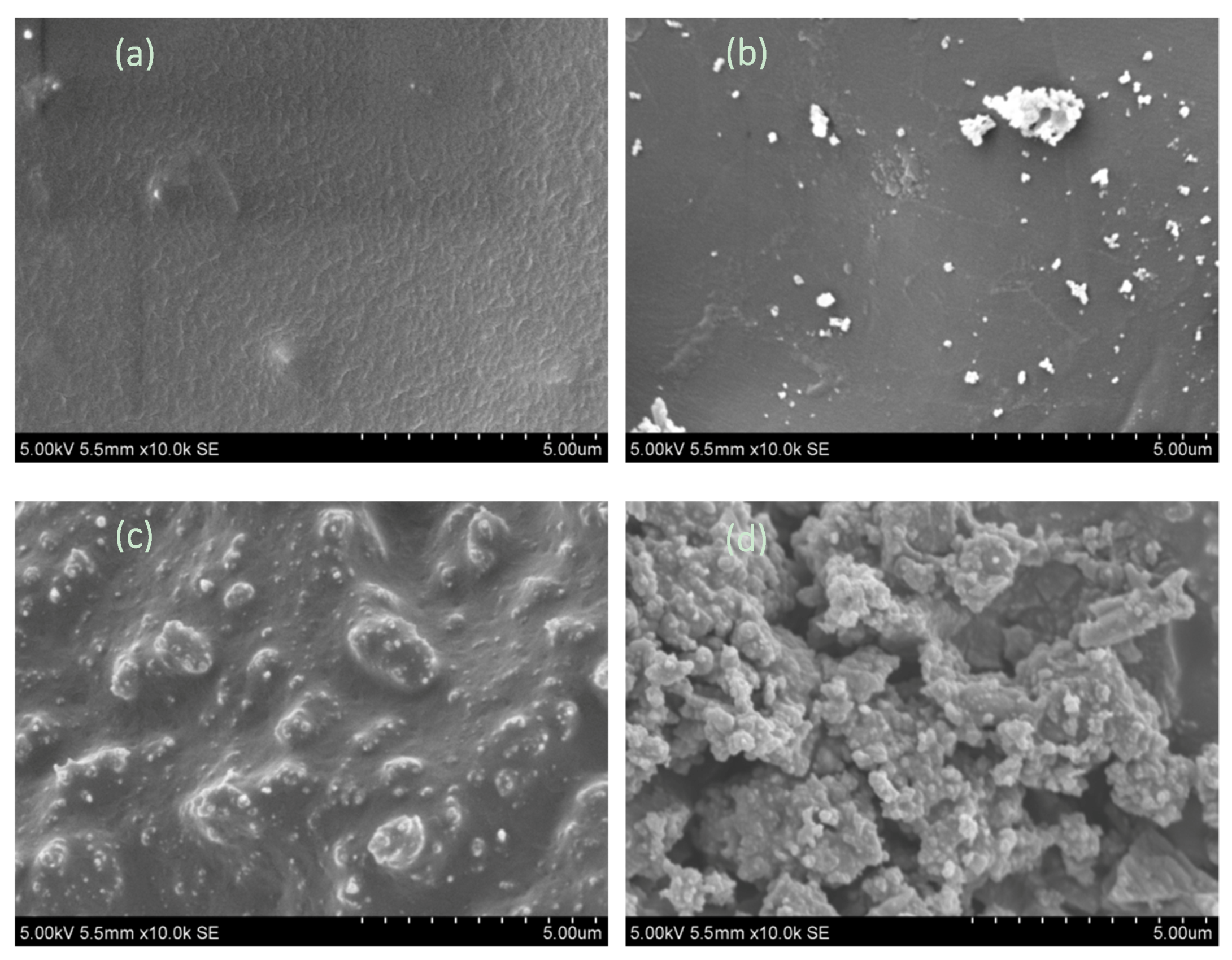
3.3. The Characteristics of Char
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gao, Y.; Yue, Q.; Gao, B.; Sun, Y.; Wang, W.; Li, Q.; Wang, Y. Preparation of high surface area-activated carbon from lignin of papermaking black liquor by KOH activation for Ni(II) adsorption. Chem. Eng. J. 2013, 217, 345–353. [Google Scholar] [CrossRef]
- Zhang, M.; Resende, F.L.P.; Moutsoglou, A. Catalytic fast pyrolysis of aspen lignin via Py-GC/MS. Fuel 2014, 116, 358–369. [Google Scholar] [CrossRef]
- Jeon, M.J.; Jeon, J.K.; Suh, D.J.; Park, S.H.; Sa, Y.J.; Joo, S.H.; Park, Y.-K. Catalytic pyrolysis of biomass components over mesoporous catalysts using Py-GC/MS. Catal. Today 2013, 204, 170–178. [Google Scholar] [CrossRef]
- De Wild, P.; Van der Laan, R.; Kloekhorst, A.; Heeres, E. Lignin Valorisation for Chemicals and (Transportation) Fuels via (Catalytic) Pyrolysis and Hydrodeoxygenation. Environ. Prog. Sustain. Energy 2010, 28, 461–469. [Google Scholar] [CrossRef]
- Stephanidis, S.; Nitsos, C.; Kalogiannis, K.; Iliopoulou, E.F.; Lappas, A.A.; Triantafyllidis, K.S. Catalytic upgrading of lignocellulosic biomass pyrolysis vapours: Effect of hydrothermal pre-treatment of biomass. Catal. Today 2011, 167, 37–45. [Google Scholar] [CrossRef]
- French, R.; Czernik, S. Catalytic pyrolysis of biomass for biofuels production. Fuel Process. Technol. 2010, 91, 25–32. [Google Scholar] [CrossRef]
- Pütün, E. Catalytic pyrolysis of biomass: Effects of pyrolysis temperature, sweeping gas flow rate and MgO catalyst. Energy 2010, 35, 2761–2766. [Google Scholar] [CrossRef]
- Zhou, L.; Yang, H.; Wu, H.; Wang, M.; Cheng, D. Catalytic pyrolysis of rice husk by mixing with zinc oxide: Characterization of bio-oil and its rheological behavior. Fuel Process. Technol. 2013, 106, 385–391. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, Q.; Zhang, Q.; Liu, Q.; Chen, L.; Li, Y.; Wang, C.; Ma, L. Aromatic fuel production from phenolics by catalytic hydrodeoxygenation over novel mo-based catalyst. Energy Procedia 2019, 158, 984–990. [Google Scholar] [CrossRef]
- Hossein, J.; Agblevor, F.A. Hydrodeoxygenation of aqueous phase catalytic pyrolysis oil to liquid hydrocarbons using multi-functional nickel catalyst. Ind. Eng. Chem. Res. 2018, 57, 13257–13268. [Google Scholar]
- Yuan, R.; Yu, S.L.; Shen, Y.F. Pyrolysis and combustion kinetics of lignocellulosic biomass pellets with calcium-rich wastes from agro-forestry residues. Waste Manag. 2019, 87, 86–96. [Google Scholar] [CrossRef]
- Veksha, A.; Giannis, A.; Oh, W.D.; Lisak, G. Catalytic processing of non-condensable pyrolysis gas from plastics: Effects of calcium supports on nickel-catalyzed decomposition of hydrocarbons and HCl sorption. Chem. Eng. Sci. 2018, 189, 311–319. [Google Scholar] [CrossRef]
- Zhou, S.; Brown, R.C.; Bai, X.L. The use of calcium hydroxide pretreatment to overcome agglomeration of technical lignin during fast pyrolysis. Green Chem. 2015, 17, 4748–4759. [Google Scholar] [CrossRef]
- Mukkamala, S.; Wheeler, M.C.; van Heiningen, A.R.; DeSisto, W.J. Formate-Assisted Fast Pyrolysis of Lignin. Energy Fuels 2012, 26, 1380–1384. [Google Scholar] [CrossRef]
- Wang, W.L.; Ren, X.Y.; Li, L.F.; Chang, J.M.; Cai, L.P.; Geng, J. Catalytic effect of metal chlorides on analytical pyrolysis of alkali lignin. Fuel Process. Technol. 2015, 134, 345–351. [Google Scholar] [CrossRef]
- Wang, W.L.; Yu, Y.X.; Chang, J.M.; Bai, T.T. Comparative analysis of micro-crystal structures and bio-oil from pyrolysis of two barks. J. Fuel Chem. Technol. 2013, 41, 1310–1315. [Google Scholar]
- Peng, C.; Zhang, G.; Yue, J.; Xu, G. Pyrolysis of lignin for phenols with alkaline additive. Fuel Process. Technol. 2014, 124, 212–221. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Pyrolysis behaviors of wood and its constituent polymers at gasification temperature. J. Anal. Appl. Pyrolysis 2007, 78, 328–336. [Google Scholar] [CrossRef]
- Raveendran, K.; Ganesh, A.; Khilar, K.C. Pyrolysis characteristics of biomass and biomass components. Fuel 1996, 75, 987–998. [Google Scholar] [CrossRef]
- Nowakowski, D.J.; Bridgwater, A.V.; Elliott, D.C.; Meier, D.; de Wild, P. Lignin fast pyrolysis: Results from an international collaboration. J. Anal. Appl. Pyrolysis 2010, 88, 53–72. [Google Scholar] [CrossRef]
- Wang, W.L.; Ren, X.Y.; Chang, J.M.; Cai, L.P.; Shi, S.Q. Characterization of bio-oils and bio-chars obtained from the catalytic pyrolysis of alkali lignin with metal chlorides. Fuel Process. Technol. 2015, 138, 605–611. [Google Scholar] [CrossRef]
- Collard, F.X.; Blin, J.; Bensakhria, A.; Valette, J. Influence of impregnated metal on the pyrolysis conversion of biomass constituents. J. Anal. Appl. Pyrolysis 2012, 95, 213–226. [Google Scholar] [CrossRef]
- López, M.B.; Blanco, C.G.; Martínez-Alonso, A.; Tascón, J.M.D. Composition of gases released during olive stones pyrolysis. J. Anal. Appl. Pyrolysis 2002, 65, 313–322. [Google Scholar] [CrossRef]
- Liu, Q.; Wang, S.; Zheng, Y.; Luo, Z.; Cen, K. Mechanism study of wood lignin pyrolysis by using TG–FTIR analysis. J. Anal. Appl. Pyrolysis 2008, 82, 170–177. [Google Scholar] [CrossRef]
- Uddin, M.A.; Tsuda, H.; Wu, S.; Sasaoka, E. Catalytic decomposition of biomass tars with iron oxide catalysts. Fuel 2008, 87, 451–459. [Google Scholar]
- Yu, Y.; Li, X.; Su, L.; Zhang, Y.; Wang, Y.; Zhang, H. The role of shape selectivity in catalytic fast pyrolysis of lignin with zeolite catalysts. Appl. Catal. A Gen. 2012, 447–448, 115–123. [Google Scholar] [CrossRef]
- Ben, H.; Ragauskas, A.J. Pyrolysis of Kraft Lignin with Additives. Energy Fuels 2011, 25, 4662–4668. [Google Scholar] [CrossRef]
- Ma, Z.; Custodis, V.; Van Bokhoven, J.A. Selective deoxygenation of lignin during catalytic fast pyrolysis. Catal. Sci. Technol. 2014, 4, 766. [Google Scholar] [CrossRef]
- Lv, G.; Wu, S.; Lou, R.; Yang, Q. Analytical Pyrolysis Characteristics of Enzymatic/Mild Acidolysis Lignin from sugarcane bagasse. Cellul. Chem. Technol. 2010, 44, 335–343. [Google Scholar]
- Wang, S.; Wang, K.; Liu, Q.; Gu, Y.; Luo, Z.; Cen, K.; Fransson, T. Comparison of the pyrolysis behavior of lignins from different tree species. Biotechnol. Adv. 2009, 27, 562–567. [Google Scholar] [CrossRef]
- Guo, D.L.; Wu, S.B.; Liu, B.; Yin, X.L.; Yang, Q. Catalytic effects of NaOH and Na2CO3 additives on alkali lignin pyrolysis and gasification. Appl. Energy 2012, 95, 22–30. [Google Scholar] [CrossRef]
- Hosoya, T.; Kawamoto, H.; Saka, S. Secondary reactions of lignin-derived primary tar components. J. Anal. Appl. Pyrolysis 2008, 83, 78–87. [Google Scholar] [CrossRef]
- Geng, J.; Wang, W.L.; Yu, Y.X.; Chang, J.M.; Cai, L.P.; Shi, S.Q. Adding Nickel Formate in Alkali Lignin to Increase Contents of Alkylphenols and Aromatics during Fast Pyrolysis. Bioresour. Technol. 2017, 227, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Chang, J.M.; Wang, W.L.; Li, B.; Ren, X.Y. Preparation of activated carbon using bio-oil phenol-formaldehyde resin. Bioresources 2015, 10, 3865–3873. [Google Scholar] [CrossRef]
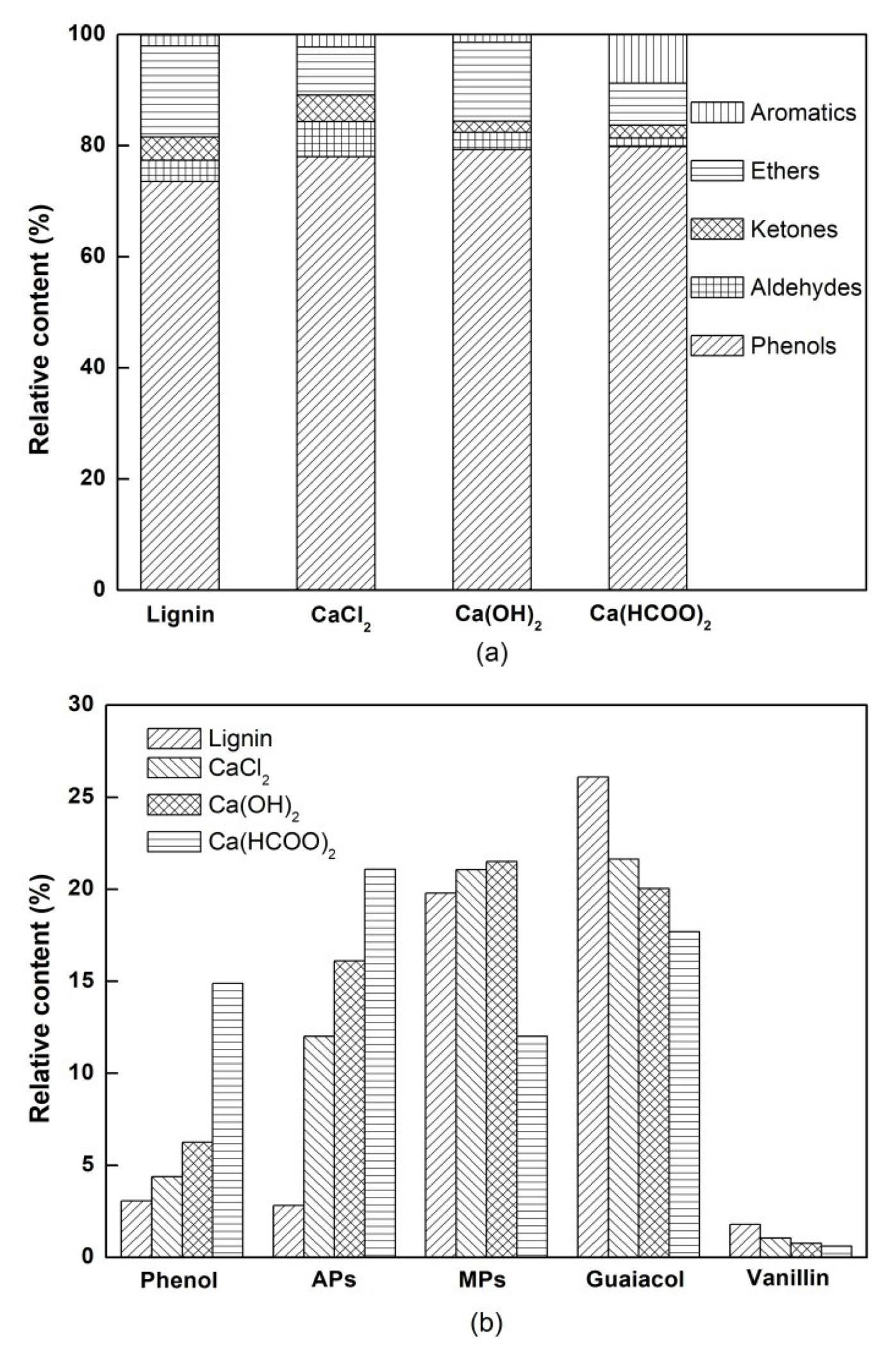
| No. | Name of Compounds | Relative Content (Area%) | |||
|---|---|---|---|---|---|
| Lignin | CaCl2 | Ca(OH)2 | Ca(HCOO)2 | ||
| Phenols | 73.65 | 78.03 | 79.29 | 79.86 | |
| 1 | Phenol | 3.08 | 4.39 | 6.25 | 14.89 |
| 2 | 2-Methylphenol | 0.82 | 1.98 | 2.19 | 3.22 |
| 3 | 4-Methylphenol | 0.34 | 1.18 | 1.81 | 3.36 |
| 4 | Guaiacol | 26.1 | 21.63 | 20.04 | 17.68 |
| 5 | 2,4-Xylenol | — | 0.56 | 1.09 | 0.98 |
| 6 | 2,6-Xylenol | — | — | 0.71 | 1.48 |
| 7 | 2-Ethylphenol | 0.78 | 0.97 | 1.3 | 1.61 |
| 8 | 4-Ethylphenol | 0.21 | 5.34 | 5.78 | 7.43 |
| 9 | 4-Ethyl-2-methylphenol | 0.67 | 1.98 | 3.22 | 3.01 |
| 10 | 2-Methoxy-5-methylphenol | 5.93 | 5.05 | 5.68 | 4.96 |
| 11 | 2-Methoxy-4-methylphenol | 9.27 | 9.68 | 7.67 | 2.13 |
| 12 | 3-Methylcatechol | 1.25 | 2.18 | 2.04 | 3.89 |
| 13 | 3-Methoxycatechol | 1.02 | 0.89 | 0.98 | 0.52 |
| 14 | 2-Methoxy-4-ethylphenol | 3.55 | 4.87 | 5.53 | 3.73 |
| 15 | 2,6-Xylohydroquinone | — | 0.61 | 0.78 | 0.68 |
| 16 | 4-Hydroxy-3-methylacetophenone | 2.37 | 3.08 | 2.1 | 1.79 |
| 17 | 2,6-Dimethoxyphenol | 5.48 | 2.83 | 2.62 | 2.01 |
| 18 | 3-Allyl-2-methoxyphenol | — | 0.79 | 1.58 | 0.95 |
| 19 | 2-Methoxy-4-propylphenol | — | 0.67 | 0.89 | 0.73 |
| 20 | Vanillin | 1.79 | 1.05 | 0.76 | 0.62 |
| 21 | 2-Methoxy-4-propenylphenol | 1.04 | 0.79 | 1.73 | 0.46 |
| 22 | cis-Isoeugenol | — | 2.05 | — | 0.19 |
| 23 | Guaiacylacetone | — | 1.22 | 0.49 | 0.74 |
| 24 | Apocynin | 1.04 | 2.91 | 1.43 | 1.71 |
| 25 | Homovanillic acid | 0.57 | 1.02 | 0.47 | 1.09 |
| 26 | Salicylaldehyde | — | 0.31 | 1.03 | — |
| 27 | Isovanillin | 8.34 | — | 1.12 | — |
| Aldehydes | 3.82 | 6.02 | 3.09 | 1.53 | |
| 1 | Veratraldehyde | 3.82 | 6.02 | 3.09 | 1.53 |
| Ketones | 4.22 | 4.78 | 2.01 | 2.32 | |
| 1 | Acetone | 1.02 | 2.76 | — | — |
| 2 | 3-Ethyl-2-hydroxy-2-cyclopenten-1-one | — | 2.02 | — | — |
| 3 | 2-Methyl-2-cyclopentenone | 1.08 | — | 2.01 | 0.92 |
| 4 | 3-Methyl-2-cyclopentenone | 2.12 | — | — | 0.78 |
| 5 | Methycyclopentenolone | — | — | — | 0.62 |
| Ethers | 16.35 | 8.61 | 14.19 | 7.56 | |
| 1 | 4-Methoxystyrene | 1.62 | — | 2.08 | 1.21 |
| 2 | 4-Methylanisole | 0.36 | — | 0.8 | 0.86 |
| 3 | Veratrole | 9.02 | 4.22 | 8.2 | 2.45 |
| 4 | 4-Ethylveratrol | 3.81 | 2.92 | 1.08 | 2.03 |
| 5 | 4-Ethyl-1,2-dimethoxybenzene | — | 0.97 | 1.64 | 1.01 |
| 6 | Methylisoeugenol | 1.54 | 0.5 | 0.39 | — |
| AromaticHydrocarbons | 1.96 | 2.56 | 1.42 | 8.73 | |
| 1 | Toluene | — | 1.21 | 0.87 | 2.63 |
| 2 | Benzene | — | 0.18 | — | 0.32 |
| 3 | 2-Isopropyltoluene | 0.41 | 1.03 | — | 2.22 |
| 4 | 3-Isopropyltoluene | 0.22 | — | 0.55 | 2.23 |
| 5 | Naphthalene | 0.81 | — | — | 0.21 |
| 6 | Anthracene | 0.52 | 0.14 | — | — |
| 7 | 2,4-Dimethyl styrene | — | — | — | 1.12 |
| Samples | SBET | Total Pore Volume | Average Pore Diameter |
|---|---|---|---|
| (m2·g−1) | (cm3·g−1) | (nm) | |
| Lignin | 40.62 | 0.035 | 3.078 |
| CaCl2 | 62.16 | 0.051 | 3.091 |
| Ca(OH)2 | 135.68 | 0.115 | 3.012 |
| Ca(HCOO)2 | 175.31 | 0.152 | 3.005 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Wang, W.; Chang, J. Study on the Product Characteristics of Pyrolysis Lignin with Calcium Salt Additives. Materials 2019, 12, 1609. https://doi.org/10.3390/ma12101609
Cui Y, Wang W, Chang J. Study on the Product Characteristics of Pyrolysis Lignin with Calcium Salt Additives. Materials. 2019; 12(10):1609. https://doi.org/10.3390/ma12101609
Chicago/Turabian StyleCui, Yong, Wenliang Wang, and Jianmin Chang. 2019. "Study on the Product Characteristics of Pyrolysis Lignin with Calcium Salt Additives" Materials 12, no. 10: 1609. https://doi.org/10.3390/ma12101609
APA StyleCui, Y., Wang, W., & Chang, J. (2019). Study on the Product Characteristics of Pyrolysis Lignin with Calcium Salt Additives. Materials, 12(10), 1609. https://doi.org/10.3390/ma12101609




