Thermal Decomposition and Ceramifying Process of Ceramifiable Silicone Rubber Composite with Hydrated Zinc Borate
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of the Ceramifiable Silicone Rubber Composites
2.3. Sample Combustion
2.4. Characterization
2.4.1. Density and Shore A hardness
2.4.2. Tensile Strength and Elongation at Break
2.4.3. Thermogravimetric Analysis
2.4.4. Fourier Transform Infrared Spectroscopy
2.4.5. X-ray Diffraction Analysis
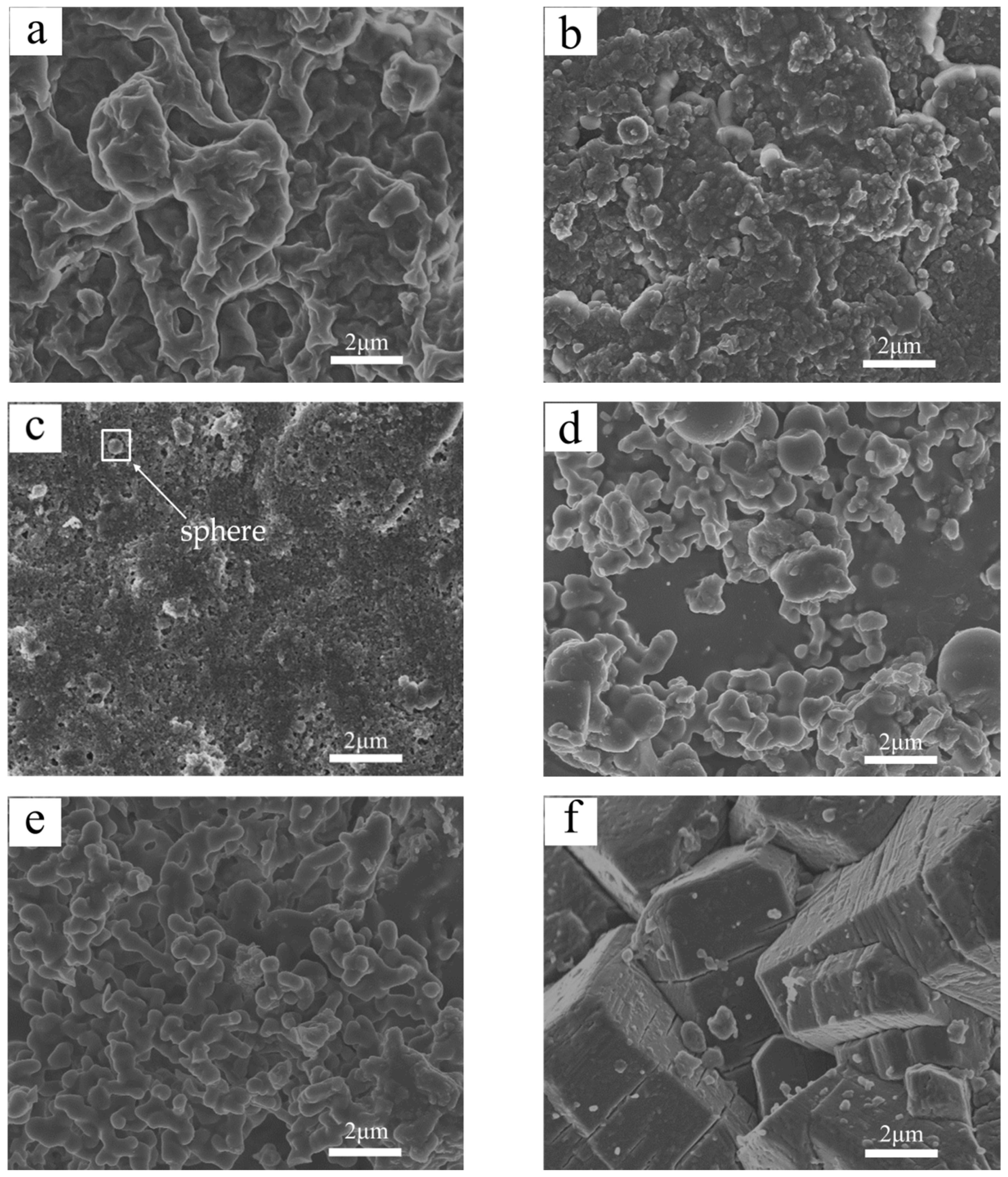
2.4.6. Morphology
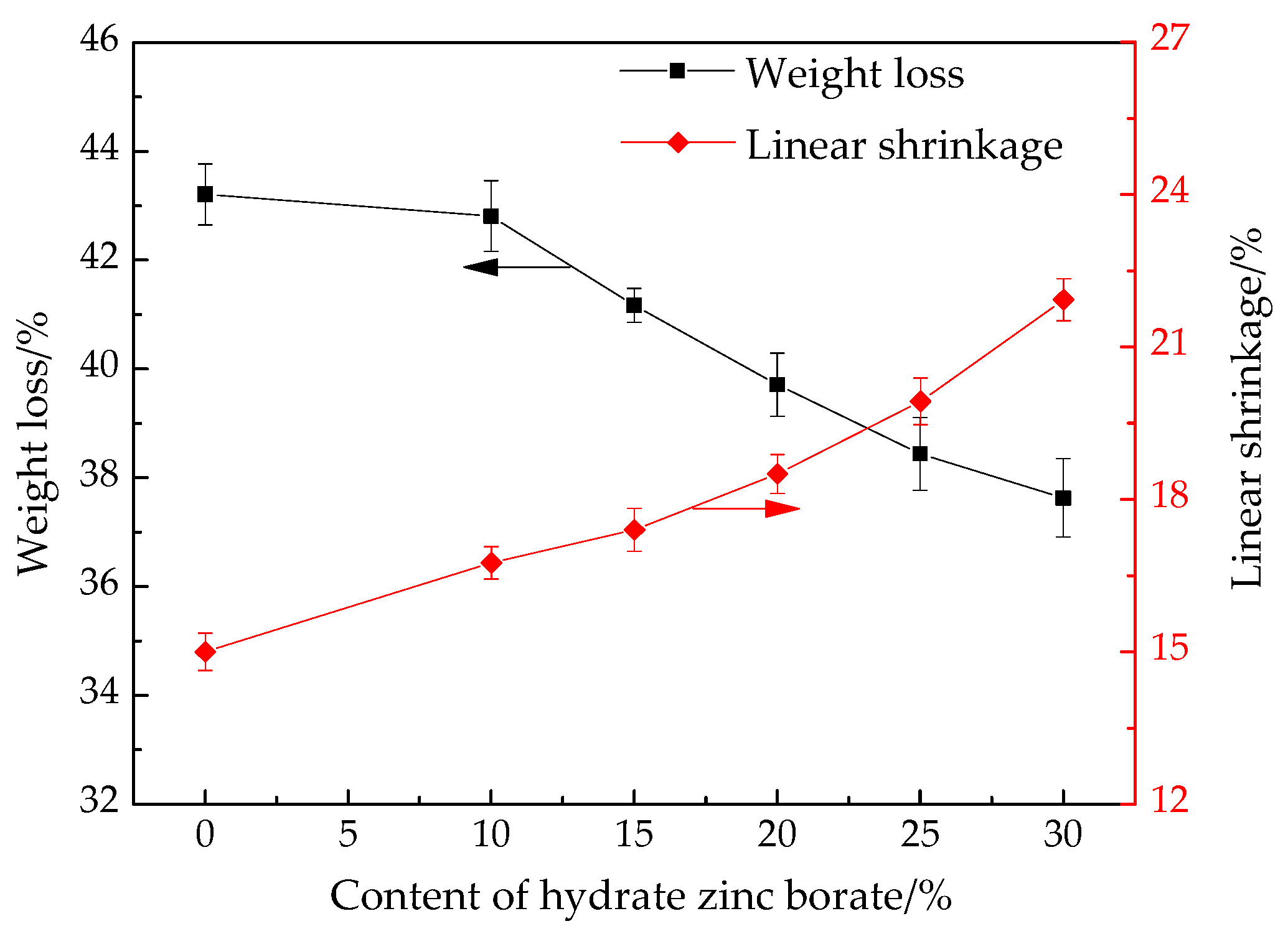
2.4.7. Weight Loss and Linear Shrinkage
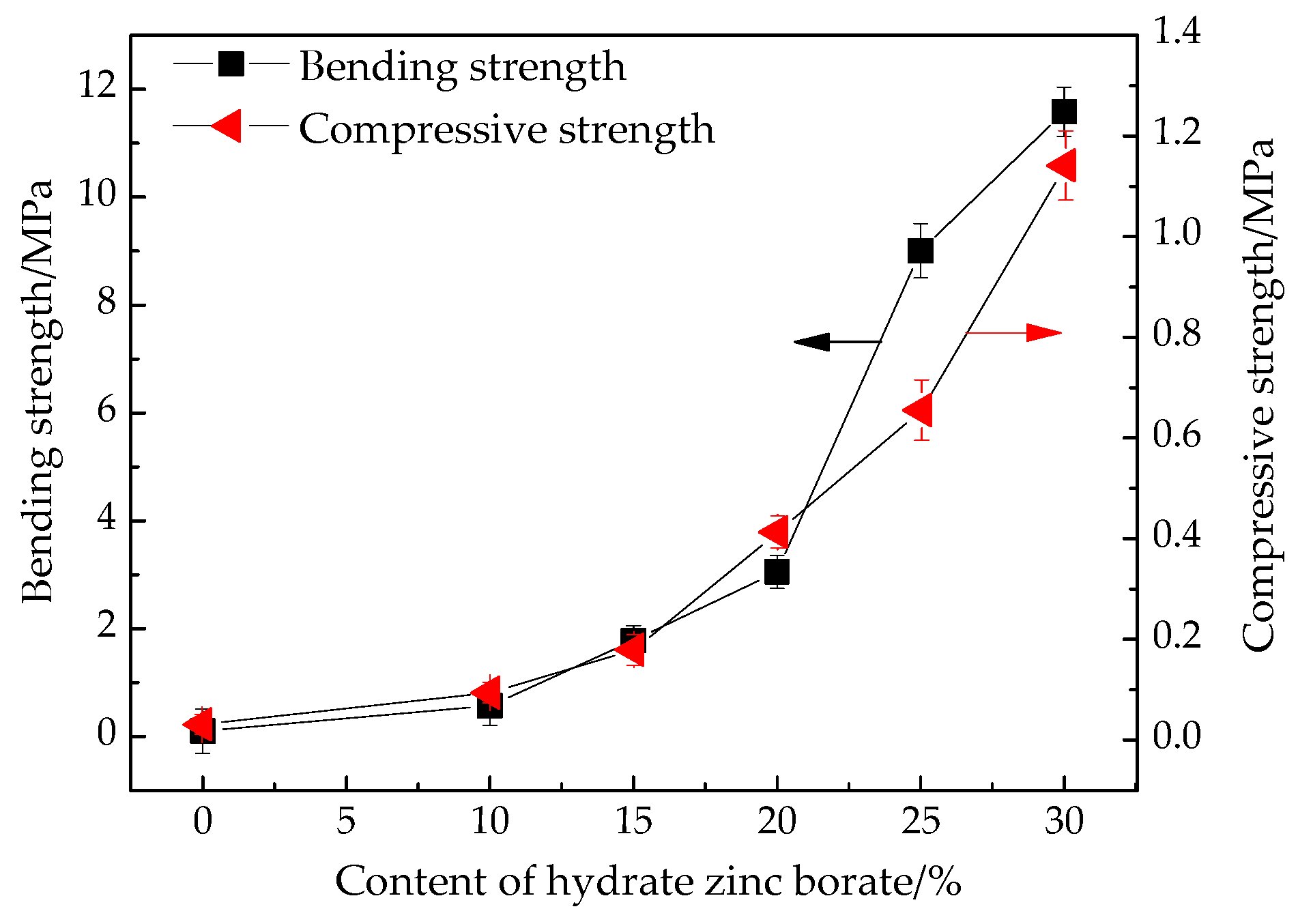
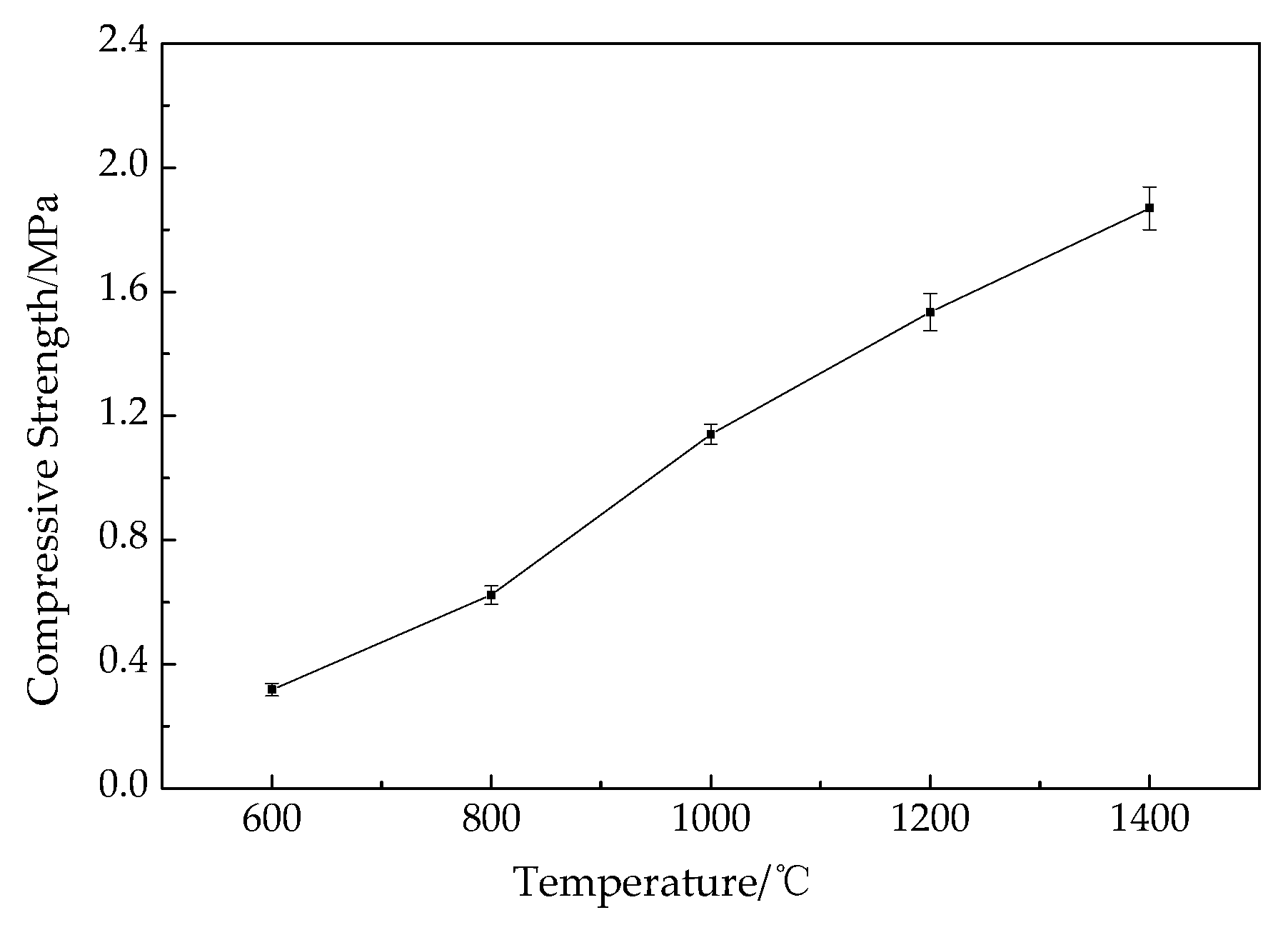
2.4.8. Bending and Compressive Strength
3. Results and Discussion
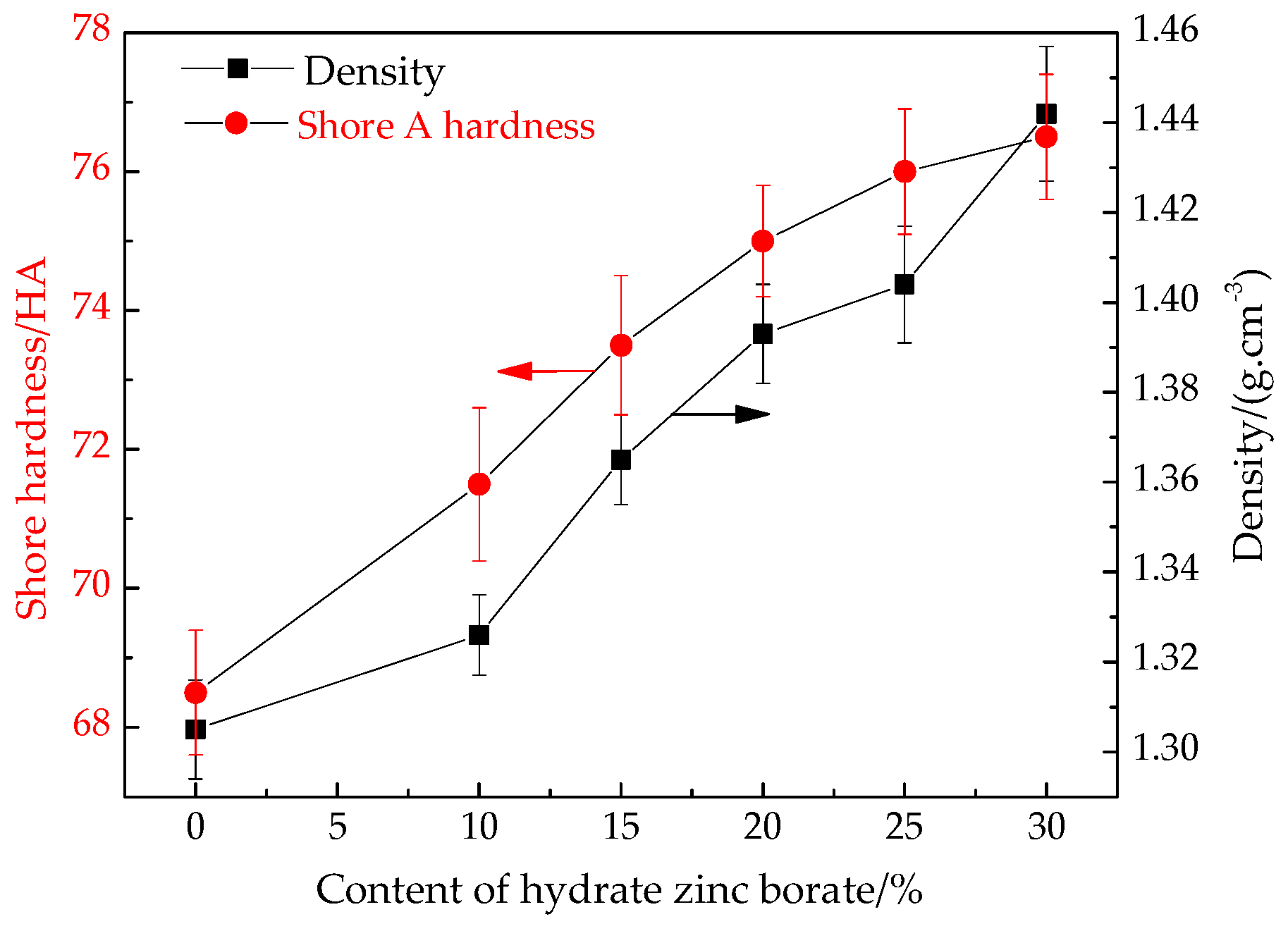
3.1. Density and Hardness of Ceramifiable Silicone Rubber Composites
3.2. Mechanical Properties of Ceramifiable Silicone Rubber Composites
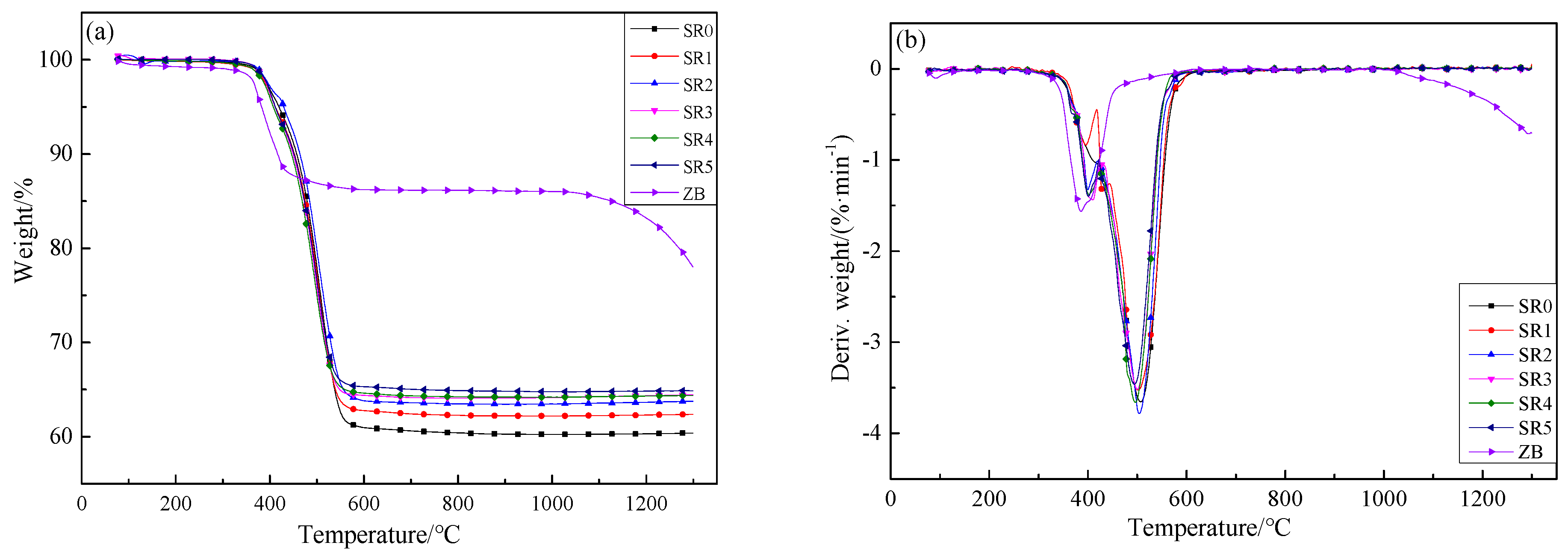
3.3. Thermal Gravimetric Analysis (TGA)
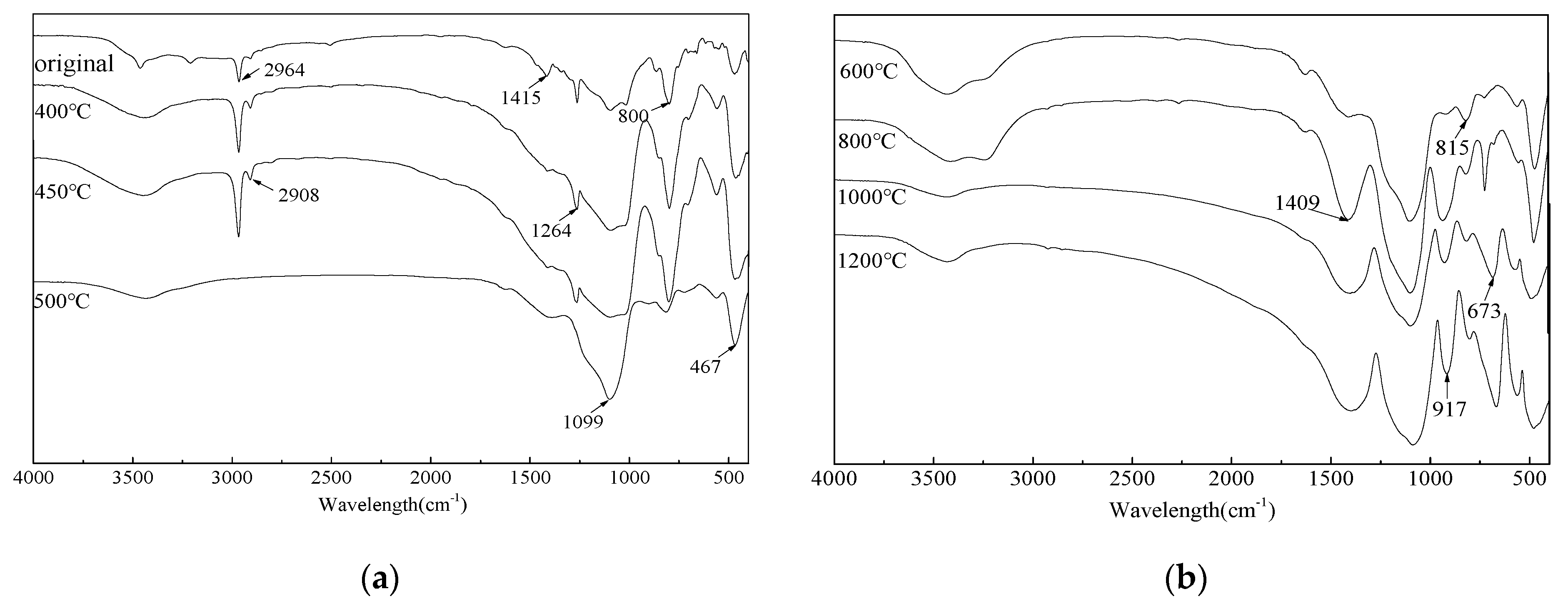
3.4. FTIR Analysis
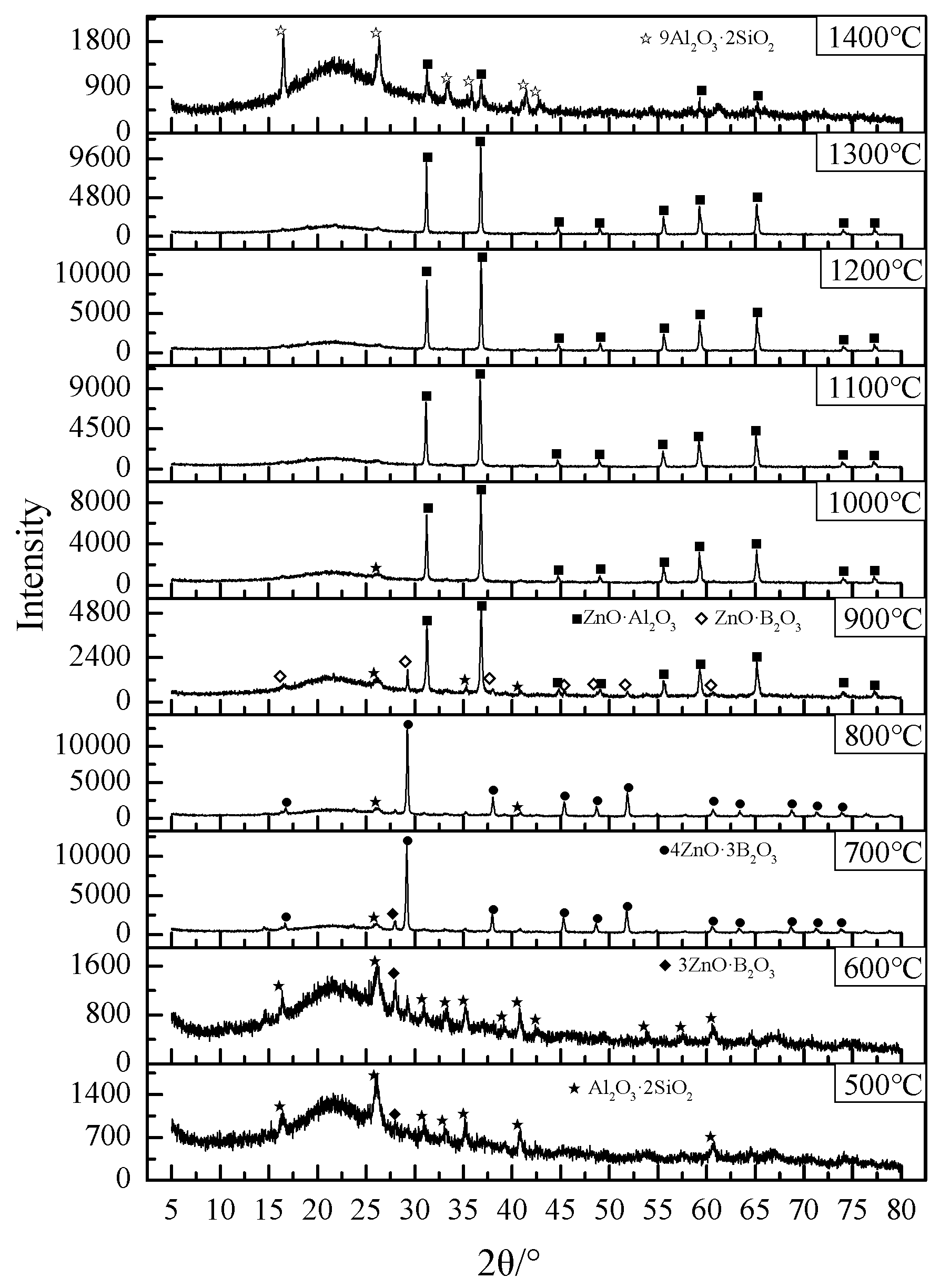
3.5. XRD Analysis
3.6. Microstructure of the Residue
3.7. Weight Loss and Linear Shrinkage of Ceramic Residue
3.8. Bending and Compressive Strength of the Residue
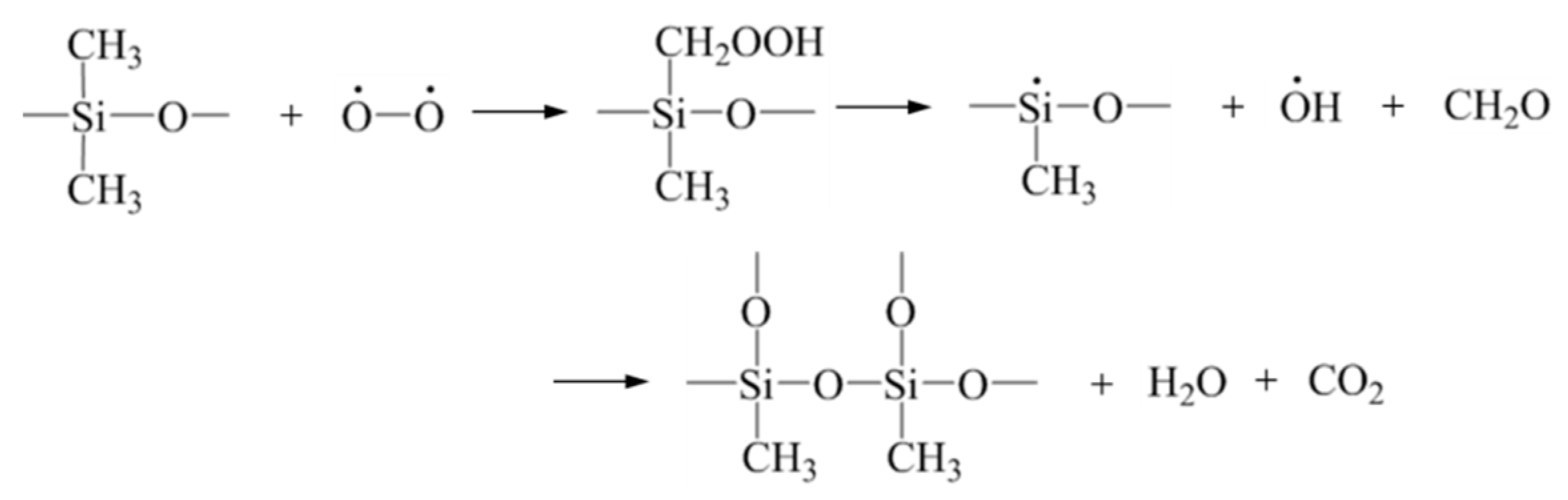
3.9. Ceramization Mechanism
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- George, K.; Panda, B.P.; Mohanty, S.; Nayak, S.K. Recent developments in elastomeric heat shielding materials for solid rocket motor casing application for future perspective. Polym. Adv. Technol. 2018, 29, 8–21. [Google Scholar] [CrossRef]
- Mansouri, J.; Wood, C.A.; Roberts, K.; Cheng, Y.B.; Burford, R.P. Investigation of the ceramifying process of modified silicone-silicate compositions. J. Mater. Sci. 2007, 42, 6046–6055. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-Cuesta, J.M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Genovese, A.; Shanks, R.A. Fire performance of poly(dimethyl siloxane) composites evaluated by cone calorimetry. Compos. Part A. 2008, 39, 398–405. [Google Scholar] [CrossRef]
- Hanu, L.G.; Simon, G.P.; Mansouri, J.; Burford, R.P.; Cheng, Y.B. Development of polymer Ceramic Composites for Improved Fire Resistance. J. Mater. Pro. Tech. 2004, 153, 401–407. [Google Scholar] [CrossRef]
- Hamdani-Devarennes, S.; Pommier, A.; Longuet, C.; Lopez-Cuesta, J.M.; Ganachaud, F. Ganachaud, Calcium and aluminium-basedfillers as flame-retardant additives in silicone matrices II. Analyses on composite residues from an industrial-based pyrolysis test. Polym. Degrad. Stab. 2011, 96, 1562–1572. [Google Scholar] [CrossRef]
- Hamdani-Devarennes, S.; Longuet, C.; Sonnier, R.; Ganachaud, F.; LopezCuesta, J.M. Calcium and aluminum-basedfillers as flame-retardant additives in silicone matrices. III. Investigations onfire reaction. Polym. Degrad. Stab. 2013, 98, 2021–2032. [Google Scholar] [CrossRef]
- Hanu, L.G.; Simon, G.P.; Cheng, Y.B. Thermal stability and flammability of silicone polymer composites. Polym. Degrad. Stab. 2006, 91, 1373–1379. [Google Scholar] [CrossRef]
- Yu, L.; Zhou, S.T.; Zou, H.W.; Liang, M. Thermal stability and ablation properties study of aluminum silicate ceramic fiber and acicular wollastonite filled silicone rubber composite. J. Appl. Polym. Sci. 2014, 131, 7. [Google Scholar] [CrossRef]
- Zhuo, D.X.; Gu, A.J.; Liang, G.Z.; Hu, J.T.; Cao, L.; Yuan, L. Flame retardancy and flame retarding mechanism of high performance hyper branched polysiloxane modified bismaleimide/cyanate ester resin. Polym. Degrad. Stab. 2011, 96, 505–514. [Google Scholar] [CrossRef]
- Hayashida, K.; Tsuge, S.; Ohtani, H. Flame retardant mechanism of polydimethylsiloxane material containing platinum compound studied by analytical pyrolysis techniques and alkaline hydrolysis gas chromatography. Polymer 2003, 44, 5611–5616. [Google Scholar] [CrossRef]
- Anyszka, R.; Bieliński, D.M.; Pedzich, Z.; Szumera, M. Influence of surfaces of modified montmorillonites on properties of silicone rubber-based ceramizable composites. J. Therm. Anal. Calorim. 2015, 119, 111–121. [Google Scholar] [CrossRef]
- Hshieh, F.Y. Shielding effects of silica-ash layer on the composition of silicones and their possible applications on the fire retardancy of organic polymers. Fire. Mater. 1998, 22, 69–76. [Google Scholar] [CrossRef]
- Zhang, G.W.; Wang, F.Z.; Huang, Z.X.; Dai, J.; Shi, M.X. Improved ablation resistance of silicone rubber composites by introducing montmorillonite and silicon carbide whisker. Materials 2016, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.W.; Wang, F.Z.; Dai, J.; Huang, Z.X. Effect of functionalization of graphene nanoplatelets on the mechanical and thermal properties of silicone rubber composites. Materials 2016, 9, 92. [Google Scholar] [CrossRef]
- Mansouri, J.; Burford, R.P.; Cheng, Y.B. Pyrolysis behaviour of silicone-based ceramifying composites. Mater. Sci. Eng. A-Struct. 2006, 425, 7–14. [Google Scholar] [CrossRef]
- Mansouri, J.; Burford, R.P.; Cheng, Y.B.; Hanu, L. Formation of strong ceramified ash from silicone-based compositions. J. Mater. Sci. 2005, 40, 5741–5749. [Google Scholar] [CrossRef]
- Hanu, L.G.; Simon, G.P.; Cheng, Y.B. Preferential orientation of muscovite in ceramifiable silicone composites. Mater. Sci. Eng. A-Struct. 2005, 398, 180–187. [Google Scholar] [CrossRef]
- Guo, J.H.; Gao, W.; Wang, Y.; Liang, D.; Li, H.J.; Zhang, X. Effect of glass frit with low softening temperature on the properties, microstructure and formation mechanism of polysiloxane elastomer-based ceramizable composites. Polym. Degrad. Stab. 2017, 136, 71–79. [Google Scholar] [CrossRef]
- Anyszka, R.; Bielinski, D.M.; Jedrzejczyk, M. Thermal behavior of silicone rubber-based ceramizable composites characterized by Fourier transform infrared (FT-IR) spectroscopy and microcalorimetry. Appl. Spectrosc. 2013, 67, 1437–1440. [Google Scholar] [CrossRef]
- Wang, J.H.; Ji, C.T.; Yan, Y.T.; Zhao, D.; Shi, L.Y. Mechanical and ceramifiable properties of silicone rubber filled with different inorganic fillers. Polym. Degrad. Stab. 2015, 121, 149–156. [Google Scholar] [CrossRef]
- Laoutid, F.; Bonnaud, L.; Alexandre, M.; Lopez-Cuesta, J.M.; Dubois, P. New prospects in flame retardant polymer materials: From fundamentals to nanocomposites. Mater. Sci. Eng. R. 2009, 63, 100–125. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, W.; Yao, R.L.; Jiang, B.Z. Thermal stability enhancement mechanism of poly(dimethylsiloxane) composite by incorporating octavinyl polyhedral oligomeric silsesquioxanes. Polym. Degrad. Stab. 2013, 98, 109–114. [Google Scholar] [CrossRef]
- Zhou, C.; Yu, L.; Luo, W.; Chen, Y.; Zou, H.W.; Liang, M. Ablation properties of aluminum silicate ceramic fibers and calcium carbonate filled silicone rubber composites. J. Appl. Polym. Sci. 2015, 132, 41619. [Google Scholar] [CrossRef]
- Amin, M.; Akbar, M.; Amin, S. Hydrophobicity of silicone rubber used for outdoor insulation (an overview). Rev. Adv. Mater. Sci. 2007, 16, 10–26. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lageard, M. Polydimethylsiloxane thermal degradation Part 2. The degradation mechanisms. Polymer 2002, 43, 2011–2015. [Google Scholar] [CrossRef]


| Sample | Ingredients in phr 1 | ||||
|---|---|---|---|---|---|
| SR | SiO2 | Kaolin | ZB | DBPH | |
| SR0 | 100 | 40 | 40 | 0 | 2 |
| SR1 | 100 | 40 | 40 | 10 | 2 |
| SR2 | 100 | 40 | 40 | 15 | 2 |
| SR3 | 100 | 40 | 40 | 20 | 2 |
| SR4 | 100 | 40 | 40 | 25 | 2 |
| SR5 | 100 | 40 | 40 | 30 | 2 |
| Sample Code | T5 1/°C | Tmax1 1/°C | Tmax2 1/°C | Residue Weight/wt% |
|---|---|---|---|---|
| SR0 | 430 | / | 506.9 | 60.39 |
| SR1 | 420 | 399.7 | 503.7 | 62.37 |
| SR2 | 412 | 395.8 | 501.3 | 63.77 |
| SR3 | 412 | 402.1 | 499.8 | 64.44 |
| SR4 | 412 | 400.8 | 493.8 | 64.37 |
| SR5 | 407 | 409.5 | 491.1 | 64.87 |
| ZB | 383 | 385.4 | / | 78.04 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.; Huang, Z.; Qin, Y.; Li, X. Thermal Decomposition and Ceramifying Process of Ceramifiable Silicone Rubber Composite with Hydrated Zinc Borate. Materials 2019, 12, 1591. https://doi.org/10.3390/ma12101591
Song J, Huang Z, Qin Y, Li X. Thermal Decomposition and Ceramifying Process of Ceramifiable Silicone Rubber Composite with Hydrated Zinc Borate. Materials. 2019; 12(10):1591. https://doi.org/10.3390/ma12101591
Chicago/Turabian StyleSong, Jiuqiang, Zhixiong Huang, Yan Qin, and Xinyi Li. 2019. "Thermal Decomposition and Ceramifying Process of Ceramifiable Silicone Rubber Composite with Hydrated Zinc Borate" Materials 12, no. 10: 1591. https://doi.org/10.3390/ma12101591
APA StyleSong, J., Huang, Z., Qin, Y., & Li, X. (2019). Thermal Decomposition and Ceramifying Process of Ceramifiable Silicone Rubber Composite with Hydrated Zinc Borate. Materials, 12(10), 1591. https://doi.org/10.3390/ma12101591





