Microstructure and Morphology Control of Potassium Magnesium Titanates and Sodium Iron Titanates by Molten Salt Synthesis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. Adjustment of Molten Salt Content
2.3. Preparation of Potassium Magnesium Titanates (KMTO)
2.4. Preparation of Sodium Iron Titanates (NFTO)
2.5. Characterizations
3. Results and Discussion
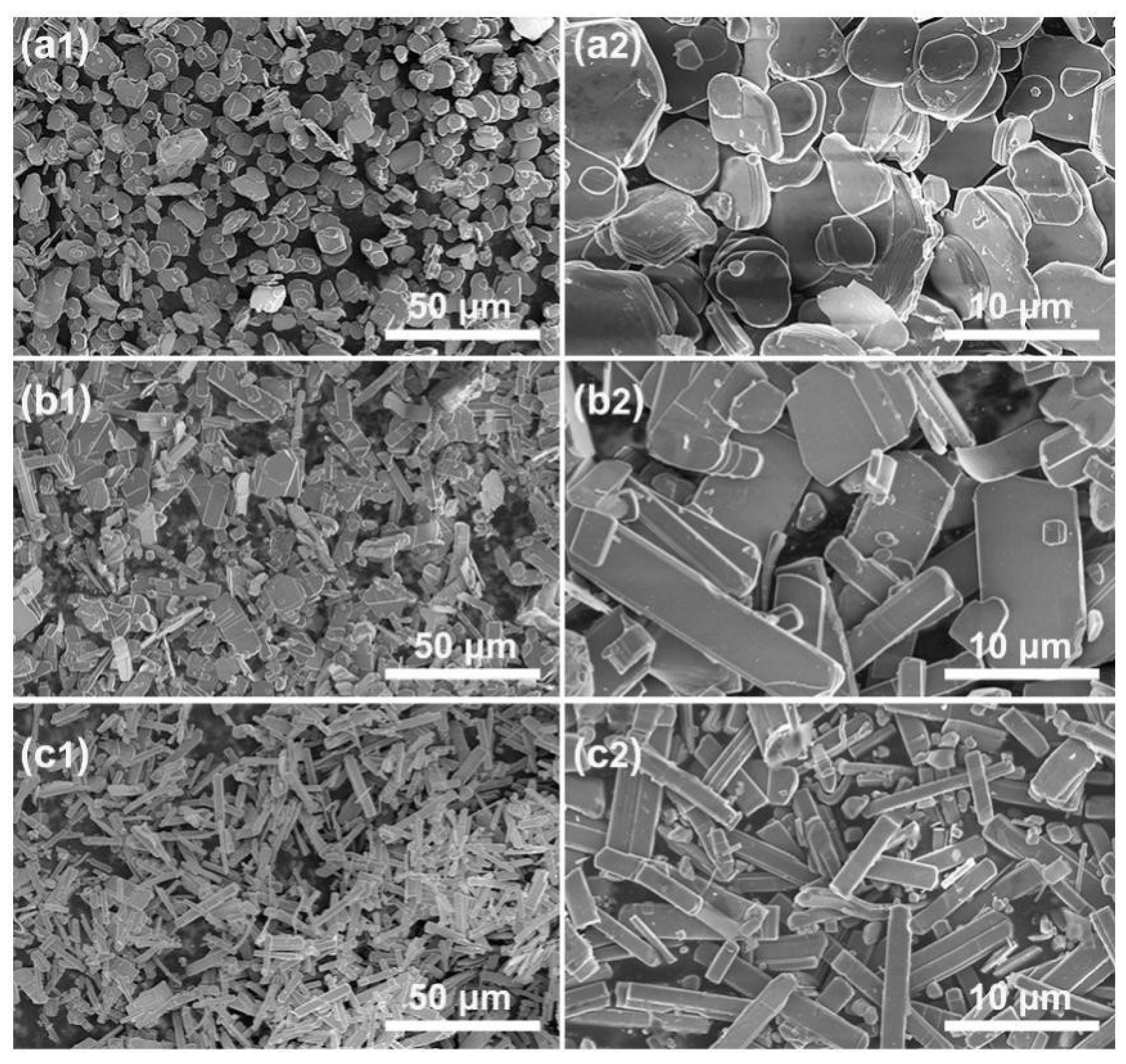
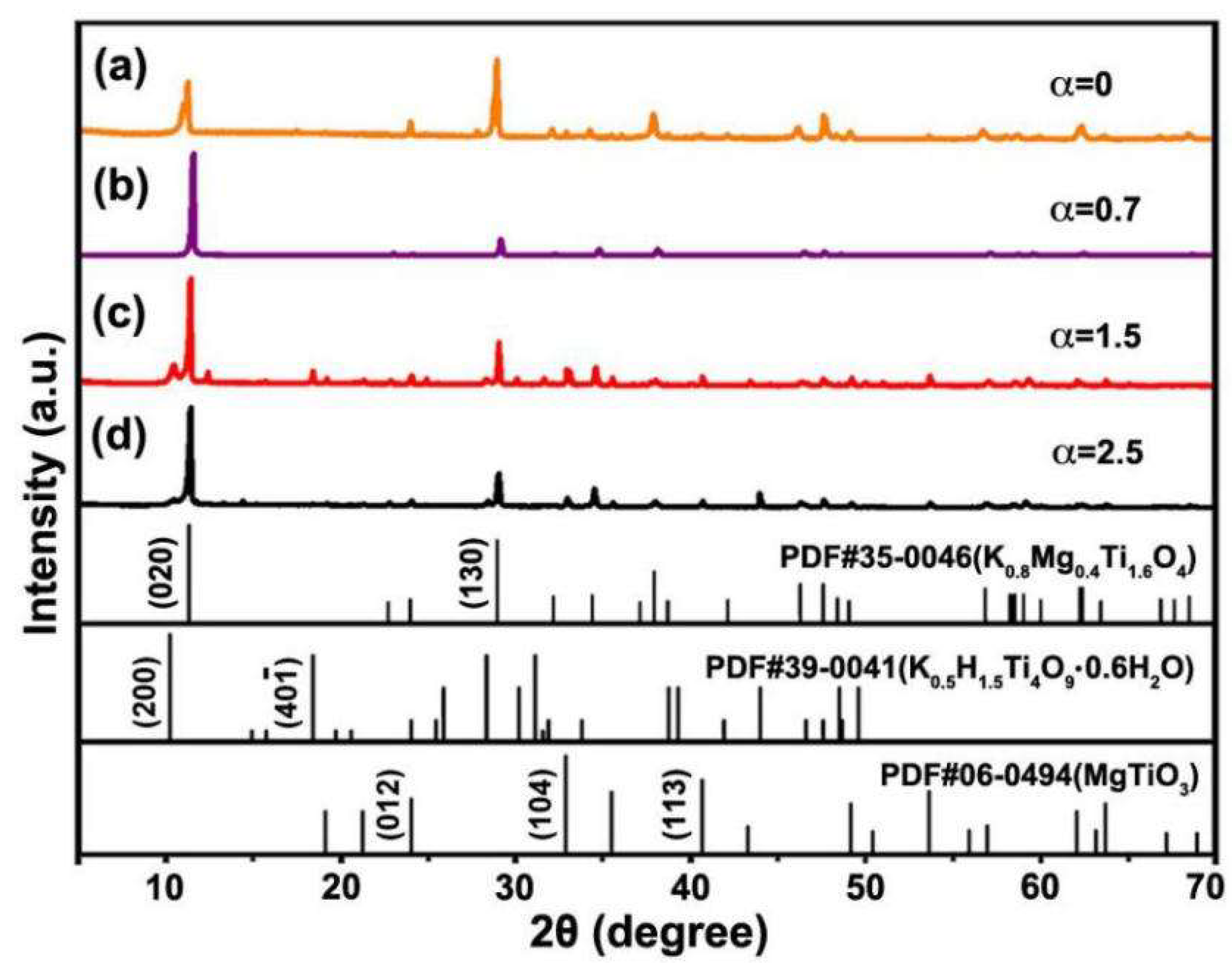
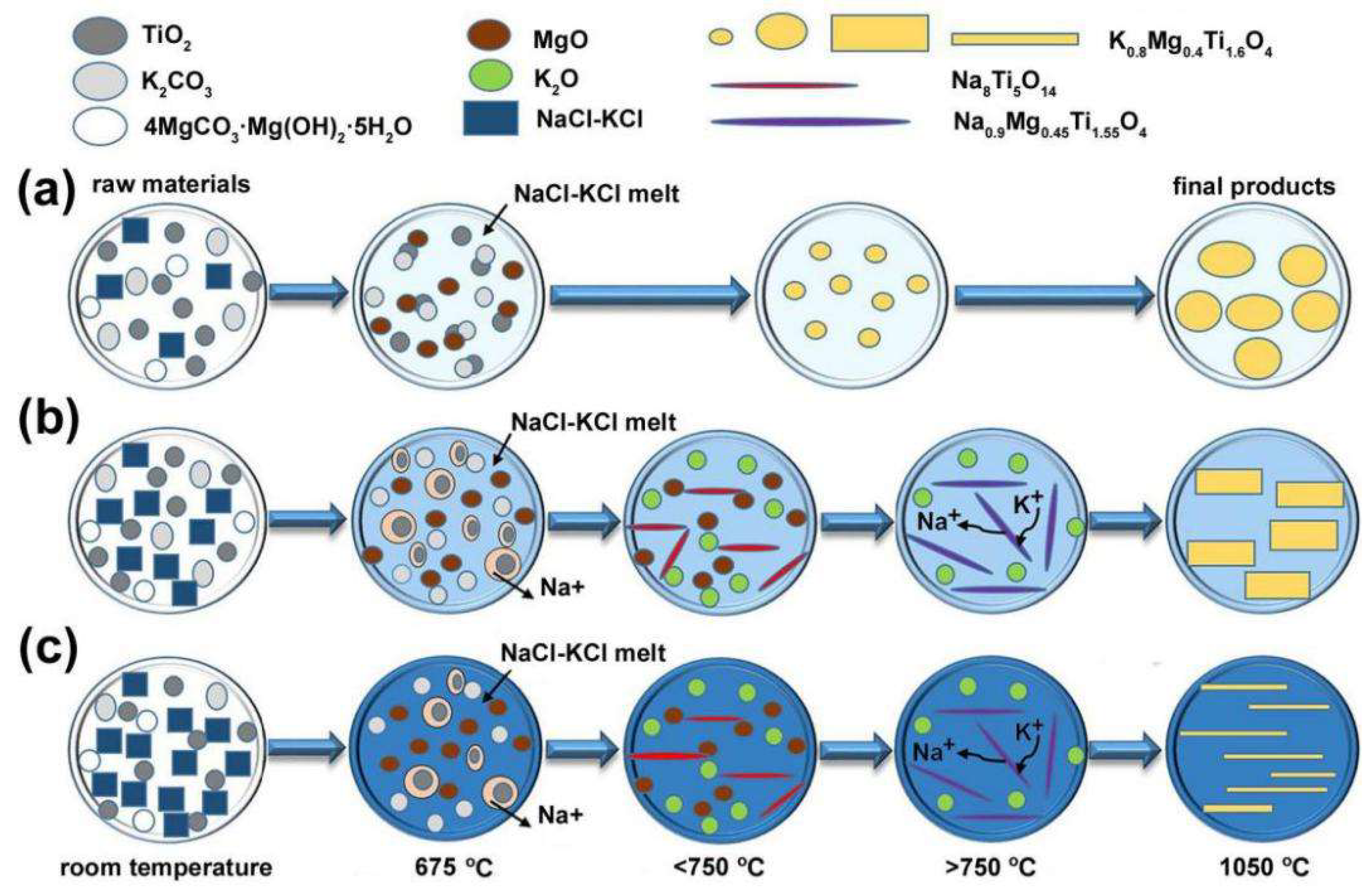
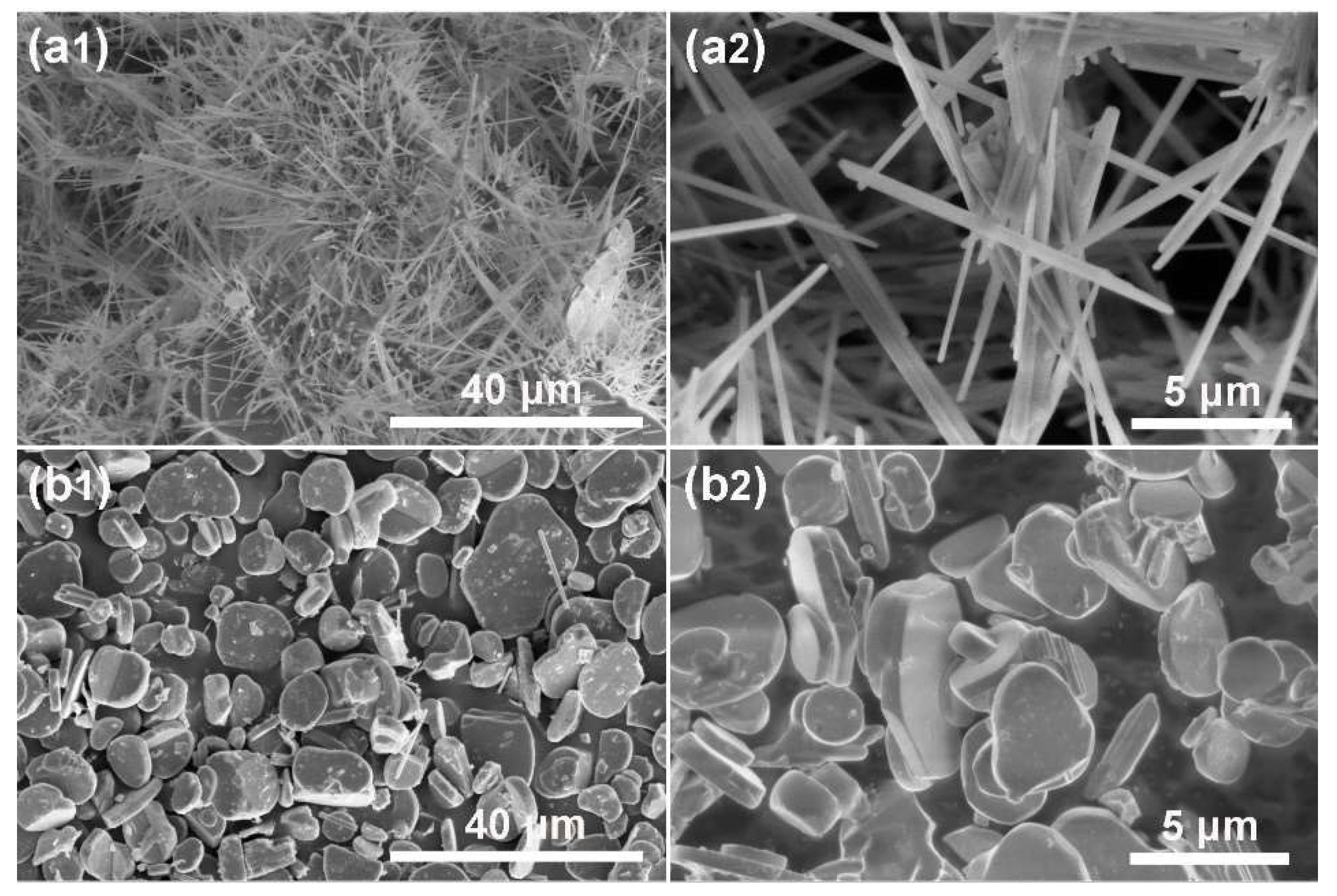
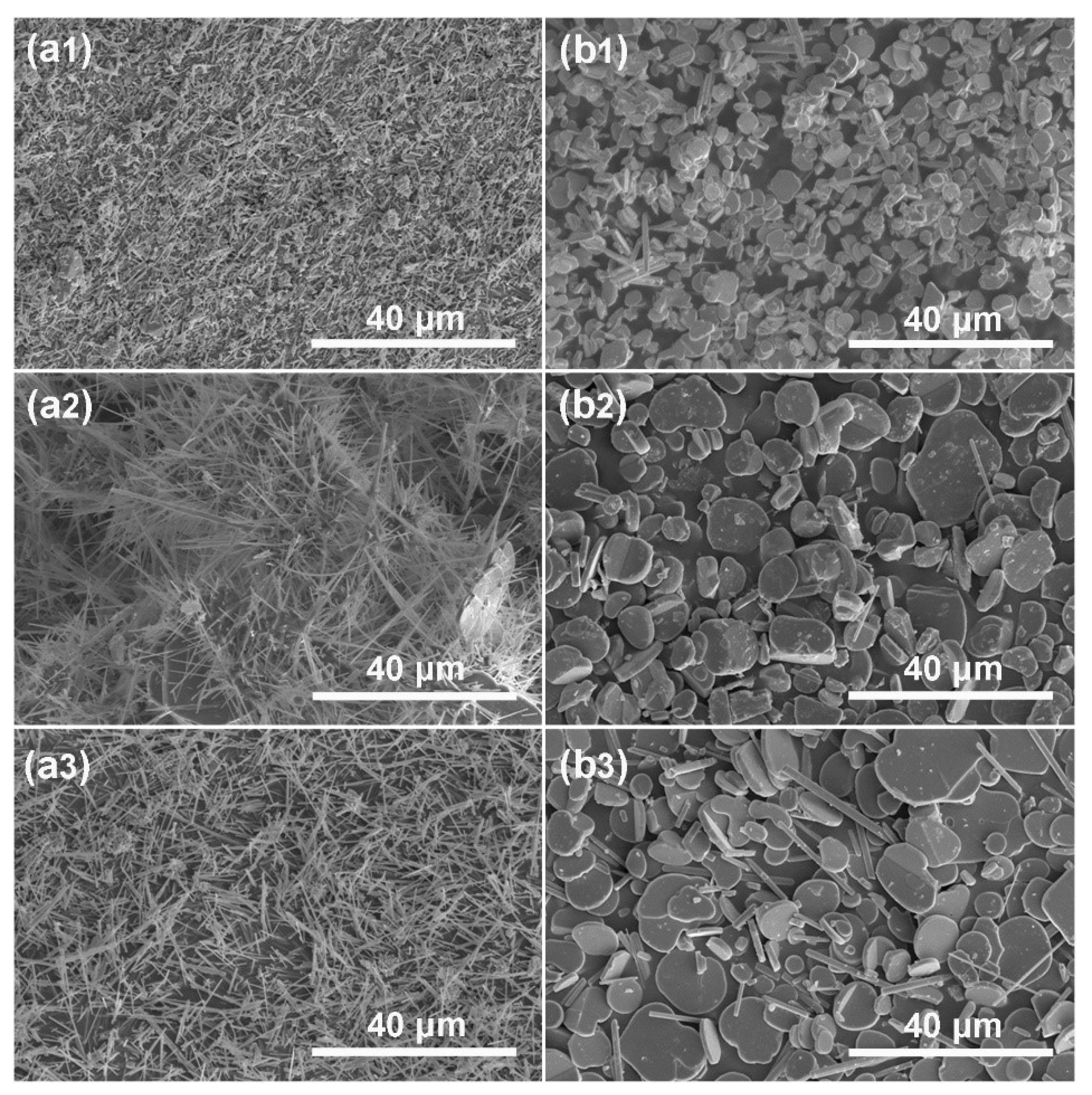
3.1. Synthesis of KMTO with Different Morphologies
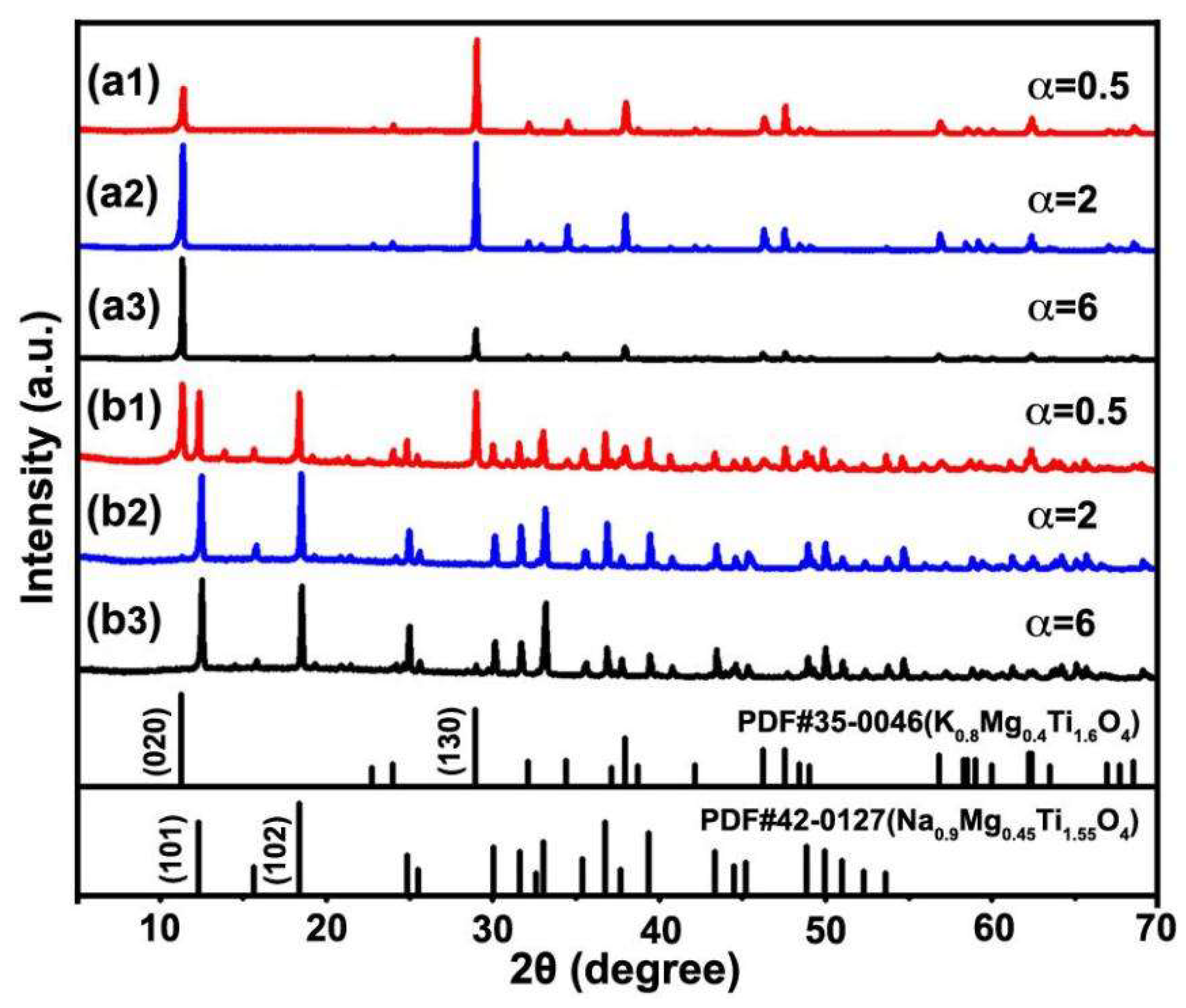
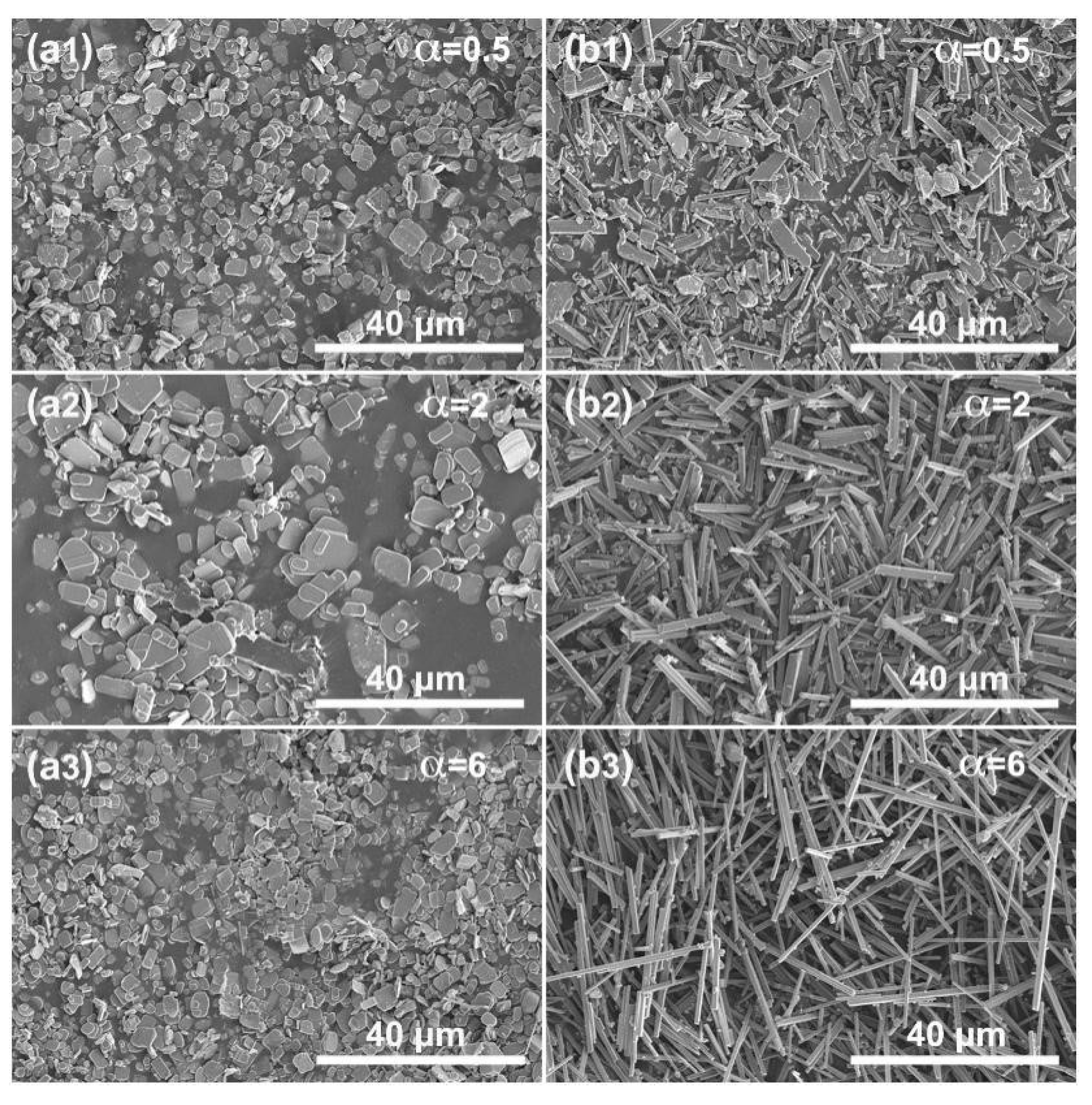
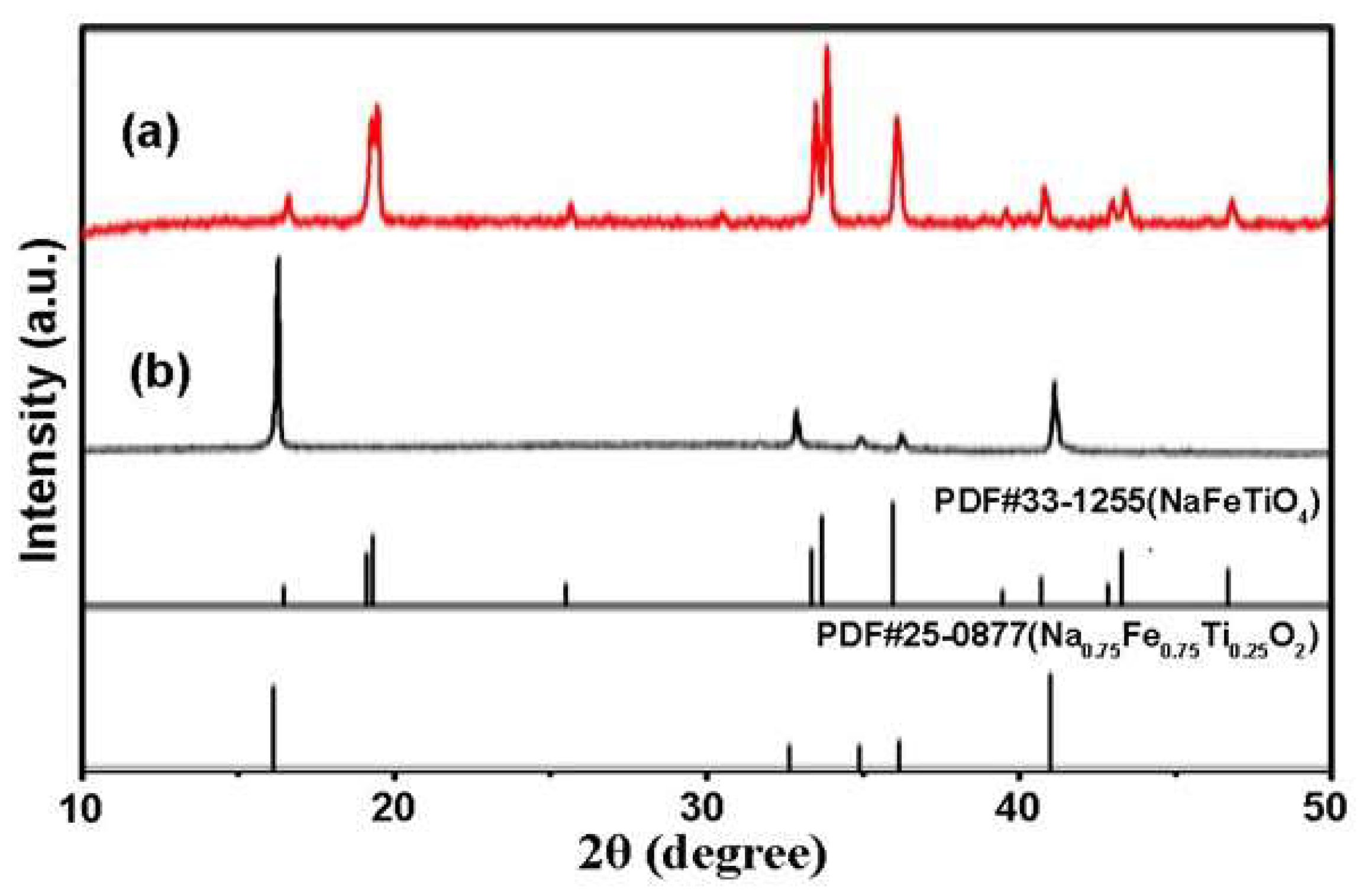
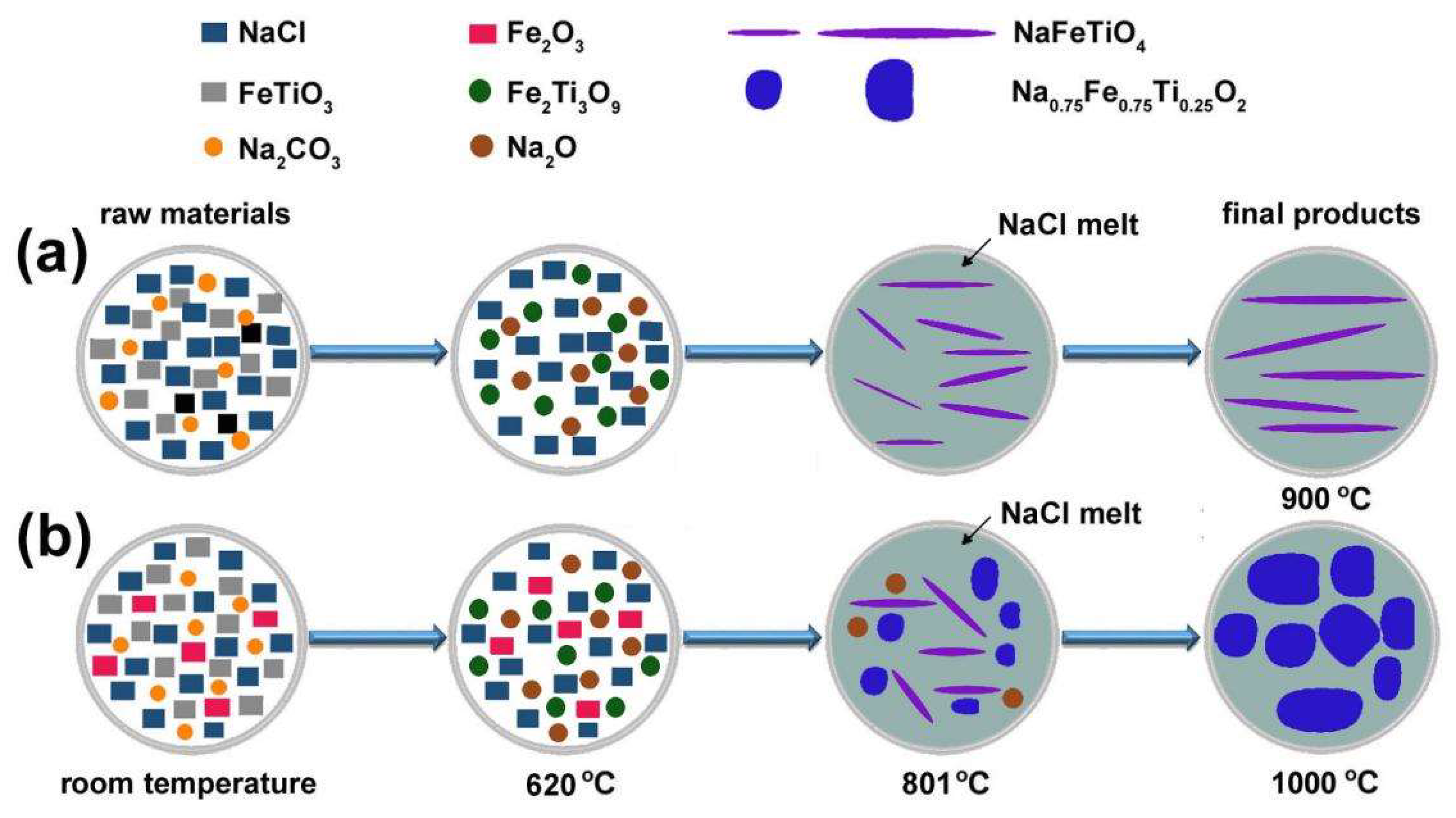
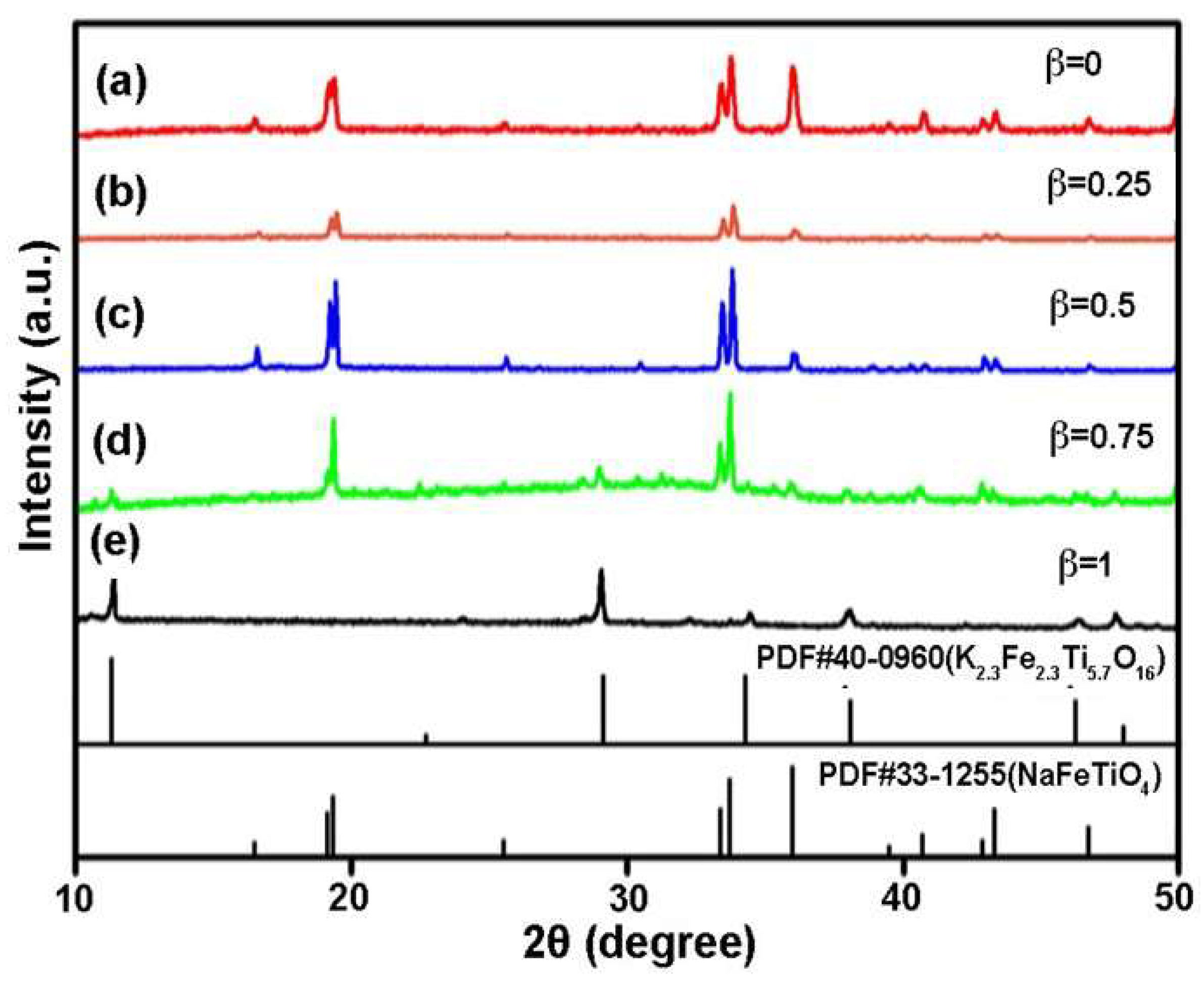
3.2. Synthesis of NFTO with Different Morphologies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ohsaka, T.; Fujiki, Y. Raman spectra in hollandite type compounds K1.6Mg0.8Ti7.2O16 and K1.6Al1.6Ti6.4O16. Solid State Commun. 1982, 44, 1325–1327. [Google Scholar] [CrossRef]
- Endo, T.; Nagayama, H.; Sato, T.; Shimada, M. Crystal growth of potassium titanates in the system K2O-Fe2O3-TiO2. J. Cryst. Growth. 1986, 78, 423–430. [Google Scholar] [CrossRef]
- Park, Y.; Terasaki, K.; Igarashi, K.; Shimizu, T. Manufacture and mechanical properties of magnesium potassium titanate short fiber/glass composite. Adv. Compos. Mater. 2001, 10, 17–28. [Google Scholar] [CrossRef]
- Chen, M.; Wang, Z.; Liu, H.; Wang, X.; Ma, Y.; Liu, J. Synthesis of potassium magnesium titanate whiskers with high near-infrared reflectivity by the flux method. Mater. Lett. 2017, 202, 59–61. [Google Scholar] [CrossRef]
- Akieh, M.N.; Lahtinen, M.; Vaisanen, A.; Sillanpaa, M. Preparation and characterization of sodium iron titanate ion exchanger and its application in heavy metal removal from waste waters. J. Hazard. Mater. 2008, 152, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Knyazev, A.V.; Chernorukov, N.G.; Ladenkov, I.V.; Belopol’skaya, S.S. Synthesis, structure, and thermal expansion of M2Fe2Ti6O16 and MFeTiO4 Compounds. Inorg. Mater. 2011, 47, 999–1005. [Google Scholar] [CrossRef]
- Hou, J.; Niu, Y.; Yi, F.; Liu, S.; Li, Y.; He, H.; Xu, M. NaTi3FeO8: A novel anode material for sodium-ion batteries. RSC Adv. 2015, 5, 44313–44316. [Google Scholar] [CrossRef]
- Archaimbault, F.; Choisnet, J. The defect solid-solution Na7/8(FeIII7/8+xTiIV9/8-2xSbxV)O4 (0 ≤ x ≤ 1/3): Evidence of Na1 mobility in the tunnels of a quadruple rutile-chain structure. J. Solid State Chem. 1991, 90, 216–227. [Google Scholar] [CrossRef]
- Mumme, W.G.; Reid, A.F. Non-stoichiometric sodium iron titanate, NaxFexTi2-xO4, (0.90 > x > 0.75). Acta Cryst. 1968, 24, 625–631. [Google Scholar] [CrossRef]
- Kuhn, A.; Leon, C.; Garcıa-Alvarado, F.; Santamarıa, J.; Moran, E.; Alario-Franco, M.A. Study of the conductivity of Nax-σFexTi2-xO4 (x = 0.875, 0 ≤ σ ≤ 0.4). J. Solid State Chem. 1998, 137, 168–173. [Google Scholar] [CrossRef]
- Tan, Y.; Song, N.; Liu, Y.; Luo, T.; Dou, Y.; Zhang, Q.; Liu, Q.; Luo, L. Synthesis of platy potassium magnesium titanate and its application in removal of copper ions from aqueous solution. Trans. Nonferrous Met. Soc. China 2015, 25, 981–990. [Google Scholar] [CrossRef]
- Liu, M.; Liu, Y.; Zhang, D.; Liu, B.; Guo, Y. Nickel ion removal by porous potassium magnesium titanate made from plate-like crystals. Adv. Appl. Ceram. 2015, 114, 456–464. [Google Scholar] [CrossRef]
- Fujishiro, Y.; Miyata, M.; Awano, Y.; Maeda, K. Thermoelectric characterization of NaxMx/2Ti1-x/2O2 (M = Co, Ni and Fe) polycrystalline materials. Ceram. Int. 2003, 28, 841–845. [Google Scholar] [CrossRef]
- Li, C.; Reid, A.F.; Saunders, S. Nonstoichiometric alkali ferrites and aluminates in the systems NaFeO2-TiO2, KFeO2-TiO2, KAlO2-TiO2, and KAlO2-SiO2. J. Solid State Chem. 1971, 3, 614–624. [Google Scholar] [CrossRef]
- Thorne, J.S.; Chowdhury, S.; Dunlap, R.A.; Obrovac, M.N. Structure and electrochemistry of NaxFexTi1-xO2 (1.0 ≥ x ≥ 0.75) for Na-Ion battery positive electrodes. J. Electrochem. Soc. 2014, 161, A1801–A1805. [Google Scholar] [CrossRef]
- Plumley, A.L.; Orr, W.C. Replacement of Potassium Ions in Solid Potassium Hexatitanate by Sodium Ions from a Chloride Flux. J. Am. Chem. Soc. 1961, 83, 1289–1291. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Li, M.; Zhou, Z.; Shen, L.; Bao, N. Microstructure and Morphology Control of Potassium Magnesium Titanates and Sodium Iron Titanates by Molten Salt Synthesis. Materials 2019, 12, 1577. https://doi.org/10.3390/ma12101577
Zhang H, Li M, Zhou Z, Shen L, Bao N. Microstructure and Morphology Control of Potassium Magnesium Titanates and Sodium Iron Titanates by Molten Salt Synthesis. Materials. 2019; 12(10):1577. https://doi.org/10.3390/ma12101577
Chicago/Turabian StyleZhang, Haoran, Mengshuo Li, Ze Zhou, Liming Shen, and Ningzhong Bao. 2019. "Microstructure and Morphology Control of Potassium Magnesium Titanates and Sodium Iron Titanates by Molten Salt Synthesis" Materials 12, no. 10: 1577. https://doi.org/10.3390/ma12101577
APA StyleZhang, H., Li, M., Zhou, Z., Shen, L., & Bao, N. (2019). Microstructure and Morphology Control of Potassium Magnesium Titanates and Sodium Iron Titanates by Molten Salt Synthesis. Materials, 12(10), 1577. https://doi.org/10.3390/ma12101577



