The Fate of Osteoblast-Like MG-63 Cells on Pre-Infected Bactericidal Nanostructured Titanium Surfaces
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Fabrication of Ti Samples
2.3. Bacterial Strains, Preparation, and Pre-Infection Procedure
2.4. Bactericidal Efficacy of Titanium Surfaces
2.5. Confocal Laser Scanning Microscopy
2.6. Scanning Electron Microscopy
2.7. MG-63 Cell Line
2.8. Immunohistochemistry
2.9. MTS Cell Proliferation Assay
2.10. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
2.11. Bicinchoninic Acid Assay (BCA)
2.12. Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
3. Results and Discussion
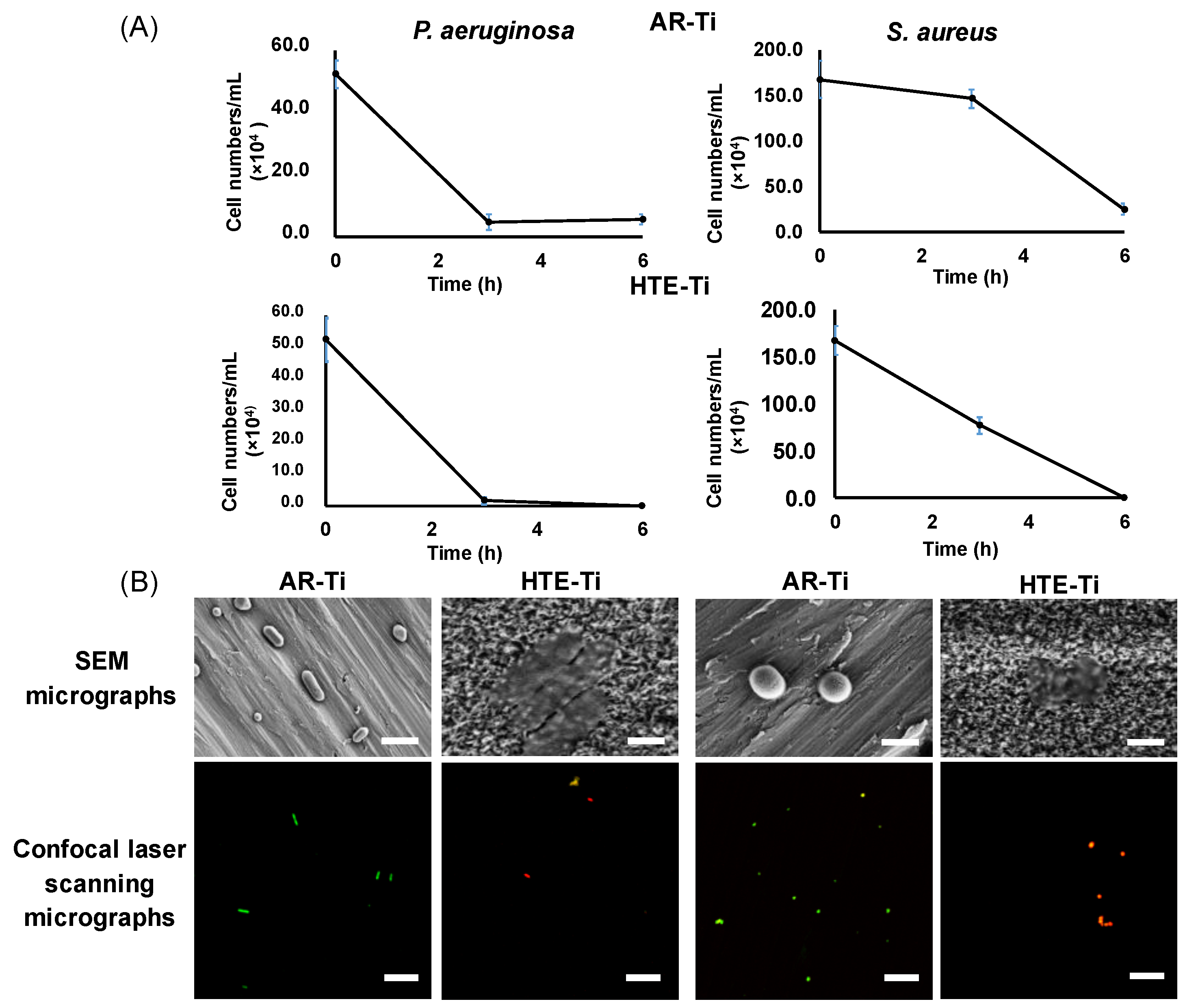
3.1. Bactericidal Activity of Hydrothermally Treated Ti (HTE-Ti)
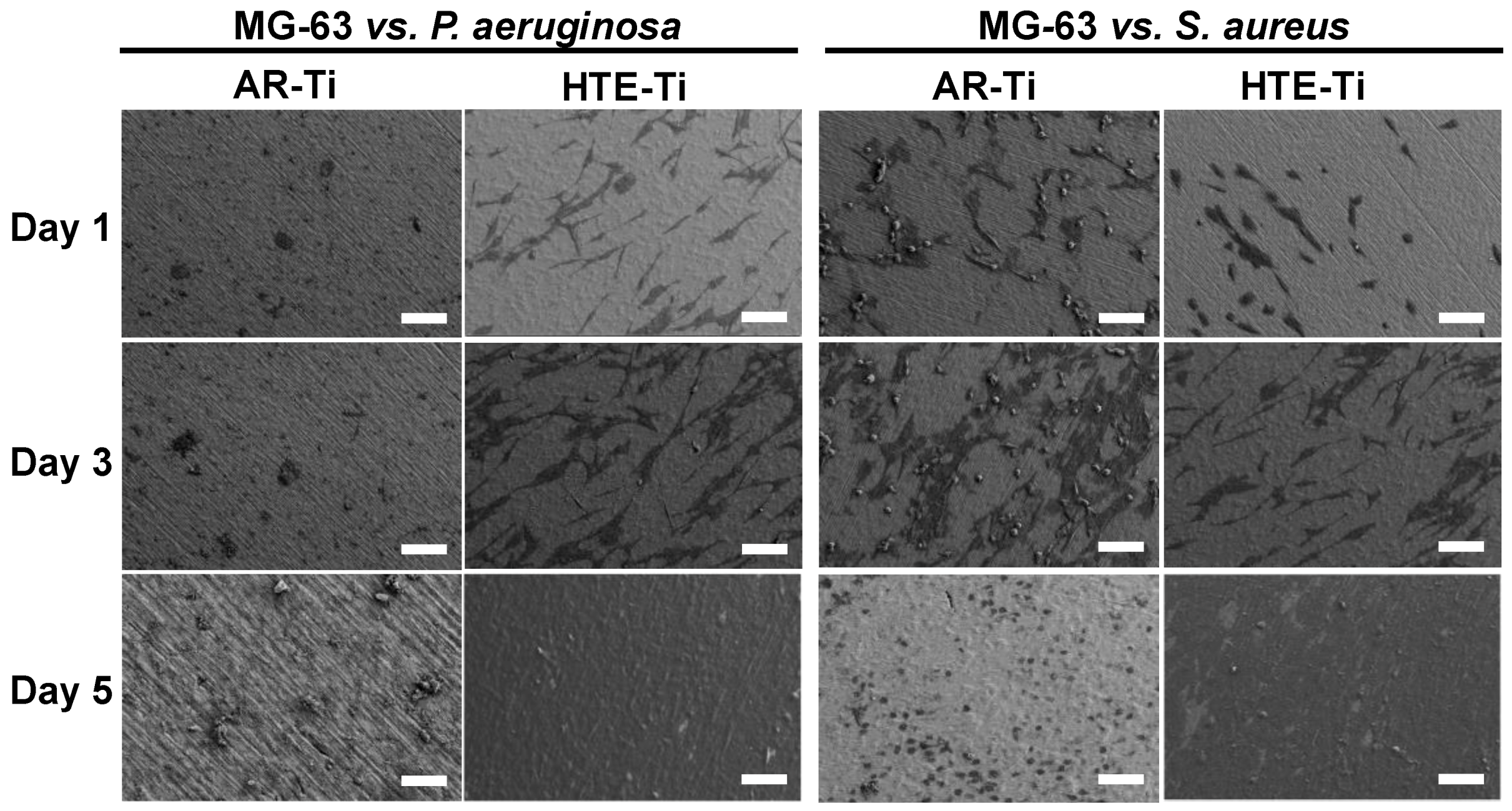
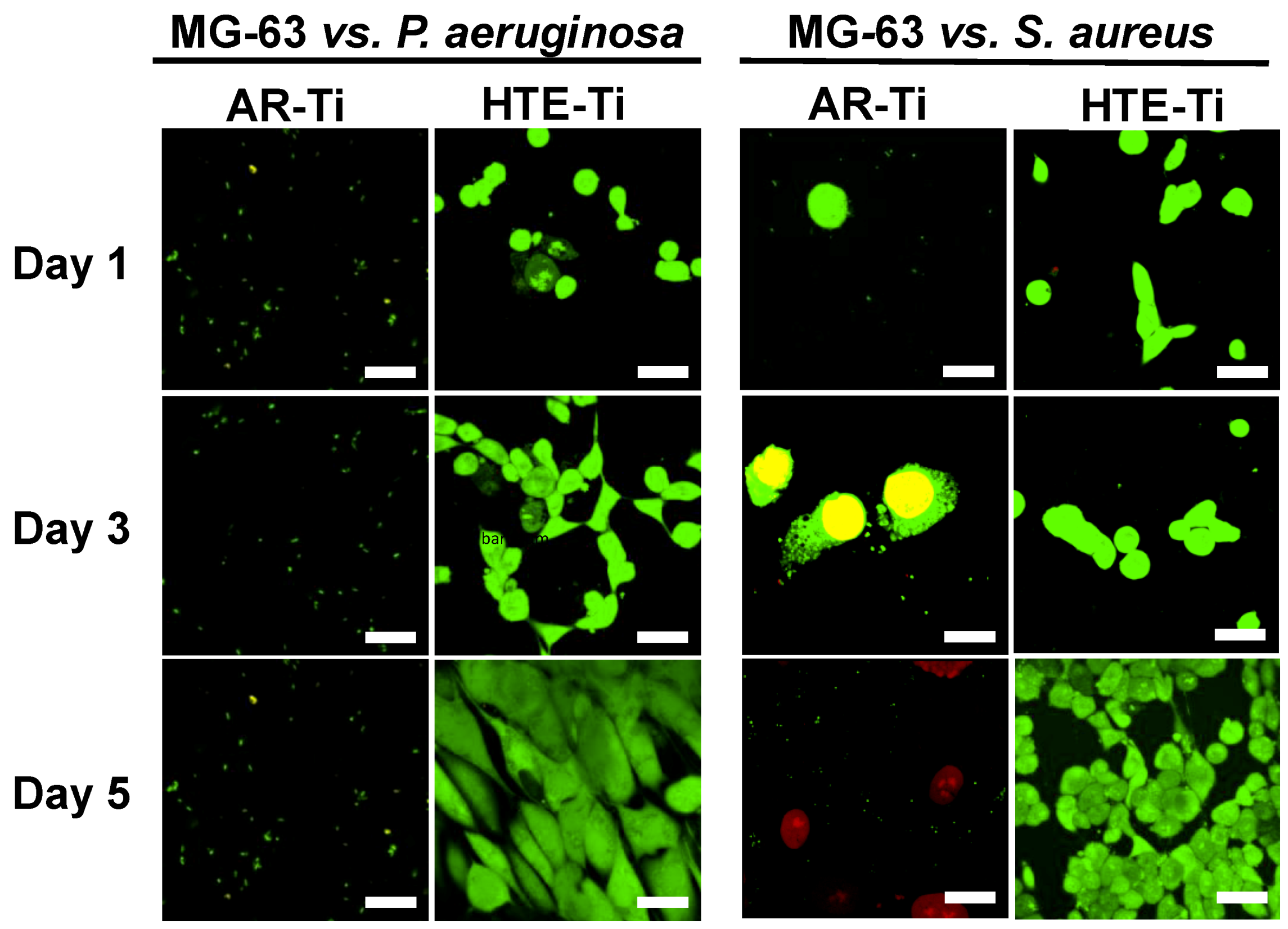
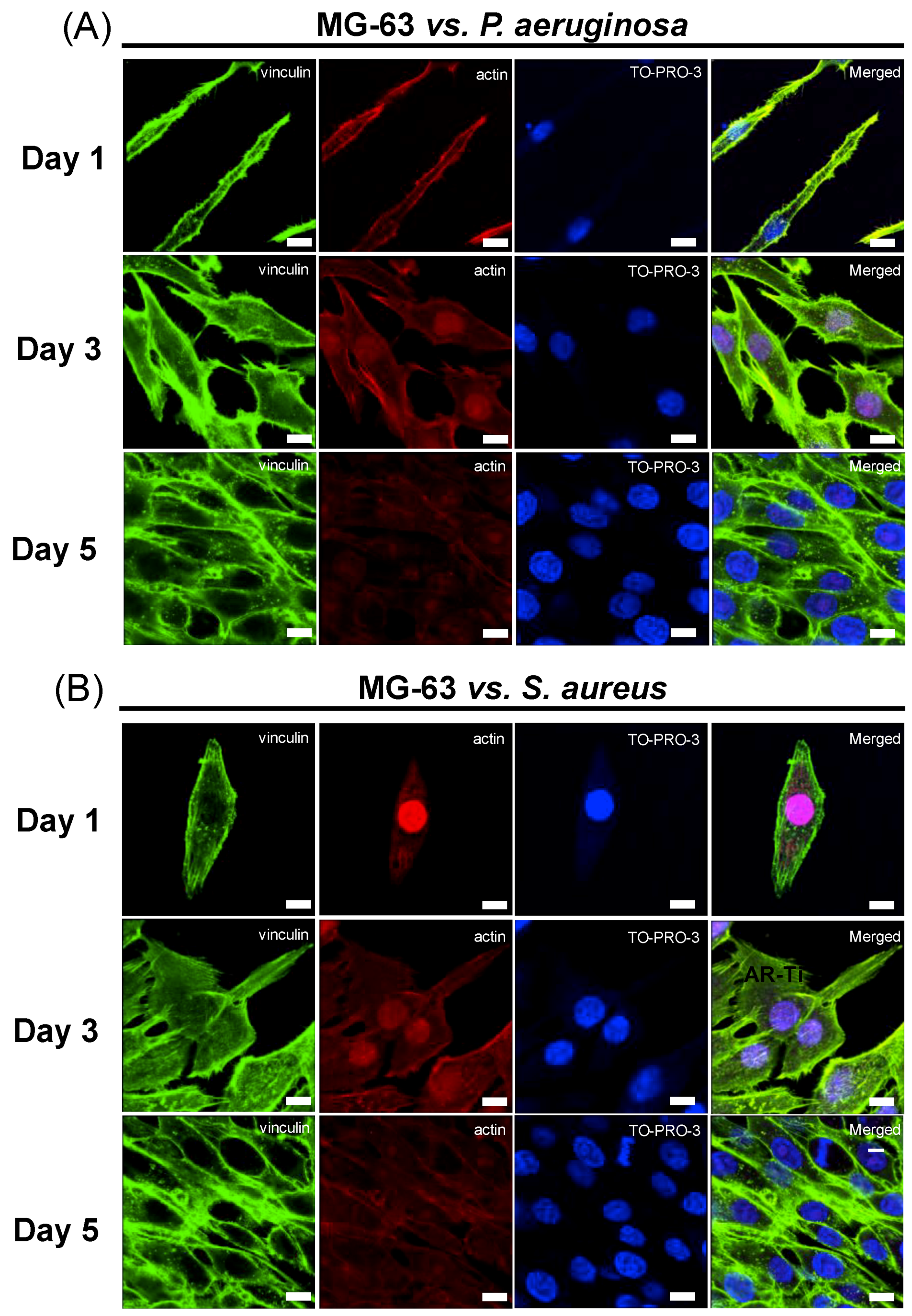
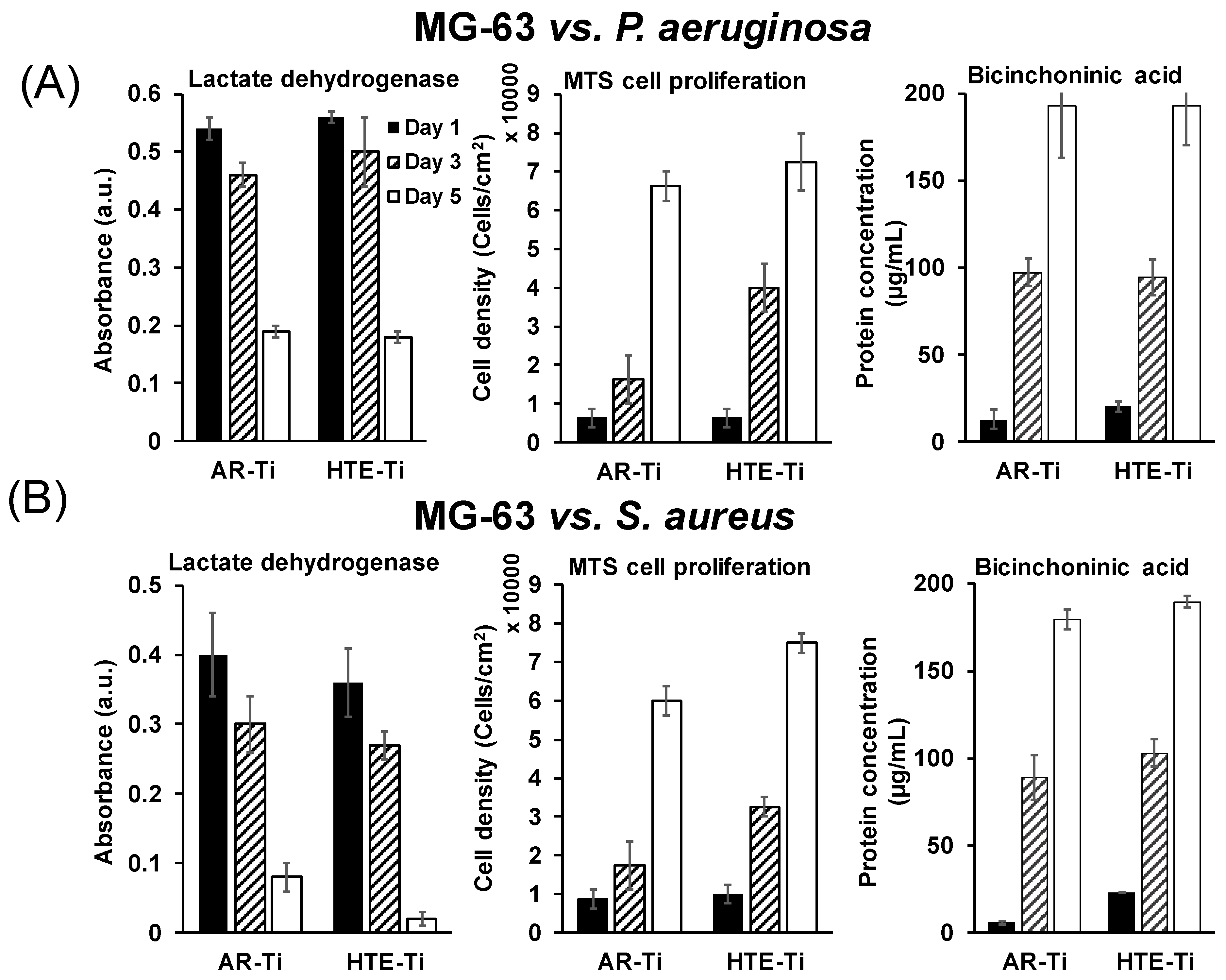
3.2. Ability of Eukaryotic Cells to Proliferate on Pre-Infected HTE-Ti
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bazaka, K.; Jacob, M.V.; Chrzanowski, W.; Ostrikov, K. Anti-bacterial surfaces: natural agents, mechanisms of action, and plasma surface modification. RSC Adv. 2015, 5, 48739–48759. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Efficient surface modification of biomaterial to prevent biofilm formation and the attachment of microorganisms. Appl. Microbiol. Biotechnol. 2012, 95, 299–311. [Google Scholar] [CrossRef] [PubMed]
- Predoi, D.; Iconaru, L.S.; Predoi, V.M. Bioceramic layers with antifungal properties. Coatings 2018, 8, 276. [Google Scholar] [CrossRef]
- Iconaru, L.S.; Prodan, M.A.; Buton, N.; Predoi, D. Structural characterization and antifungal studies of zinc-doped hydroxyapatite coatings. Molecules 2017, 22, 604. [Google Scholar] [CrossRef]
- Groza, A.; Ciobanu, S.C.; Popa, L.C.; Iconaru, L.S.; Chapon, P.; Luculescu, C.; Ganciu, M.; Predoi, D. Structural properties and antifungal activity against candida albicans biofilm of different composite layers based on ag/zn doped hydroxyapatite-polydimethylsiloxanes. Polymers 2016, 8, 131. [Google Scholar] [CrossRef] [PubMed]
- Iconaru, S.L.; Prodan, A.M.; Turculet, C.S.; Beuran, M.; Ghita, R.V.; Costescu, A.; Groza, A.; Chifiriuc, M.C.; Chapon, P.; Gaiaschi, S.; et al. Enamel based composite layers deposited on titanium substrate with antifungal activity. J. Spectrosc. 2016, 2016, 13. [Google Scholar] [CrossRef]
- Jeong, W.-S.; Kwon, J.-S.; Lee, J.-H.; Uhm, S.-H.; Ha Choi, E.; Kim, K.-M. Bacterial attachment on titanium surfaces is dependent on topography and chemical changes induced by nonthermal atmospheric pressure plasma. Biomed. Mater. 2017, 12, 045015. [Google Scholar] [CrossRef]
- Doll, K.; Fadeeva, E.; Stumpp Nico, S.; Grade, S.; Chichkov Boris, N.; Stiesch, M. Reduced bacterial adhesion on titanium surfaces micro-structured by ultra-short pulsed laser ablation. BioNanoMaterials 2016, 17, 53–57. [Google Scholar] [CrossRef]
- Gristina, A.G. Biomaterial-centered infection: Microbial adhesion versus tissue integration. Science 1987, 237, 1588. [Google Scholar] [CrossRef]
- Grassi, L.; Maisetta, G.; Esin, S.; Batoni, G. Combination strategies to enhance the efficacy of antimicrobial peptides against bacterial biofilms. Front. Microbiol. 2017, 8, 2409. [Google Scholar] [CrossRef]
- Del Pozo, J.L. Biofilm-related disease. Expert Rev. Anti-Infect. Therapy 2018, 16, 51–65. [Google Scholar] [CrossRef]
- Stewart, P.S.; William Costerton, J. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Tîlmaciu, C.-M.; Mathieu, M.; Lavigne, J.-P.; Toupet, K.; Guerrero, G.; Ponche, A. In vitro and in vivo characterization of antibacterial activity and biocompatibility: A study on silver-containing phosphonate monolayers on titanium. Acta Biomater. 2015, 15, 266–277. [Google Scholar] [CrossRef]
- Norambuena, G.A.; Patel, R.; Karau, M.; Wyles, C.C.; Jannetto, P.J.; Bennet, K.E. Antibacterial and biocompatible titanium-copper oxide coating may be a potential strategy to reduce periprosthetic infection: An in vitro study. Clin. Orthop. Relat. Res. 2017, 475, 722–732. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Crawford, R.J.; Nazarenko, E.L.; Ivanova, E.P. Bacterial Extracellular Polysaccharides. In Bacterial adhesion; Springer: Dordrecht, The Netherlands, 2011; pp. 213–226. [Google Scholar]
- Prasad, K.; Bazaka, O.; Chua, M.; Rochford, M.; Fedrick, L.; Spoor, J. Metallic Biomaterials: Current Challenges and Opportunities. Materials 2017, 10, 884. [Google Scholar] [CrossRef] [PubMed]
- Jäger, M.; Jennissen, H.P.; Dittrich, F.; Fischer, A.; Köhling, H.L. Antimicrobial and Osseointegration Properties of Nanostructured Titanium Orthopaedic Implants. Materials 2017, 10, 1302. [Google Scholar] [CrossRef]
- Jiang, Y.; Mehta, C.K.; Hsu, T.-Y.; Alsulaimani, F.F.H. Bacteria induce osteoclastogenesis via an osteoblast-independent pathway. Infect. Immun. 2002, 70, 3143–3148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Rossi, A.; Fukada, S.Y.; De Rossi, M.; da Silva, R.A.B.; Queiroz, A.M.; Nelson-Filho, P. Cementocytes express receptor activator of the nuclear factor kappa-B ligand in response to endodontic infection in mice. J. Endod. 2016, 42, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Leibbrandt, A.; Penninger, J.M. RANK(L) as a Key Target for Controlling Bone Loss. In Therapeutic Targets of the TNF Superfamily; Springer: New York, NY, USA, 2009; pp. 130–145. [Google Scholar]
- Jäger, M.; Zilkens, C.; Zanger, K.; Krauspe, R. Significance of nano- and micro-topography for cell-surface interactions in orthopaedic implants. J. Biomed. & Biotechnol. 2007, 2007, 69036. [Google Scholar]
- Prasad, K.; Lekshmi, G.S.; Ostrikov, K.; Lussini, V.; Blinco, J.; Mohandas, M. Synergic bactericidal effects of reduced graphene oxide and silver nanoparticles against Gram-positive and Gram-negative bacteria. Sci. Rep. 2017, 7, 1591. [Google Scholar] [CrossRef]
- Bazaka, K.; Crawford, R.J.; Ivanova, E.P. Do bacteria differentiate between degrees of nanoscale surface roughness? Biotechnol. J. 2011, 6, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A. Natural bactericidal surfaces: Mechanical rupture of Pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Phong Nguyen, T.H. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wan, Y.; Ren, B.; Liu, Z. Fabrication of an orderly micro/nanostructure on titanium surface and its effect on cell proliferation. Mater. Lett. 2018, 212, 247–250. [Google Scholar] [CrossRef]
- Li, N.B.; Sun, S.J.; Bai, H.Y.; Xiao, G.Y.; Xu, W.H.; Zhao, J.H.; Zhang, Y.L. Preparation of well-distributed Titania nanopillar arrays on Ti6Al4V surface by induction heating for enhancing osteogenic differentiation of stem cells. Nanotechnology 2018, 29, 045101. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Qiu, J.; Du, L.Q.; Jia, L.; Liu, H.; Ge, S. TiO2 nanorod arrays modified Ti substrates promote the adhesion, proliferation and osteogenic differentiation of human periodontal ligament stem cells. Mater. Sci. Eng. C 2017, 76, 684–691. [Google Scholar] [CrossRef]
- Liu, L.; Bhatia, R.; Webster, T.J. Atomic layer deposition of nano-TiO2 thin films with enhanced biocompatibility and antimicrobial activity for orthopedic implants. Int. J. Nanomed. 2017, 12, 8711–8723. [Google Scholar] [CrossRef]
- Tian, B.; Chen, W.; Yu, D.; Lei, Y.; Ke, Q.; Guo, Y. Fabrication of silver nanoparticle-doped hydroxyapatite coatings with oriented block arrays for enhancing bactericidal effect and osteoinductivity. J. Mech. Behav. Biomed. Mater. 2016, 61, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Bao, N.-R.; Chen, S.; Zhao, J.-N. Antimicrobial design of titanium surface that kill sessile bacteria but support stem cells adhesion. Appl. Surf. Sci. 2016, 389, 7–16. [Google Scholar] [CrossRef]
- Pérez-Tanoira, R.; Han, X.; Soininen, A.; Aarnisalo, A.A.; Tiainen, V.-M.; Eklund, K.K. Competitive colonization of prosthetic surfaces by Staphylococcus aureus and human cells. J. Biomed. Mater. Res. Part A 2016, 105, 62–72. [Google Scholar] [CrossRef]
- Martinez-Perez, M.; Perez-Jorge, C.; Lozano, D.; Portal-Nuñez, S.; Perez-Tanoira, R.; Conde, A. Evaluation of bacterial adherence of clinical isolates of Staphylococcus sp. using a competitive model. Bone & Joint Res. 2017, 6, 315–322. [Google Scholar]
- Bhadra, C.M.; Truong, V.K.; Pham, V.T.; Al Kobaisi, M.; Seniutinas, G.; Wang, J.Y. Antibacterial titanium nano-patterned arrays inspired by dragonfly wings. Sci. Rep. 2015, 5, 16817. [Google Scholar] [CrossRef]
- Govindaraj, S.; Muthuraman, M.S. Systematic review on sterilization methods of implants and medical devices. Int. J. Chem. Tech. Res. 2015, 8, 897–911. [Google Scholar]
- Ivanova, E.P.; Nguyen, S.H.; Guo, Y.; Baulin, V.A.; Webb, H.K.; Truong, V.K. Bactericidal activity of self-assembled palmitic and stearic fatty acid crystals on highly ordered pyrolytic graphite. Acta Biomater. 2017, 59, 148–157. [Google Scholar] [CrossRef]
- Walderhaug, M. Bad Bug Book: Foodborne Pathogenic Microorganisms and Natural Toxins Handbook; International Medical Publishing: Hyderabad, India, 2014. [Google Scholar]
- Ivanova, E.P.; Truong, V.K.; Wang, J.Y.; Berndt, C.C.; Jones, R.T.; Yusuf, I.I. Impact of nanoscale roughness of titanium thin film surfaces on bacterial retention. Langmuir 2010, 26, 1973–1982. [Google Scholar] [CrossRef] [PubMed]
- Marinucci, L.; Bodo, M.; Balloni, S.; Locci, P.; Baroni, T. Sub-toxic nicotine concentrations affect extracellular matrix and growth factor signaling gene expressions in human osteoblasts. J. Cell. Physiol. 2014, 229, 2038–2048. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time uantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Wandiyanto, V.J.; Linklater, D.; Tharushi Perera, G.P.; Orlowska, A.; Truong, K.V.; Thissen, H. Pheochromocytoma (PC12) cell response on mechanobactericidal titanium surfaces. Materials 2018, 11, 605. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Chen, X.; Cheng, K.; Dong, L.; Weng, W.; Wang, H. Enhanced cellular osteogenic differentiation on Zn-containing bioglass incorporated TiO2 nanorod films. J. Mater. Sci. Mater. Med. 2018, 29, 136. [Google Scholar] [CrossRef] [PubMed]
- Schmid-Hempel, P.; Frank, S.A. Pathogenesis, virulence, and infective dose. PLOS Pathogens. 2007, 3, e147. [Google Scholar] [CrossRef] [PubMed]
- Sewell, D.L. Laboratory-associated infections and biosafety. Clin. Microbiol. Rev. 1995, 8, 389. [Google Scholar] [CrossRef]
- Emmerson, M. A microbiologist’s view of factors contributing to infection. New Horiz. Sci. Pract. Acute Med. 1998, 6, S3–S10. [Google Scholar]
- Linklater, D.P.; De Volder, M.; Baulin, V.A.; Werner, M.; Jessl, S.; Golozar, M. High aspect ratio nanostructures kill bacteria via storage and release of mechanical energy. ACS Nano 2018, 12, 6657–6667. [Google Scholar] [CrossRef]
- Veesenmeyer, J.L.; Hauser, A.R.; Lisboa, T.; Rello, J. Pseudomonas aeruginosa virulence and therapy: Evolving translational strategies. Critic. Care Med. 2009, 37, 1777–1786. [Google Scholar] [CrossRef]
- Pavlovskis, O.R.; Iglewski, B.H.; Pollack, M. Mechanism of action of Pseudomonas aeruginosa exotoxin A in experimental mouse infections: Adenosine diphosphate ribosylation of elongation factor 2. Infect. Immun. 1978, 19, 29–33. [Google Scholar] [PubMed]
- Grashoff, C.; Hoffman, B.D.; Brenner, M.D.; Zhou, R.; Parsons, M.; Yang, M.T. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 2010, 466, 263. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, T.; Kashiwakura, Y.; Ishiwata, A.; Madoiwa, S.; Mimuro, J.; Furukawa, Y. Vinculin is indispensable for repopulation by hematopoietic stem cells, independent of integrin function. J. Biologic. Chem. 2010, 285, 31763–31773. [Google Scholar] [CrossRef]
- Alenghat, F.J.; Fabry, B.; Tsai, K.Y.; Goldmann, W.H.; Ingber, D.E. Analysis of cell mechanics in single vinculin-deficient cells using a magnetic tweezer. Biochem. Biophys. Res. Commun. 2000, 277, 93–99. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Denhardt, D.T.; Noda, M. Osteopontin expression and function: Role in bone remodeling. J. Cell. Biochem. 1998, 72, 92–102. [Google Scholar] [CrossRef]
- Giachelli, C.M.; Steitz, S. Osteopontin: A versatile regulator of inflammation and biomineralization. Matrix Biology 2000, 19, 615–622. [Google Scholar] [CrossRef]
- Hsieh, I.S.; Yang, R.-S.; Fu, W.-M. Osteopontin upregulates the expression of glucose transporters in osteosarcoma cells. PloS ONE 2014, 9, e109550. [Google Scholar] [CrossRef]
- Agustina, H.; Asyifa, I.; Aziz, A.; Hernowo, B.S. The role of osteocalcin and alkaline phosphatase immunohistochemistry in osteosarcoma diagnosis. Pathol. Res. Int. 2018, 2018, 6346409. [Google Scholar] [CrossRef]
- Tsai, S.-W.; Liou, H.-M.; Lin, C.-J.; Kuo, K.-L.; Hung, Y.-S.; Weng, R.-C. MG63 osteoblast-like cells exhibit different behavior when grown on electrospun collagen matrix versus electrospun gelatin matrix. PloS ONE 2012, 7, e31200. [Google Scholar] [CrossRef]
- Lajeunesse, D.; Kiebzak, G.M.; Frondoza, C.; Sacktor, B. Regulation of osteocalcin secretion by human primary bone cells and by the human osteosarcoma cell line MG-63. Bone Mine. 1991, 14, 237–250. [Google Scholar] [CrossRef]
- Tsao, Y.-T.; Huang, Y.-J.; Wu, H.-H.; Liu, Y.-A.; Liu, Y.-S.; Lee, O.K. Osteocalcin mediates biomineralization during osteogenic maturation in human mesenchymal stromal cells. Int. J. Mol. Sci. 2017, 18, 159. [Google Scholar] [CrossRef]
- Bazaka, K.; Jacob, V.M. Implantable Devices: Issues and Challenges. Electronics 2013, 2, 1–34. [Google Scholar] [CrossRef]
- Bazaka, K.; Baranov, O.; Cvelbar, U.; Podgornik, B.; Wang, Y.; Huang, S. Oxygen plasmas: a sharp chisel and handy trowel for nanofabrication. Nanoscale 2018, 10, 17494–17511. [Google Scholar] [CrossRef]
- Baranov, O.; Levchenko, I.; Bell, J.M.; Lim, J.W.M.; Huang, S.; Xu, L. From nanometre to millimetre: A range of capabilities for plasma-enabled surface functionalization and nanostructuring. Mater. Horiz. 2018, 5, 765–798. [Google Scholar] [CrossRef]
- Levchenko, I.; Bazaka, K.; Keidar, M.; Xu, S.; Fang, J. Hierarchical Multicomponent Inorganic Metamaterials: Intrinsically Driven Self-Assembly at the Nanoscale. Adv. Mater. 2018, 30, 1702226. [Google Scholar] [CrossRef]
- Baranov, O.; Bazaka, K.; Kersten, H.; Keidar, M.; Cvelbar, U.; Xu, S. Plasma under control: Advanced solutions and perspectives for plasma flux management in material treatment and nanosynthesis. Appl. Phys. Rev. 2017, 4, 041302. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wandiyanto, J.V.; Truong, V.K.; Al Kobaisi, M.; Juodkazis, S.; Thissen, H.; Bazaka, O.; Bazaka, K.; Crawford, R.J.; Ivanova, E.P. The Fate of Osteoblast-Like MG-63 Cells on Pre-Infected Bactericidal Nanostructured Titanium Surfaces. Materials 2019, 12, 1575. https://doi.org/10.3390/ma12101575
Wandiyanto JV, Truong VK, Al Kobaisi M, Juodkazis S, Thissen H, Bazaka O, Bazaka K, Crawford RJ, Ivanova EP. The Fate of Osteoblast-Like MG-63 Cells on Pre-Infected Bactericidal Nanostructured Titanium Surfaces. Materials. 2019; 12(10):1575. https://doi.org/10.3390/ma12101575
Chicago/Turabian StyleWandiyanto, Jason V., Vi Khanh Truong, Mohammad Al Kobaisi, Saulius Juodkazis, Helmut Thissen, Olha Bazaka, Kateryna Bazaka, Russell J. Crawford, and Elena P. Ivanova. 2019. "The Fate of Osteoblast-Like MG-63 Cells on Pre-Infected Bactericidal Nanostructured Titanium Surfaces" Materials 12, no. 10: 1575. https://doi.org/10.3390/ma12101575
APA StyleWandiyanto, J. V., Truong, V. K., Al Kobaisi, M., Juodkazis, S., Thissen, H., Bazaka, O., Bazaka, K., Crawford, R. J., & Ivanova, E. P. (2019). The Fate of Osteoblast-Like MG-63 Cells on Pre-Infected Bactericidal Nanostructured Titanium Surfaces. Materials, 12(10), 1575. https://doi.org/10.3390/ma12101575






