Mineral Trioxide Aggregate Mixed with 5-Aminolevulinic Acid for the Photodynamic Antimicrobial Strategy in Hard Tissue Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of MTA/ALA Specimens
2.2. Setting Time and Mechanical Properties
2.3. Phase Composition and Morphology
2.4. In Vitro Soaking
2.5. Antibacterial Properties
2.6. Cell Isolation and Culture
2.7. Cell Viability
2.8. Fluorescent Staining
2.9. Cell Cycle
2.10. Western Blot
2.11. Osteogenesis Assay
2.12. Alizarin Red S Stain
2.13. Statistical Analyses
3. Results
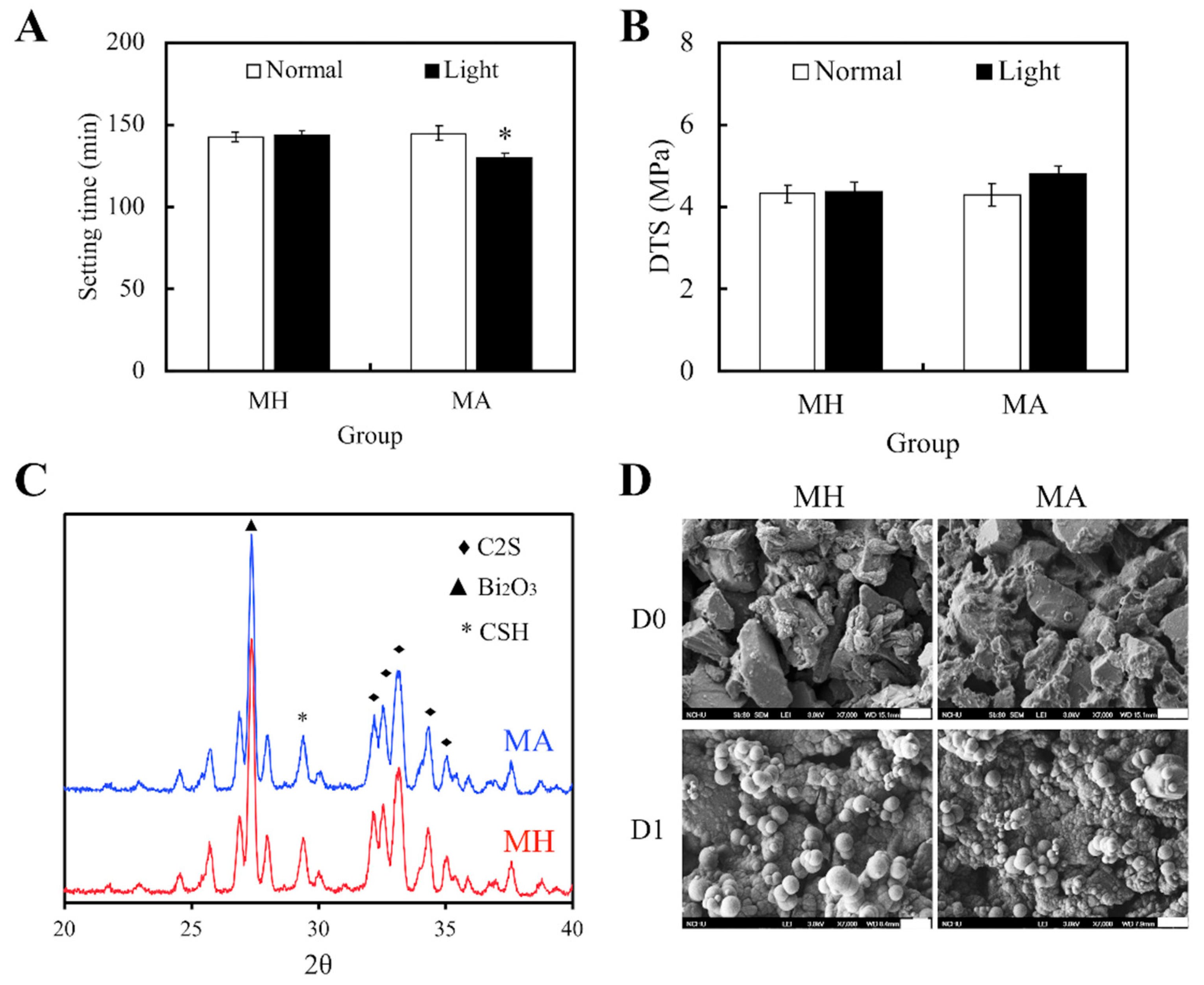
3.1. Physicochemical Properties
3.2. Antibacterial Properties
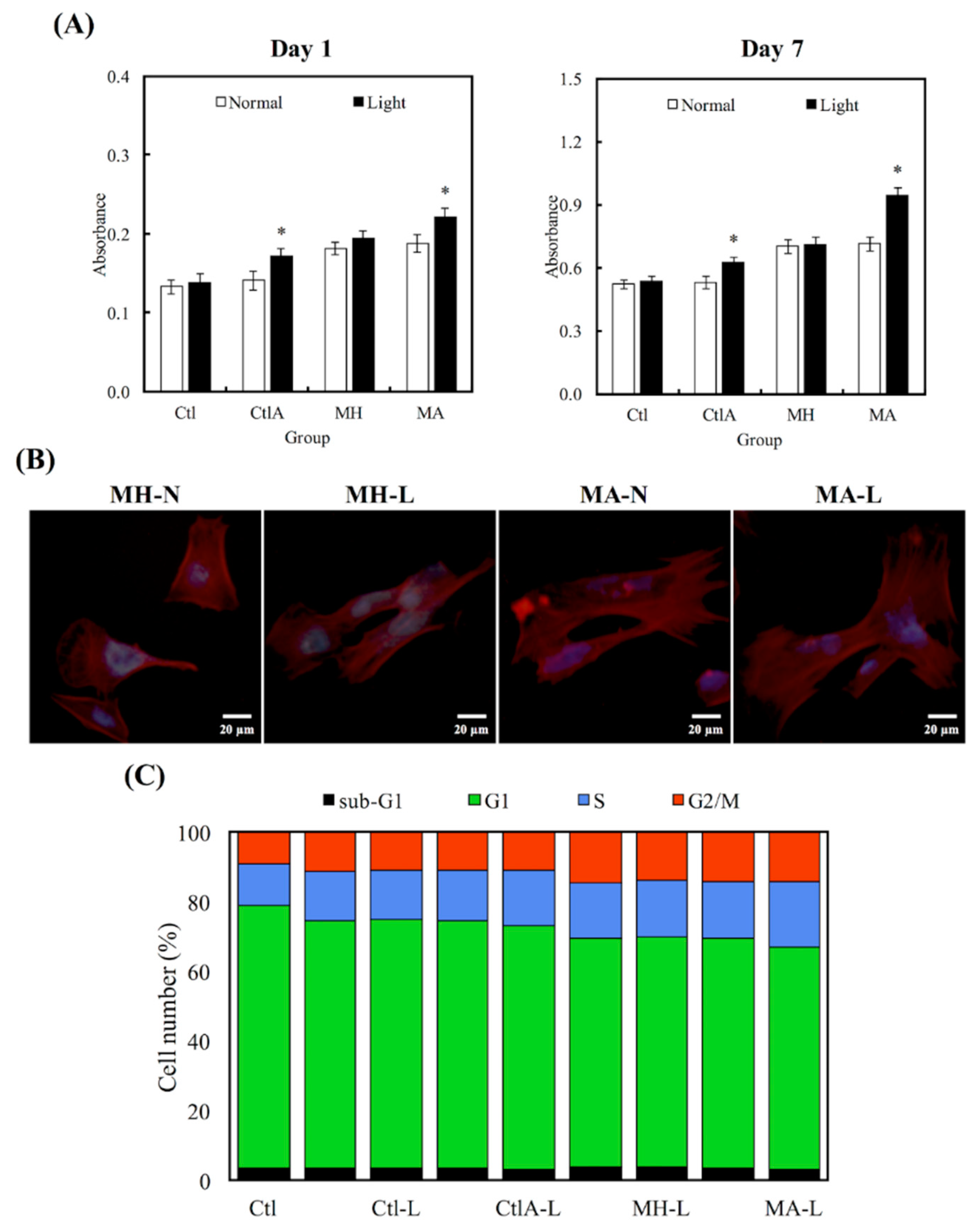
3.3. Cell viability
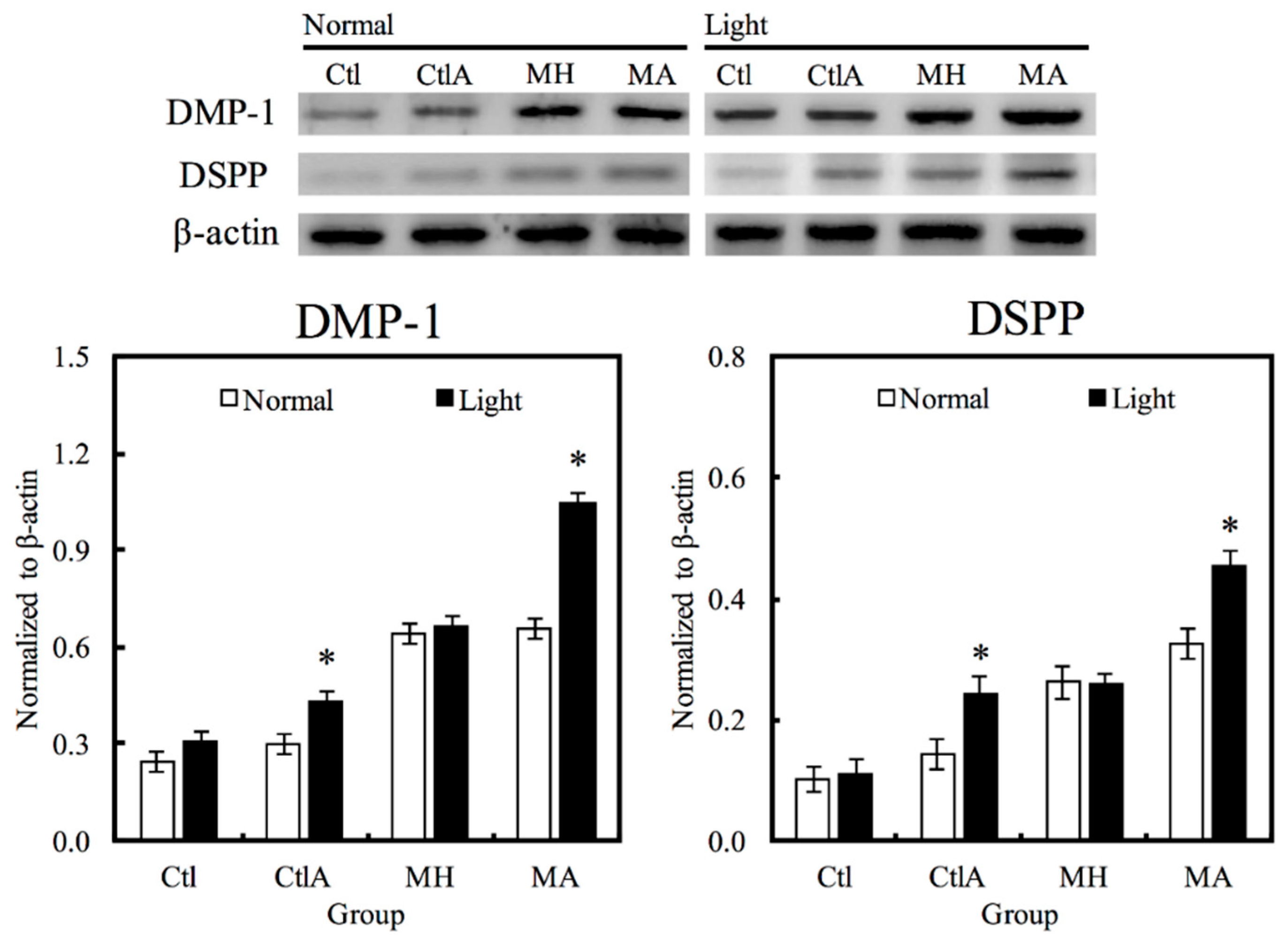
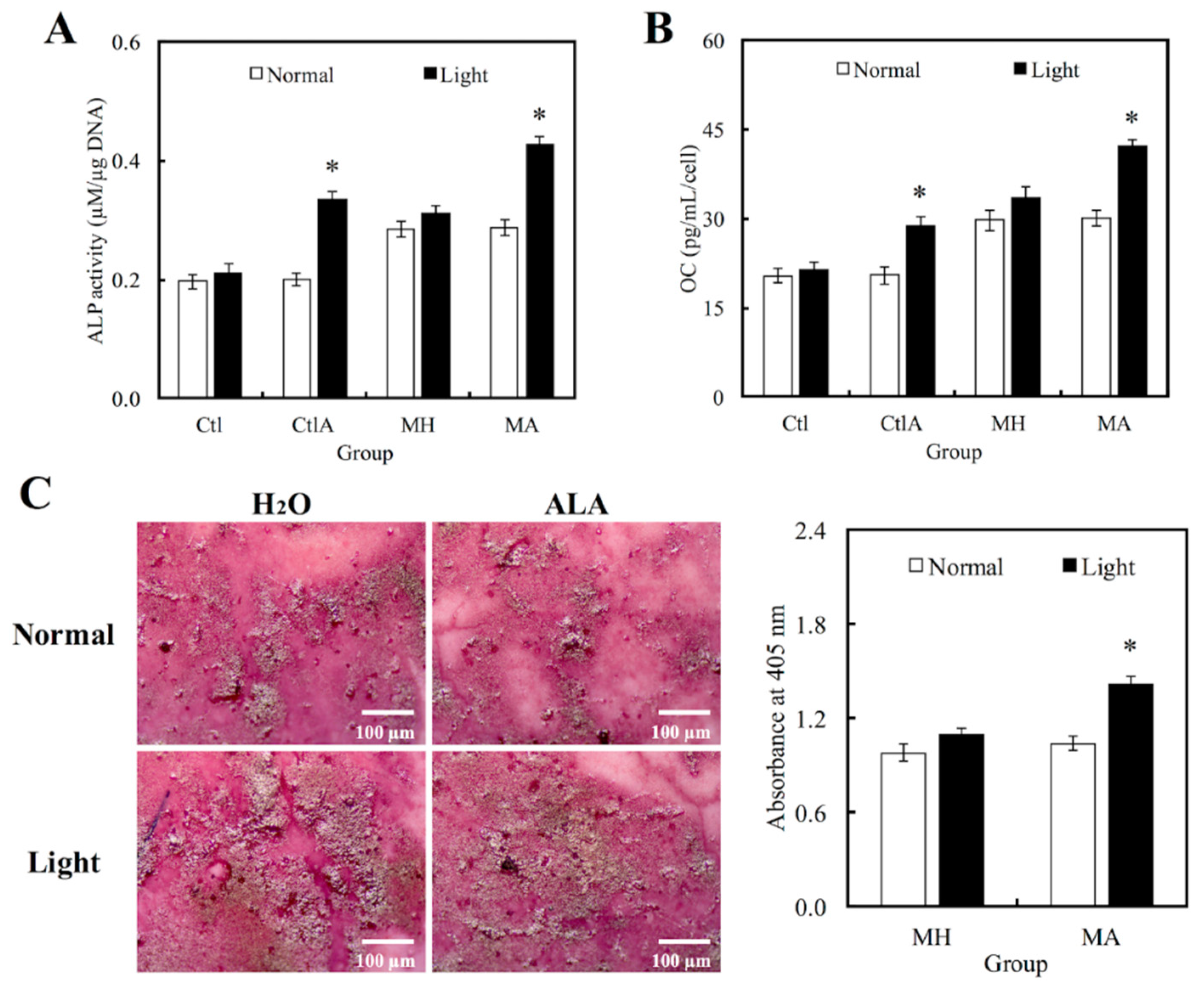
3.4. Odontogenic Differentiation in hDPCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nair, P.N. On the causes of persistent apical periodontitis, a review. Int. Endod. J. 2006, 39, 249–281. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr. Aetiology of root canal treatment failure: Why well-treated teeth can fail. Int. Endod. J. 2001, 34, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Sun, Y.; Fu, Y.; Chang, D.; Fu, C.; Wang, G.; Liu, Y.; Tay, F.R.; Zhou, Y. Bioinspired collagen-apatite nanocomposites for bone regeneration. J. Endod. 2016, 42, 1226–1232. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.A.; Versiani, M.A.; De-Deus, G.; Dummer, P.M.H. A new system for classifying root and root canal morphology. Int. Endod. J. 2017, 50, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.T.; Yeh, C.H.; Kao, C.T.; Chen, Y.W.; Huang, T.H.; Yang, J.J.; Shie, M.Y. Antibacterial and odontogenesis efficacy of mineral trioxide aggregate combined with CO2 laser treatment. J. Endod. 2015, 41, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Siboni, F.; Taddei, P.; Zamparini, F.; Prati, C.; Gandolfi, M.G. Properties of BioRoot RCS, a tricalcium silicate endodontic sealer modified with povidone and polycarboxylate. Int. Endod. J. 2017, 50, e120–e136. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Huang, T.H.; Kao, C.T.; Wu, Y.H.; Chen, W.C.; Shie, M.Y. Mesoporous calcium silicate nanoparticles with drug delivery and odontogenesis properties. J. Endod. 2017, 43, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.N.; Henry, S.; Cano, V.; Vera, J. Microbial status of apical root canal system of human mandibular first molars with primary apical periodontitis after “one-visit” endodontic treatment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 99, 231–252. [Google Scholar] [CrossRef] [PubMed]
- Al-Hezaimi, K.; Al-Shalan, T.A.; Naghshbandi, J. Antibacterial effect of two mineral trioxide aggregate (MTA) preparations against Enterococcus faecalis and Streptococcus sanguis in vitro. J. Endod. 2006, 32, 1053–1056. [Google Scholar] [CrossRef] [PubMed]
- McMichael, G.E.; Primus, C.M.; Opperman, L.A. Dentinal tubule penetration of tricalciums silicate sealers. J. Endod. 2016, 42, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Parrilli, A.P.; Fini, M.; Prati, C.; Dummer, P.M.H. 3D micro-CT analysis of the interface voids associated with Thermafil root fillings used with AH Plus or a flowable MTA sealer. Int. Endod. J. 2013, 46, 253–263. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Yeh, C.H.; Shie, M.Y. Stimulatory effects of the fast setting and degradable Ca–Si–Mg cement on both cementogenesis and angiogenesis differentiation of human periodontal ligament cells. J. Mater. Chem. B 2015, 3, 7099–7108. [Google Scholar] [CrossRef]
- Prati, C.; Gandolfi, M.G. Calcium silicate bioactive cements: Biological perspectives and clinical applications. Dent. Mater. 2015, 31, 351–370. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Fang, H.Y.; Hsu, T.T.; Lin, C.Y.; Shie, M.Y. The characteristics of Mineral Trioxide Aggregate/polycaprolactone 3-dimensional scaffold with osteogenesis properties for tissue regeneration. J. Endod. 2017, 43, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Ho, C.C.; Huang, T.H.; Hsu, T.T.; Shie, M.Y. The ionic products from mineral trioxide aggregate–induced odontogenic differentiation of dental pulp cells via activation of the Wnt/β-catenin signaling pathway. J. Endod. 2016, 42, 1062–1069. [Google Scholar] [CrossRef] [PubMed]
- Huang, T.H.; Shie, M.Y.; Kao, C.T.; Ding, S.J. The effect of setting accelerator on properties of mineral trioxide aggregate. J. Endod. 2008, 34, 590–593. [Google Scholar] [CrossRef] [PubMed]
- Shie, M.Y.; Chiang, W.H.; Chen, I.W.P.; Liu, W.Y.; Chen, Y.W. Synergistic acceleration in the osteogenic and angiogenic differentiation of human mesenchymal stem cells by calcium silicate–graphene composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 73, 726–735. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Hsu, T.T.; Wang, K.; Shie, M.Y. Preparation of the fast setting and degrading Ca-Si-Mg cement with both odontogenesis and angiogenesis differentiation of human periodontal ligament cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 60, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Cetenovic, B.; Prokic, B.; Vasilijic, S.; Dojcinovic, B.; Magic, M.; Jokanovic, V.; Markovic, D. Biocompatibility investigation of new endodontic materials based on nanosynthesized calcium silicates combined with different radiopacifiers. J. Endod. 2017, 43, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Tosun, E.; Tasar, F.; Strauss, R.; Kıvanc, D.G.; Ungor, C. Comparative evaluation of antimicrobial effects of Er:YAG, diode, and CO2 lasers on titanium discs: An experimental study. J. Oral Maxillofac. Surg. 2012, 70, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Kishen, A. The effect of tissue inhibitors on the antibacterial activity of chitosan nanoparticles and photodynamic therapy. J. Endod. 2012, 38, 1275–1278. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, R.F.; McCarron, P.A.; Tunney, M.M. Antifungal photodynamic therapy. Microbiol. Res. 2008, 163, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Garcez, A.S.; Nuñez, S.C.; Hamblim, M.R.; Suzuki, H.; Ribeiro, M.S. Photodynamic therapy associated with conventional endodontic treatment in patients with antibiotic-resistant microflora: A preliminary report. J. Endod. 2010, 36, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Chrepa, V.; Kotsakis, G.A.; Pagonis, T.C.; Hargreaves, K.M. The effect of photodynamic therapy in root canal disinfection: A systematic review. J. Endod. 2014, 40, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Fotinos, N.; Convert, M.; Piffaretti, J.C.; Gurny, R.; Lange, N. Effects on gram-negative and gram-positive bacteria mediated by 5-aminolevulinic Acid and 5-aminolevulinic acid derivatives. Antimicrob. Agents Chemother. 2008, 52, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.M.; Huang, Y.H.; Chen, C.P.; Hsieh, B.C.; Tsai, T. 5-Aminolevulinic acid induced photodynamic inactivation on Staphylococcus aureus and Pseudomonas aeruginosa. J. Food Drug Anal. 2014, 22, 350–355. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Lee, H.J.; Kim, S.H.; Lee, S.K.; Kim, H.R.; Pae, H.O.; Chung, H.T.; Shin, H.I.; Lee, S.-K.; Kim, E.C. Hydrogen peroxide induces heme oxygenase–1 and dentin sialophosphoprotein mRNA in human pulp cells. J. Endod. 2008, 34, 983–989. [Google Scholar] [CrossRef] [PubMed]
- Min, K.S.; Hwang, Y.H.; Ju, H.J.; Chang, H.S.; Kang, K.H.; Pi, S.H.; Lee, S.K.; Lee, S.K.; Kim, E.C. Heme oxygenase-1 mediates cytoprotection against nitric oxide-induced cytotoxicity via the cGMP pathway in human pulp cells. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006, 102, 803–808. [Google Scholar] [CrossRef] [PubMed]
- Katara, G.; Hemvani, N.; Chitnis, S.; Chitnis, V.; Chitnis, D.S. Katara Surface disinfection by exposure to germicidal UV light. Indian J. Med. Microbiol. 2008, 26, 241–242. [Google Scholar] [PubMed]
- Shie, M.Y.; Ding, S.J. Integrin binding and MAPK signal pathways in primary cell responses to surface chemistry of calcium silicate cements. Biomaterials 2013, 34, 6589–6606. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Wu, B.C.; Kao, C.T.; Huang, T.H.; Hung, C.J.; Shie, M.Y. Role of the p38 pathway in mineral trioxide aggregate-induced cell viability and angiogenesis-related proteins of dental pulp cell in vitro. Int. Endod. J. 2015, 48, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Asnaashari, M.; Godiny, M.; Azari-Marhabi, S.; Tabatabaei, F.S.; Barati, M. Comparison of the antibacterial effect of 810 nm diode laser and photodynamic therapy in reducing the microbial flora of root canal in endodontic retreatment in patients with periradicular lesions. J. Lasers Med. Sci. 2016, 7, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chen, Y.; Wang, X.; Feng, X.; Wang, P.; Liu, Q. Comparison of protoporphyrin IX produced cell proliferation inhibition between human breast cancer MCF-7 and MDA-MB-231 cells. Pharmazie 2014, 69, 621–628. [Google Scholar] [PubMed]
- Josefsen, L.B.; Boyle, R.W. Unique diagnostic and therapeutic roles of porphyrins and phthalocyanines in photodynamic therapy, imaging and theranostics. Theranostics 2012, 2, 916–966. [Google Scholar] [CrossRef] [PubMed]
- Dietel, W.; Pottier, R.; Pfister, W.; Schleier, P.; Zinner, K. 5-Aminolaevulinic acid (ALA) induced formation of different fluorescent porphyrins: A study of the biosynthesis of porphyrins by bacteria of the human digestive tract. J. Photochem. Photobiol. B 2007, 86, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Akilov, O.E.; Kosaka, S.; O’Riordan, K.; Hasan, T. Parasiticidal effect of δ-aminolevulinic acid-based photodynamic therapy for cutaneous leishmaniasis is indirect and mediated through the killing of the host cells. Exp. Dermatol. 2007, 16, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Fabbrocini, G.; Di Costanzo, M.P.; Riccardo, A.M.; Quarto, M.; Colasanti, A.; Roberti, G.; Monfrecola, G. Photodynamic therapy with topical δ-aminolaevulinic acid for the treatment of plantar warts. J. Photochem. Photobiol. B 2001, 61, 30–34. [Google Scholar] [CrossRef]
- Zawislak, A.; Donnelly, R.F.; McCluggage, W.G.; Price, J.H.; McClelland, H.R.; Woolfson, A.D.; Dobbs, S.; Maxwell, P.; McCarron, P.A. Clinical and immunohistochemical assessment of vulval intraepithelial neoplasia following photodynamic therapy using a novel bioadhesive patch-type system loaded with 5-aminolevulinic acid. Photodiagnosis Photodyn. Ther. 2009, 6, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.C.; Fang, H.Y.; Wang, B.; Huang, T.H.; Shie, M.Y. The effects of Biodentine/polycaprolactone 3D-scaffold with odontogenesis properties on human dental pulp cells. Int. Endod. J. 2018, 51, e291–e300. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.G.; Ho, C.C.; Hsu, T.T.; Huang, T.H.; Lin, M.J.; Shie, M.Y. Mineral Trioxide Aggregate with mussel-inspired surface nanolayers for stimulating odontogenic differentiation of dental pulp cells. J. Endod. 2018, 44, 963–970. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, M.G.; Siboni, F.; Prati, C. Properties of a novel polysiloxane-guttapercha calcium silicate-bioglass-containing root canal sealer. Dent. Mater. 2016, 32, e113–e126. [Google Scholar] [CrossRef] [PubMed]
- Hsu, T.T.; Kao, C.T.; Chen, Y.W.; Huang, T.H.; Yang, J.J.; Shie, M.Y. The synergistic effects of CO2 laser treatment with calcium silicate cement of antibacterial, osteogenesis and cementogenesis efficacy. Laser Phys. Lett. 2015, 12, 055602. [Google Scholar] [CrossRef]
- Antunes, H.S.; Gominho, L.F.; Andrade Junior, C.V.; Dessaune Neto, N.; Alves, F.R.F.; Rôças, I.N.; Siqueira, J.F. Sealing ability of two root-end filling materials in a bacterial nutrient leakage model. Int. Endod. J. 2016, 49, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Shalavi, S.; Yazdizadeh, M. Antimicrobial activity of calcium hydroxide in endodontics: A review. Chonnam Med. J. 2012, 48, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Farhad, A.; Mohammadi, Z. Calcium hydroxide: A review. Int. Dent. J. 2005, 55, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Moghaddame-Jafari, S.; Mantellini, M. Effect of ProRoot MTA on pulp cell apoptosis and proliferation in vitro. J. Endod. 2005, 31, 387–391. [Google Scholar] [CrossRef] [PubMed]
- Wyld, L.; Smith, O.; Lawry, J.; Reed, M.W.; Brown, N.J. Cell cycle phase influences tumour cell sensitivity to aminolaevulinic acid-induced photodynamic therapy in vitro. Br. J. Cancer 1998, 78, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C.; Chiang, C.P.; Chen, H.M.; Huang, S.B.; Wang, C.W.; Lee, M.I.; Hsu, Y.C.; Chen, C.T.; Tsai, T. Photodynamic therapy of oral dysplasia with topical 5-aminolevulinic acid and light-emitting diode array. Lasers Surg. Med. 2004, 34, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.; Salhab, I.; Setzer, F.C.; Kim, S.; Nah, H.D. A new calcium silicate–based bioceramic material promotes human osteo- and odontogenic stem cell proliferation and survival via the extracellular signal-regulated kinase signaling pathway. J. Endod. 2016, 42, 480–486. [Google Scholar] [CrossRef] [PubMed]
- Rifaey, H.S.; Villa, M.; Zhu, Q.; Wang, Y.H.; Safavi, K.; Chen, I.P. Comparison of the osteogenic potential of mineral trioxide aggregate and endosequence root repair material in a 3-dimensional culture system. J. Endod. 2016, 42, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Karamzadeh, R.; Baghaban Eslaminejad, M.; Sharifi-Zarchi, A. Comparative in vitro evaluation of human dental pulp and follicle stem cell commitment. Cell J. 2017, 18, 609–618. [Google Scholar] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, Y.-F.; Huang, T.-H.; Ng, H.-Y.; Fang, H.-Y.; Hsu, T.-T. Mineral Trioxide Aggregate Mixed with 5-Aminolevulinic Acid for the Photodynamic Antimicrobial Strategy in Hard Tissue Regeneration. Materials 2018, 11, 1734. https://doi.org/10.3390/ma11091734
Shen Y-F, Huang T-H, Ng H-Y, Fang H-Y, Hsu T-T. Mineral Trioxide Aggregate Mixed with 5-Aminolevulinic Acid for the Photodynamic Antimicrobial Strategy in Hard Tissue Regeneration. Materials. 2018; 11(9):1734. https://doi.org/10.3390/ma11091734
Chicago/Turabian StyleShen, Yu-Fang, Tsui-Hsien Huang, Hooi-Yee Ng, Hsin-Yuan Fang, and Tuan-Ti Hsu. 2018. "Mineral Trioxide Aggregate Mixed with 5-Aminolevulinic Acid for the Photodynamic Antimicrobial Strategy in Hard Tissue Regeneration" Materials 11, no. 9: 1734. https://doi.org/10.3390/ma11091734
APA StyleShen, Y.-F., Huang, T.-H., Ng, H.-Y., Fang, H.-Y., & Hsu, T.-T. (2018). Mineral Trioxide Aggregate Mixed with 5-Aminolevulinic Acid for the Photodynamic Antimicrobial Strategy in Hard Tissue Regeneration. Materials, 11(9), 1734. https://doi.org/10.3390/ma11091734



