Ion Transport Properties and Ionicity of 1,3-Dimethyl-1,2,3-Triazolium Salts with Fluorinated Anions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
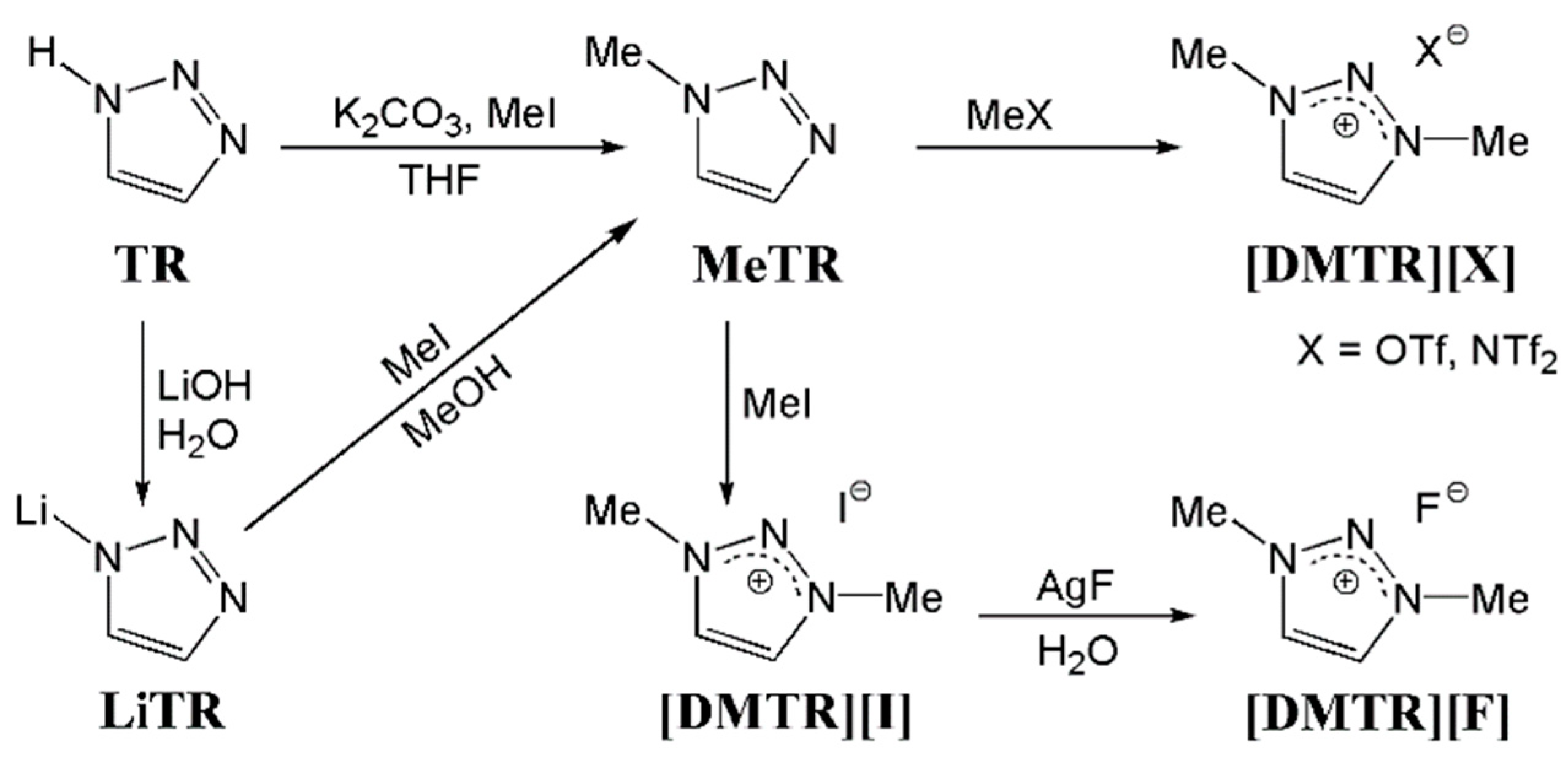
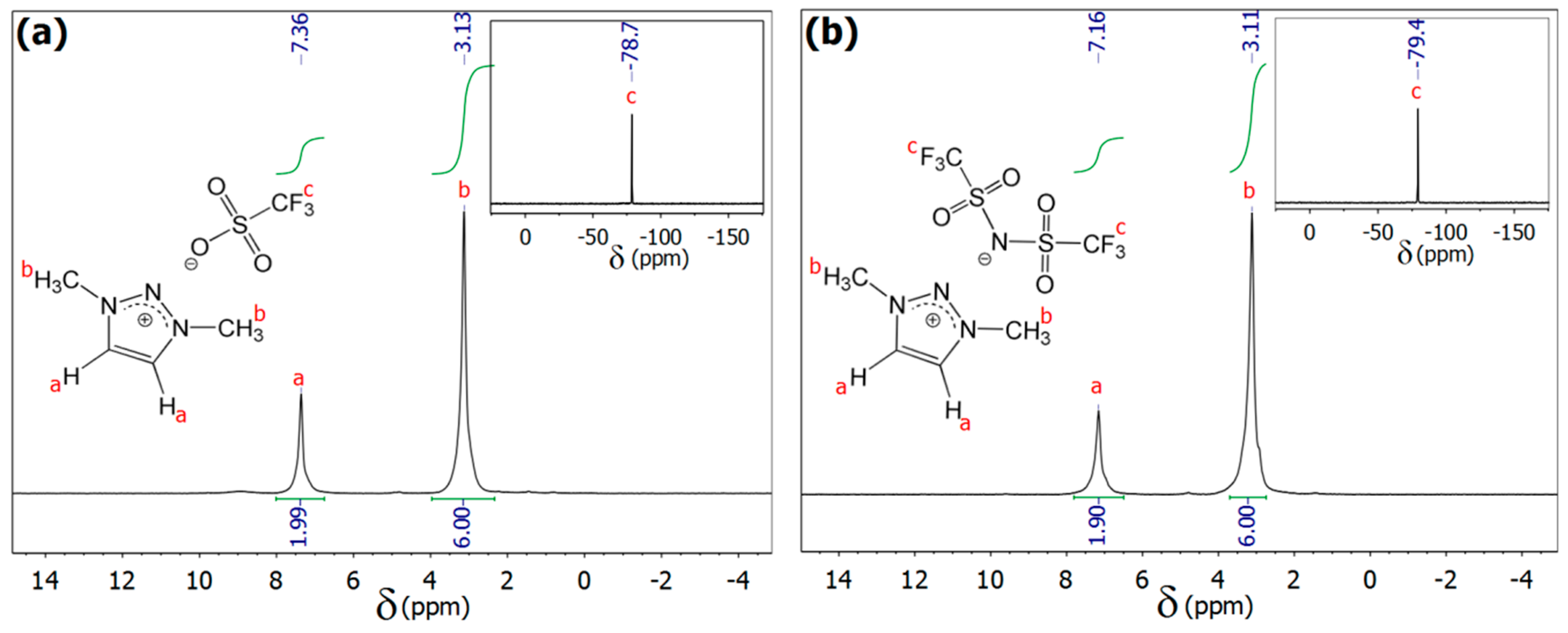
2.2. Methods
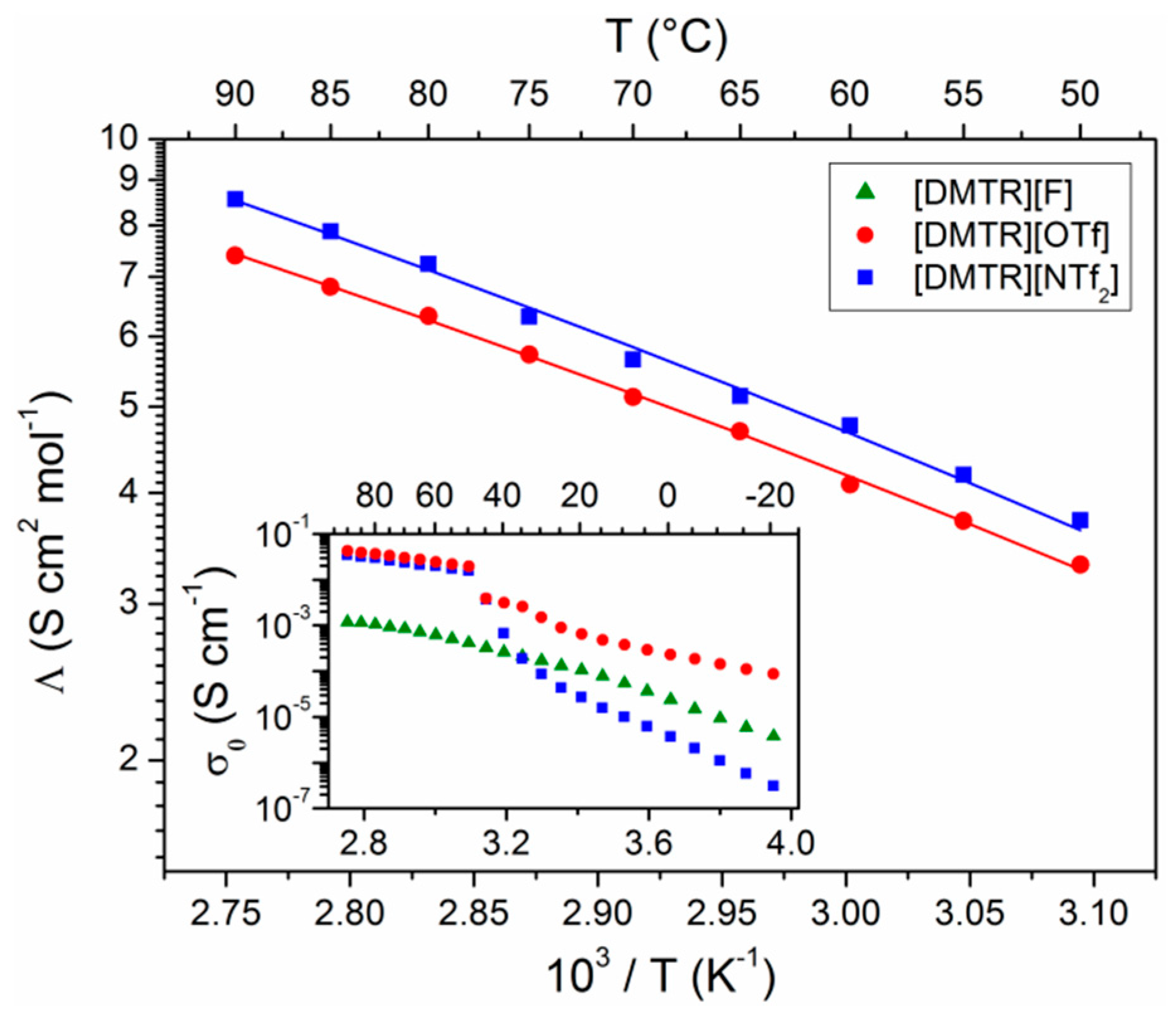
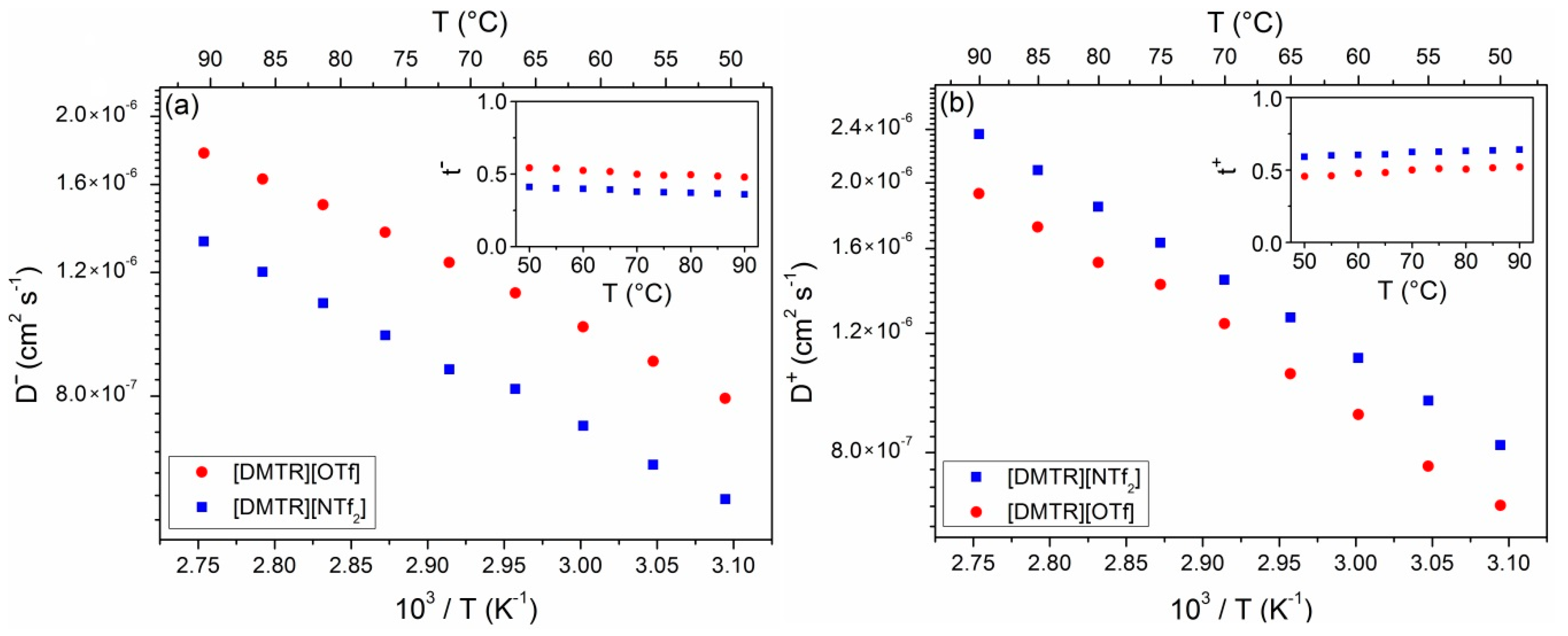
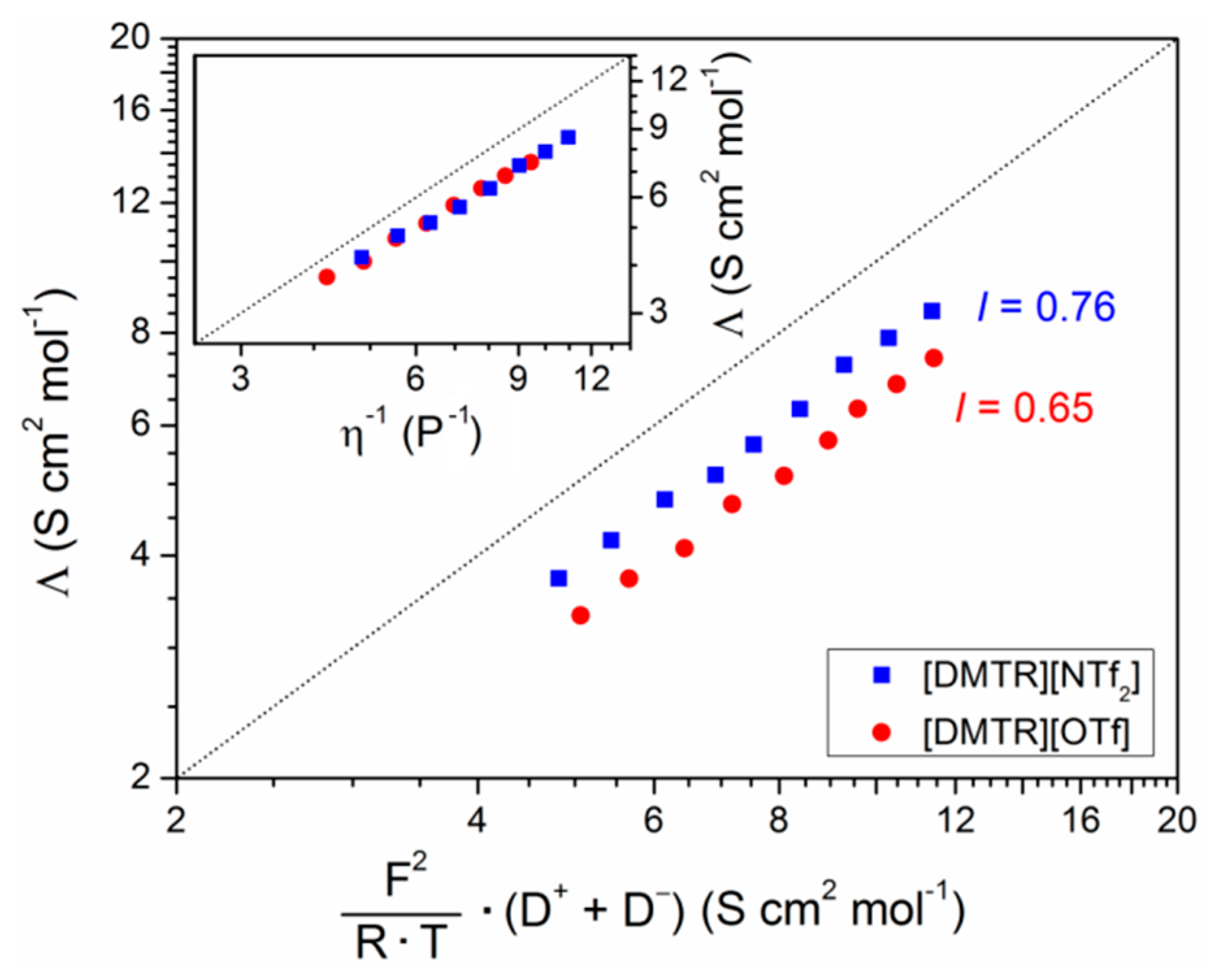
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yacob, Z.; Liebscher, J. 1,2,3-Triazolium Salts as a Versatile New Class of Ionic Liquids. In Ionic Liquids–Classes and Properties; Handy, S.T., Ed.; InTech: Rijeka, Croatia, 2011. [Google Scholar]
- Riduan, S.N.; Zhang, Y. Imidazolium salts and their polymeric materials for biological applications. Chem. Soc. Rev. 2013, 42, 9055–9070. [Google Scholar] [CrossRef] [PubMed]
- Aizpurua, J.M.; Fratila, R.M.; Monasterio, Z.; Pérez-Esnaola, N.; Andreieff, E.; Irastorza, A.; Sagartzazu-Aizpurua, M. Triazolium cations: From the “click” pool to multipurpose applications. New J. Chem. 2014, 38, 474–480. [Google Scholar] [CrossRef]
- Obadia, M.M.; Drockenmuller, E. Poly(1,2,3-triazolium)s: A new class of functional polymer electrolytes. Chem. Commun. 2016, 52, 2433–2450. [Google Scholar] [CrossRef] [PubMed]
- Schulze, B.; Schubert, U.S. Beyond Click Chemistry–Supramolecular Interactions of 1,2,3-Triazoles. Chem. Soc. Rev. 2014, 43, 2522–2571. [Google Scholar] [CrossRef] [PubMed]
- Mirjafari, A. Ionic liquid syntheses via click chemistry: Expeditious routes toward versatile functional materials. Chem. Commun. 2018, 54, 2944–2961. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.; Ryu, J.-S. Synthesis of 1,3-Dialkyl-1,2,3-triazolium Ionic Liquids and Their Applications to the Baylis−Hillman Reaction. J. Org. Chem. 2010, 75, 4183–4191. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-Y.; Chen, C.-Y.; Cheng, H.-T.; Chu, Y.-H. Exploiting 1,2,3-Triazolium Ionic Liquids for Synthesis of Tryptanthrin and Chemoselective Extraction of Copper(II) Ions and Histidine-Containing Peptides. Molecules 2016, 21, 1355. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Li, Q.; Dong, F.; Zhang, J.; Luan, F.; Wei, L.; Chen, Y.; Guo, Z. Novel cationic chitosan derivative bearing 1,2,3-triazolium and pyridinium: Synthesis, characterization, and antifungal property. Carbohydr. Polym. 2018, 182, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Cuervo-Rodríguez, A.M.-B.R.; López-Fabal, F.; Gómez-Garcés, J.L.; Fernández-García, M. Antimicrobial Porous Surfaces Prepared by Breath Figures Approach. Materials 2018, 11, 1266. [Google Scholar]
- Coutrot, F. A Focus on Triazolium as a Multipurpose Molecular Station for pH-Sensitive Interlocked Crown-Ether-Based Molecular Machines. Chem. Open 2015, 4, 556–576. [Google Scholar]
- Clavel, C.; Fournel-Marotte, K.; Coutrot, F. A pH-Sensitive Peptide-Containing Lasso Molecular Switch. Molecules 2013, 18, 11553–11575. [Google Scholar] [CrossRef] [PubMed]
- Nayal, A.; Pandey, P.S. Bile acid-based triazole and triazolium receptors for colorimetric sensing of anions. Tetrahedron 2015, 71, 6991–6996. [Google Scholar] [CrossRef]
- Steiner, I.; Stojanovic, N.; Bolje, A.; Brozovic, A.; Polancec, D.; Ambriovic-Ristov, A.; Stojkovic, M.R.; Piantanida, I.; Eljuga, D.; Kosmrlj, J.; et al. Discovery of ‘click’ 1,2,3-triazolium salts as potential anticancer drugs. Radiol. Oncol. 2016, 50, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, K.F.; Petronilho, A.; Albrecht, M. Application of 1,2,3-triazolylidenes as versatile NHC-type ligands: Synthesis, properties, and application in catalysis and beyond. Chem. Commun. 2013, 49, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Hettmanczyk, L.; Schmid, B.; Hohloch, S.; Sarkar, B. Palladium(II)-Acetylacetonato Complexes with Mesoionic Carbenes: Synthesis, Structures and Their Application in the Suzuki-Miyaura Cross Coupling Reaction. Molecules 2016, 21, 1561. [Google Scholar] [CrossRef] [PubMed]
- Sanghi, S.; Willett, E.; Versek, C.; Tuominen, M.; Coughlin, E.B. Physicochemical properties of 1,2,3-triazolium ionic liquids. RSC Adv. 2012, 2, 848–853. [Google Scholar] [CrossRef]
- M’sahel, M.; Obadia, M.M.; Medimagh, R.; Serghei, A.; Zina, M.S.; Drockenmuller, E. Biosourced 1,2,3-triazolium ionic liquids derived from isosorbide. New J. Chem. 2016, 40, 740–747. [Google Scholar] [CrossRef]
- Stappert, K.; Ünal, D.; Mallicka, B.; Mudring, A.-V. New triazolium based ionic liquid crystals. J. Mater. Chem. C 2014, 2, 7976–7986. [Google Scholar] [CrossRef]
- Martini, M.; Hegger, P.S.; Schädel, N.; Minsky, B.B.; Kirchhof, M.; Scholl, S.; Southan, A.; Tovar, G.E.M.; Boehm, H.; Laschat, S. Charged Triazole Cross-Linkers for Hyaluronan-Based Hybrid Hydrogels. Materials 2016, 9, 810. [Google Scholar] [CrossRef] [PubMed]
- Tokuda, H.; Tsuzuk, S.; Susan, M.A.B.H.; Hayamizu, K.; Watanabe, M. How Ionic Are Room-Temperature Ionic Liquids? An Indicator of the Physicochemical Properties. J. Phys. Chem. B 2006, 110, 19593–19600. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Forsyth, M.; Izgorodina, E.I.; Abbott, A.P.; Annata, G.; Frasera, K. On the concept of ionicity in ionic liquids. Phys. Chem. Chem. Phys. 2009, 11, 4962–4967. [Google Scholar] [CrossRef] [PubMed]
- Gouverneur, M.; Kopp, J.; van Wüllen, L.; Schönhoff, M. Direct determination of ionic transference numbers in ionic liquids by electrophoretic NMR. Phys. Chem. Chem. Phys. 2015, 17, 30680–30686. [Google Scholar] [CrossRef] [PubMed]
- Hollóczki, O.; Malberg, F.; Welton, T.; Kirchner, B. On the origin of ionicity in ionic liquids. Ion pairing versus charge transfer. Phys. Chem. Chem. Phys. 2014, 16, 16880–16890. [Google Scholar] [CrossRef] [PubMed]
- Lartey, M.; Meyer-Ilse, J.; Watkins, J.D.; Roth, E.A.; Bowser, S.; Kusuma, V.A.; Damodaran, K.; Zhou, X.; Haranczyk, M.; Albenze, E.; et al. Branched isomeric 1,2,3-triazolium-based ionic liquids: New insight into structure–property relationships. Phys. Chem. Chem. Phys. 2015, 17, 29834–29843. [Google Scholar] [CrossRef] [PubMed]
- Reeder, Z.K.; Adler, A.M.; Miller, K.M. 1-Alkyl-3-methyl-1,2,3-triazolium [NTf2] ionic liquids: Synthesis and properties. Tetrahedron Lett. 2016, 57, 206–209. [Google Scholar] [CrossRef]
- Watkins, J.D.; Roth, E.A.; Lartey, M.; Albenze, E.; Zhong, M.; Luebke, D.R.; Nulwala, H.B. Ionic liquid regioisomers: Structure effect on the thermal and physical properties. New J. Chem. 2015, 39, 1563–1566. [Google Scholar] [CrossRef]
- Singh, D.; Gardas, R.L. Influence of Cation Size on the Ionicity, Fluidity, and Physiochemical Properties of 1,2,4-Triazolium Based Ionic Liquids. J. Phys. Chem. B 2016, 120, 4834–4842. [Google Scholar] [CrossRef] [PubMed]
- Begtrup, M.; Kristensen, P.A. Reactions between Azolium Salts and Nucleophilic Reagents I. Bromo-1,2,3-triazolium Salts as Brominating Reagents. Acta Chem. Scand. 1969, 23, 2733–2740. [Google Scholar] [CrossRef]
- Benson, F.R.; Savell, W.L. The Chemistry of the Vicinal Triazoles. Chem. Rev. 1950, 46, 1–68. [Google Scholar] [CrossRef] [PubMed]
- Dehaen, W.; Bakulev, V.A. (Eds.) Chemistry of 1,2,3-Triazoles; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Pulst, M.; Balko, J.; Golitsyn, Y.; Reichert, D.; Busse, K.; Kressler, J. Proton conductivity and phase transitions in 1,2,3-triazole. Phys. Chem. Chem. Phys. 2016, 18, 6153–6163. [Google Scholar] [CrossRef] [PubMed]
- Pulst, M.; Elgabarty, H.; Sebastiani, D.; Kressler, J. The annular tautomerism of lithium 1,2,3-triazolate. New J. Chem. 2017, 41, 1430–1435. [Google Scholar] [CrossRef]
- Riedel, E.; Janiak, C. Anorganische Chemie, 7th ed.; deGruyter: Berlin, Germany, 2007. [Google Scholar]
- Freire, M.G.; Teles, A.R.R.; Rocha, M.A.A.; Schröder, B.; Neves, C.M.S.S.; Carvalho, P.J.; Evtuguin, D.V.; Santos, L.M.N.B.F.; Coutinho, J.A.P. Thermophysical Characterization of Ionic Liquids Able to Dissolve Biomass. J. Chem. Eng. Data 2011, 56, 4813–4822. [Google Scholar] [CrossRef]
- Gardas, R.L.; Coutinho, J.A.P. Extension of the Ye and Shreeve group contribution method for density estimation of ionic liquids in a wide range of temperatures and pressures. Fluid Phase Equilib. 2008, 263, 26–32. [Google Scholar] [CrossRef]
- Dyre, J.C. A Simple Model of AC Hopping Conductivity in Disordered Solids. Phys. Lett. A 1985, 108, 457–461. [Google Scholar] [CrossRef]
- Diederichsen, K.M.; Buss, H.G.; McCloskey, B.D. The Compensation Effect in the Vogel-Tammann-Fulcher (VTF) Equation for Polymer-Based Electrolytes. Macromolecules 2017, 50, 3831–3840. [Google Scholar] [CrossRef]
- Compaan, K.; Haven, Y. Correlation factors for diffusion in solids. Part 2. Indirect interstitial mechanism. Trans. Faraday Soc. 1958, 54, 1498–1508. [Google Scholar] [CrossRef]
- Stejskal, E.O.; Tanner, J.E. Spin Diffusion Measurements: Spin Echoes in the Presence of a Time Dependent Field Gradient. J. Chem. Phys. 1965, 42, 288–292. [Google Scholar] [CrossRef]
| Triazolium Salt | Cation | Anion | V+ (Å3) | V− (Å3) | Tm (°C) | Td10 (°C) | ρ (g cm−3) |
|---|---|---|---|---|---|---|---|
| [DMTR][F] | 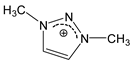 | 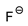 | 154 1 | 10 2 | --- 5 | 155 | --- |
| [DMTR][OTf] | 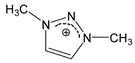 | 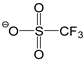 | 154 1 | 129 3 | 49.0 | 360 | 1.467 |
| [DMTR][NTf2] |  |  | 154 1 | 248 4 | 46.1 | 385 | 1.583 |
| Ionic Liquid | Λ (S cm2 mol−1) | I | Λ∞ (S cm2 mol−1) | Ea (kJ mol−1) | T0 (K) |
|---|---|---|---|---|---|
| [DMTR][OTf] | 3.3 | 0.65 | 172 | 5.06 | 169 |
| [DMTR][NTf2] | 3.7 | 0.76 | 264 | 5.74 | 162 |
| [DMIM][NTf2] 1 | 2.5 | 0.76 | --- | --- | --- |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pulst, M.; Golitsyn, Y.; Reichert, D.; Kressler, J. Ion Transport Properties and Ionicity of 1,3-Dimethyl-1,2,3-Triazolium Salts with Fluorinated Anions. Materials 2018, 11, 1723. https://doi.org/10.3390/ma11091723
Pulst M, Golitsyn Y, Reichert D, Kressler J. Ion Transport Properties and Ionicity of 1,3-Dimethyl-1,2,3-Triazolium Salts with Fluorinated Anions. Materials. 2018; 11(9):1723. https://doi.org/10.3390/ma11091723
Chicago/Turabian StylePulst, Martin, Yury Golitsyn, Detlef Reichert, and Jörg Kressler. 2018. "Ion Transport Properties and Ionicity of 1,3-Dimethyl-1,2,3-Triazolium Salts with Fluorinated Anions" Materials 11, no. 9: 1723. https://doi.org/10.3390/ma11091723
APA StylePulst, M., Golitsyn, Y., Reichert, D., & Kressler, J. (2018). Ion Transport Properties and Ionicity of 1,3-Dimethyl-1,2,3-Triazolium Salts with Fluorinated Anions. Materials, 11(9), 1723. https://doi.org/10.3390/ma11091723






