Composition, Structure and Morphology Evolution of Octadecylamine (ODA)–Reduced Graphene Oxide and Its Dispersion Stability under Different Reaction Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Modification of GO
2.3. Material Characterizations
3. Results
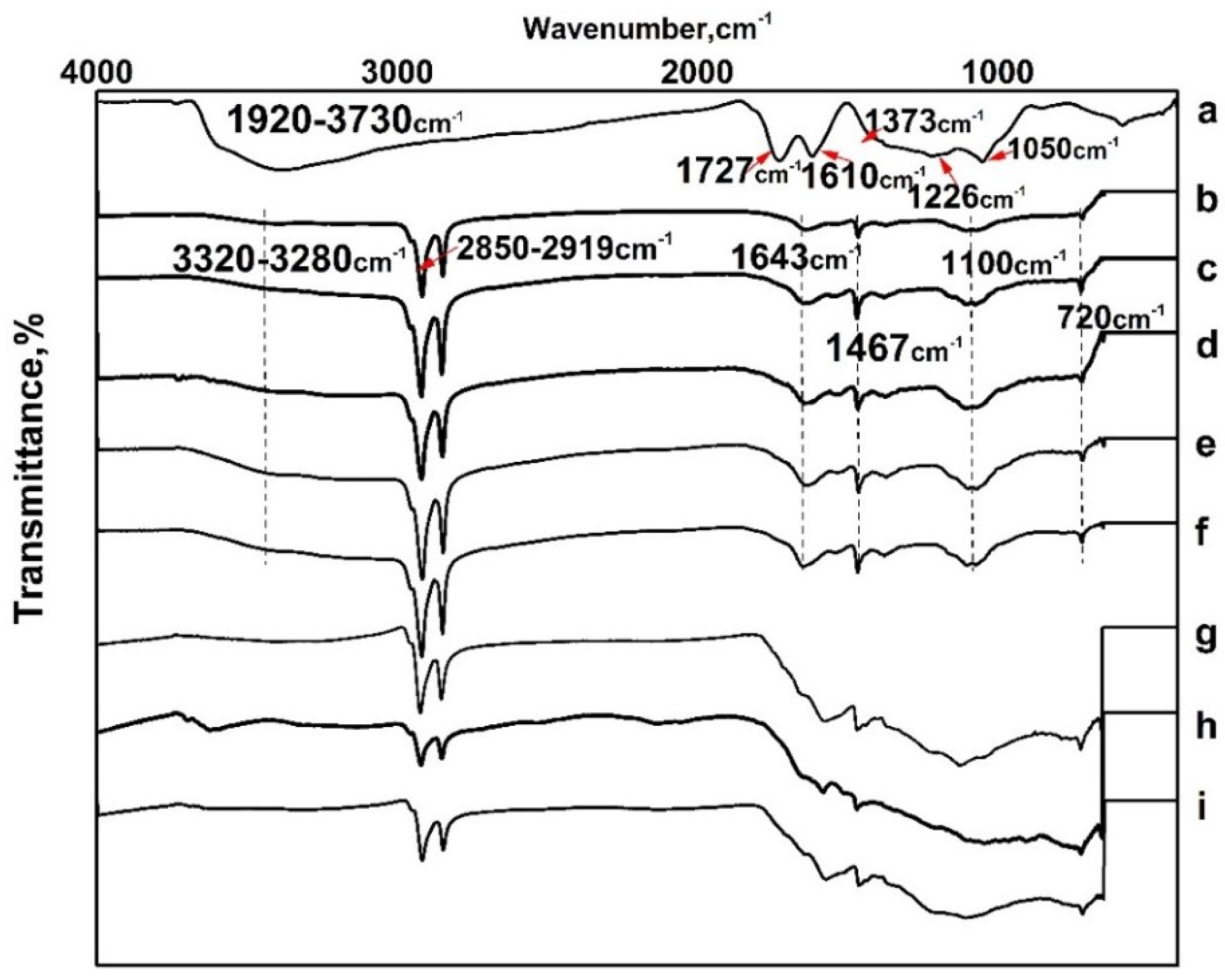
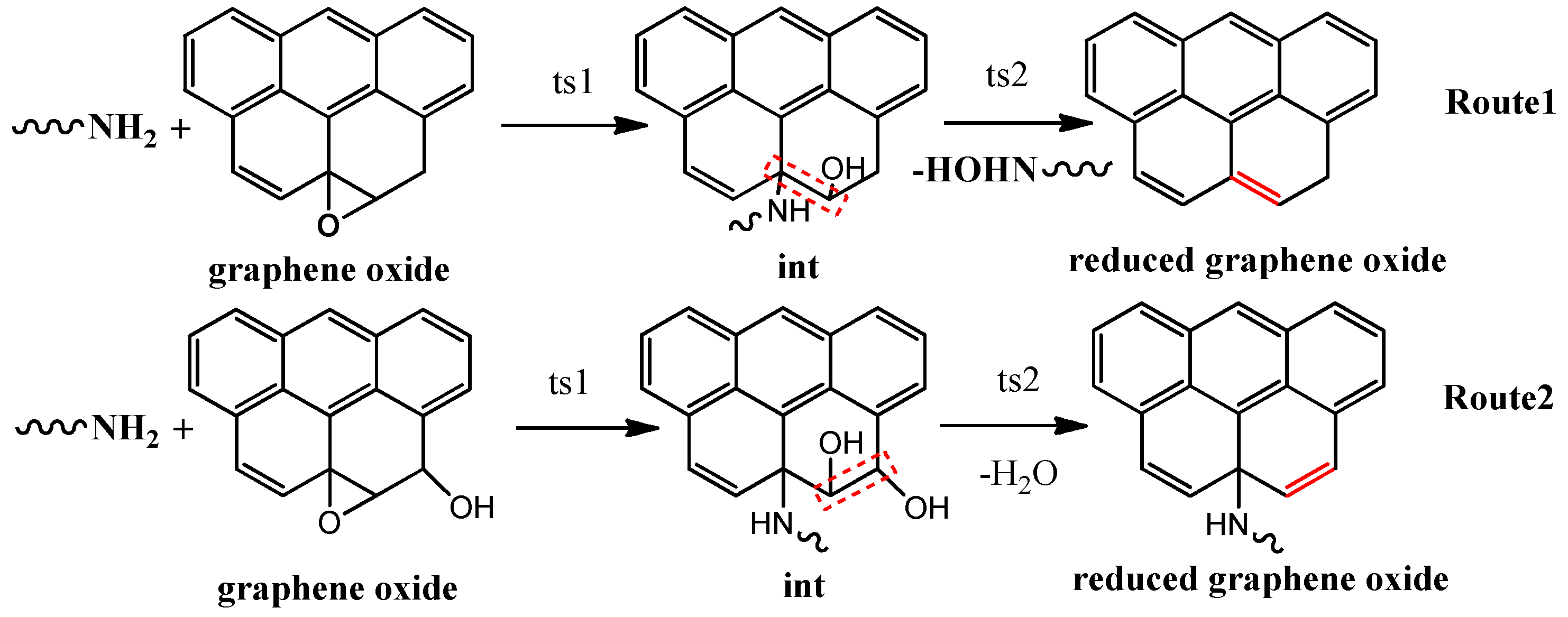
3.1. Composition and Chemical Structure of ORGOs
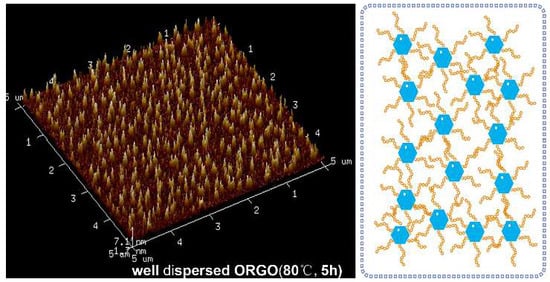
3.2. Morphology Evolution of ORGOs with Different Conditions
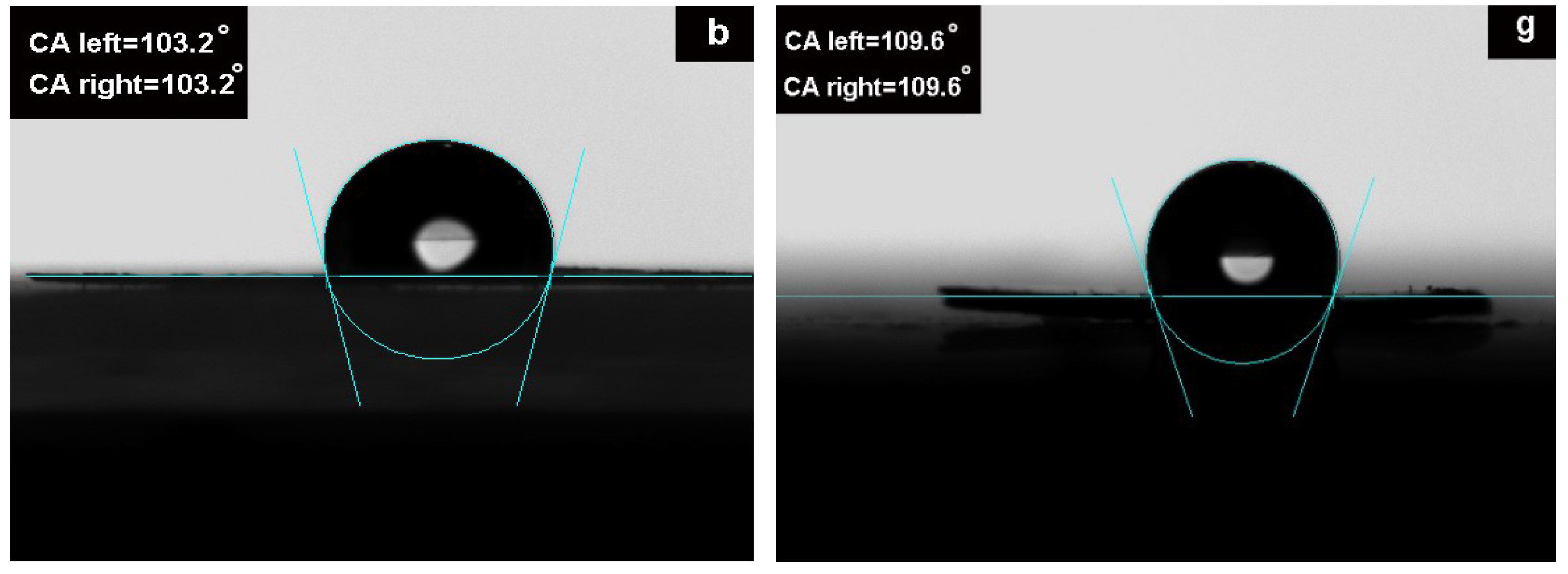
3.3. Dispersion Stability of ORGOs
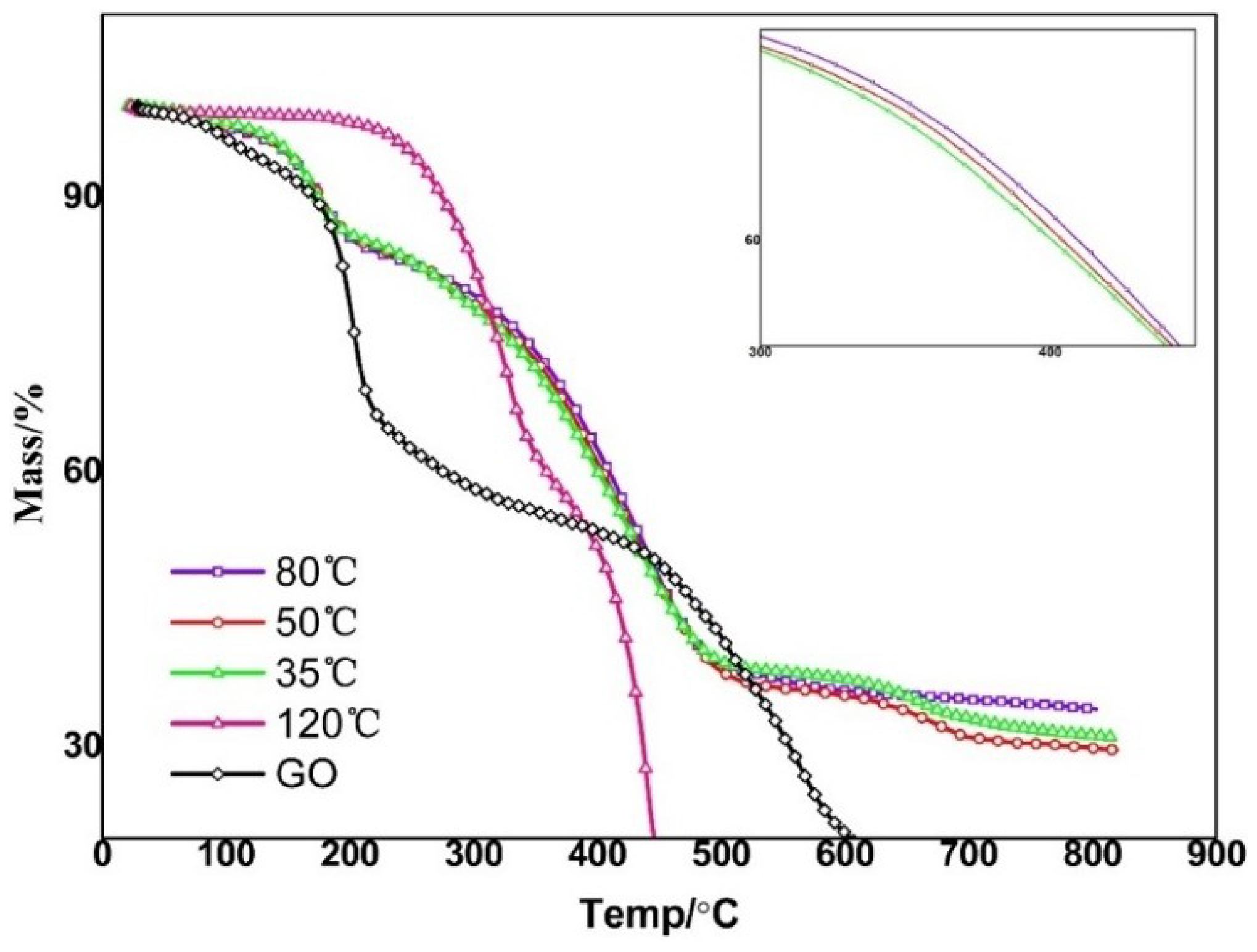
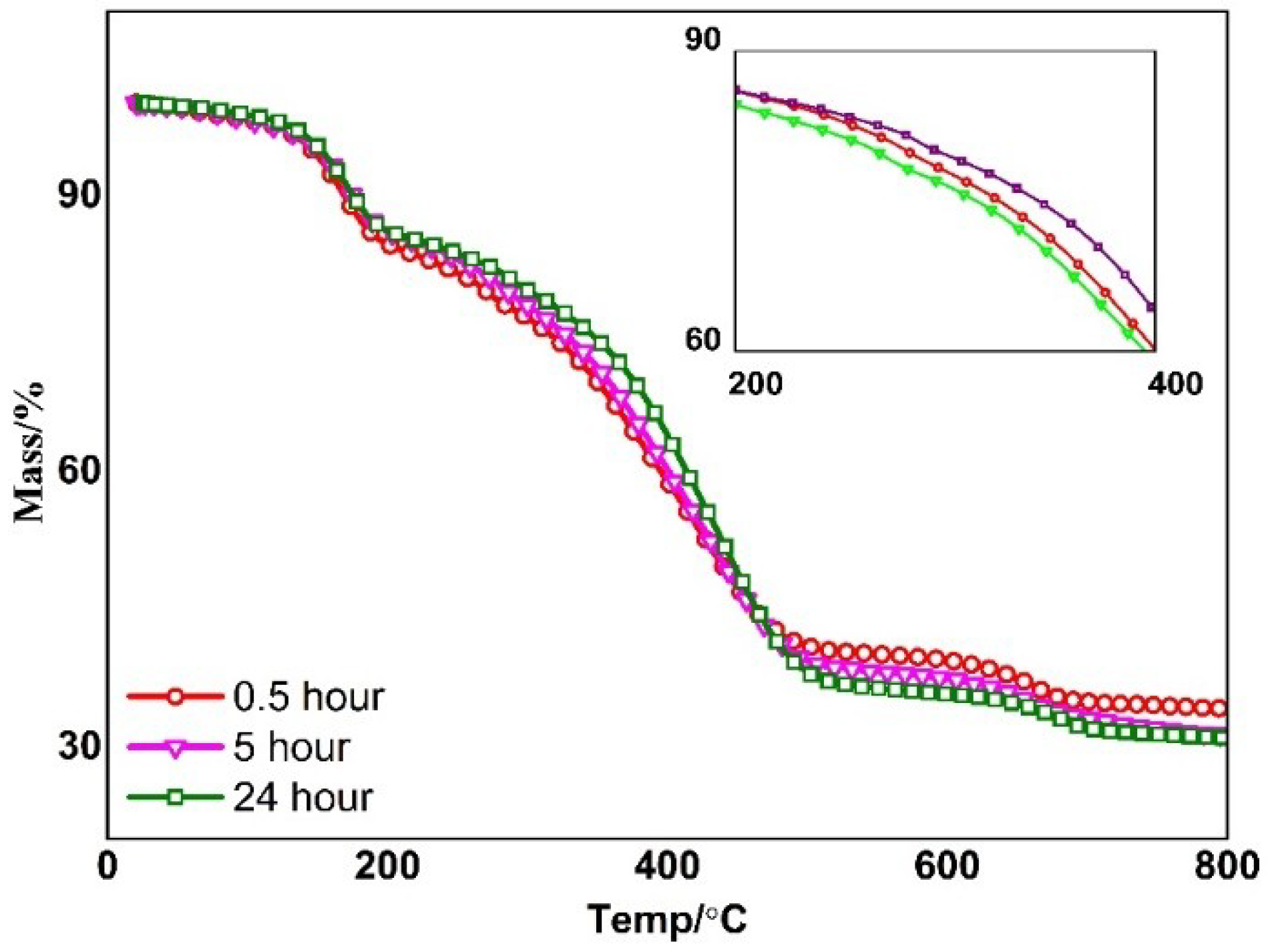
3.4. Heat Resistant Properties of ORGOs
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Geim, A.K.; Novoselov, K.S. The rise of grapheme. Nat. Mater. 2007, 6, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene–based composite materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Mccarthy, J.E.; Baranov, A.; Gun’ko, Y.K. Development of Graphene Nano-Platelet Based Counter Electrodes for Solar Cells. Materials 2015, 8, 5953–5973. [Google Scholar] [CrossRef] [PubMed]
- Eda, G.; Fanchini, G.; Chhowalla, M. Large-Area ultrathin films of reduced graphene oxide as a transparent and flexible electronic material. Nat. Nanotechnol. 2008, 3, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, C.; Ma, J. Applications of Graphene-Modified Electrodes in Microbial Fuel Cells. Materials 2016, 9, 807. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Wang, F.; Dai, J.; Huang, Z. Effect of Functionalization of Graphene Nanoplatelets on the Mechanical and Thermal Properties of Silicone Rubber Composites. Materials 2016, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Ruoff, R.S. Chemical methods for the production of graphenes. Nat. Nanotechnol. 2009, 4, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Muller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Velamakanni, A.; Bozoklu, G.; Park, S.; Stoller, M.; Piner, R.D.; Stankovich, S.; Jung, I.; Field, D.A.; Ventrice, C.A., Jr.; et al. Chemical analysis of graphene oxide films after heat and chemical treatments by X-ray photoelectron and micro–Raman spectroscopy. Carbon 2009, 47, 145–152. [Google Scholar] [CrossRef]
- Park, S.; An, J.; Jung, I.; Piner, R.D.; Jin, S.A.; Li, X.; Velamakanni, A.; Ruoff, R.S. Colloidal suspensions of highly reduced graphene oxide in a wide variety of organic solvents. Nano Lett. 2009, 9, 1593–1597. [Google Scholar] [CrossRef] [PubMed]
- Jeanphilippe, T.; Mark, A.B. Dispersion of alkyl–chain–functionalized reduced graphene oxide sheets in nonpolar solvents. Langmuir 2012, 16, 6691–6697. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Stable Aqueous Dispersions of Graphitic Nanoplatelets via the Reduction of Exfoliated Graphite Oxide in the Presence of Poly(sodium 4-styrenesulfonate). J. Mater. Chem. 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Xu, C.; Wu, X.; Zhu, J.; Wang, X. Synthesis of amphiphilic graphite oxide. Carbon 2008, 46, 386–389. [Google Scholar] [CrossRef]
- Stankovich, S.; Piner, R.D.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis and exfoliation of isocyanate–treated graphene oxide nanoplatelets. Carbon 2006, 44, 3342–3347. [Google Scholar] [CrossRef]
- Kuila, T.; Bose, S.; Hong, C.E.; Uddin, M.E.; Khanra, P.; Kim, N.H.; Lee, J.H. Preparation of functionalized graphene/linear low–density polyethylene composites by a solution mixing method. Carbon 2011, 49, 1033–1037. [Google Scholar] [CrossRef]
- Bourlinos, A.B.; Gournis, D.; Petridis, D.; Szabo, T.; Szeri, A.; Dekany, I. Graphite oxide: Chemical reduction to graphite and surface modification with primary aliphatic amines and amino acids. Langmuir 2003, 19, 6050–6055. [Google Scholar] [CrossRef]
- Niyogi, S.; Bekyarova, E.; Itkis, M.E.; McWilliams, J.L.; Hamon, M.A.; Haddon, R.C. Solution properties of graphite and grapheme. J. Am. Chem. Soc. 2006, 128, 7720–7721. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.W.; Feng, J.C.; Wu, P.Y. Alkyl–functionalized graphene nanosheets with improved lipophilicity. Carbon 2010, 48, 1683–1685. [Google Scholar] [CrossRef]
- Wang, G.; Shen, X.; Wang, B.; Yao, J.; Park, J. Synthesis and characterization of hydrophilic and organophilic graphene nanosheets. Carbon 2009, 47, 1359–1364. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Liu, Y.; Wong, C.P. Facile fabrication of superhydrophobic octadecylamine–functionalized graphite oxide film. Langmuir 2010, 26, 16110–16114. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, X.Z.; Zhang, H.B. Simultaneous surface functionalization and reduction of graphene oxide with octadecylamine for electrically conductive polystyrene composites. Carbon 2011, 49, 4724–4730. [Google Scholar] [CrossRef]
- Hummers William, S.; Offeman Richard, E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 6, 1339. [Google Scholar] [CrossRef]
- Xu, Y.; Bai, H.; Lu, G.W.; Li, C.; Shi, G.Q. Flexible graphene films via the filtration of water–soluble noncovalent functionalized graphene sheets. J. Am. Chem. Soc. 2008, 130, 5856–5857. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lim, S.; Lim, E. Synthesis of aqueous dispersion of graphenes via reduction of graphite oxide in the solution of conductive polymer. J. Phys. Chem. Solids 2010, 71, 483–486. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.Y.; Wu, Y.; Nguyen, S.B.T.; Ruoff, R.S. Synthesis of graphene–based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Park, S.; Dikin, D.A.; Nguyen, S.B.T.; Ruoff, R.S. Graphene oxide sheets chemically cross–Linked by polyallylamine. J. Phys. Chem. C 2009, 113, 15801–15804. [Google Scholar] [CrossRef]
- Biniak, S.; Szymański, G.; Siedlewski, J.; Swiatkowski, A. The characterization of activated carbons with oxygen and nitrogen surface groups. Carbon 1997, 35, 1799–1810. [Google Scholar] [CrossRef]
- Gao, J.; Liu, F.; Liu, Y.L.; Ma, N.; Wang, Z.Q.; Zhang, X. Environment–friendly method to produce graphene that employs vitamin C and amino acid. Chem. Mater. 2010, 22, 2213–2218. [Google Scholar] [CrossRef]
- Li, Z.; Yao, Y.G.; Lin, Z.Y.; Moon, K.S.; Lin, W.; Wong, C.P. Ultrafast, dry microwave synthesis of graphene sheets. J. Mater. Chem. 2010, 20, 4781–4783. [Google Scholar] [CrossRef]
- Skoog, D.A.; Holler, F.J.; Nieman, T.A. Principles of Instrumental Analysis; Hartcourt Brace & Company: Philadelphia, PA, USA, 1998; Chapter 13. [Google Scholar]
- JParedes, I.; Rodil, S.V.; Martínez–Alonso, A.; Tascon, J.M.D. Graphene oxide dispersions in organic solvents. Langmuir 2008, 24, 10560–10564. [Google Scholar] [CrossRef] [PubMed]
- Verwey, E.J.W.; Overbeek, J.T.G. Theory of the Stability of Lyophobic Colloids, 1st ed.; Elsevier Publishing Company Inc.: New York, NY, USA, 1948. [Google Scholar]
- Hansen, C.M. Hansen Solubility Parameters: A User’s Handbook; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Shen, J.; Hu, Y.; Shi, M.; Lu, X.; Qin, C.; Li, C.; Ye, M.X. Fast and Facile Preparation of Graphene Oxide and Reduced Graphene Oxide Nanoplatelets. Chem. Mater. 2009, 21, 3514–3520. [Google Scholar] [CrossRef]
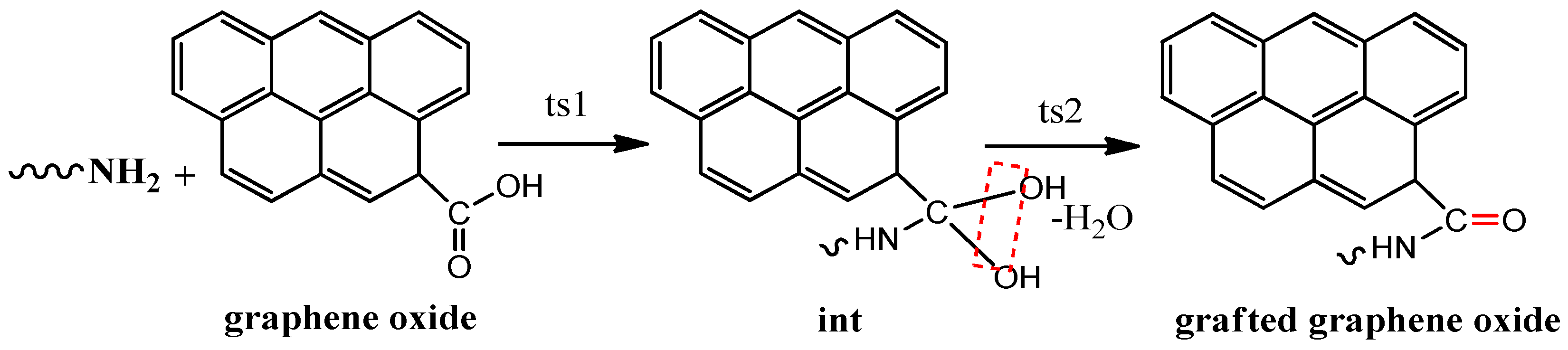
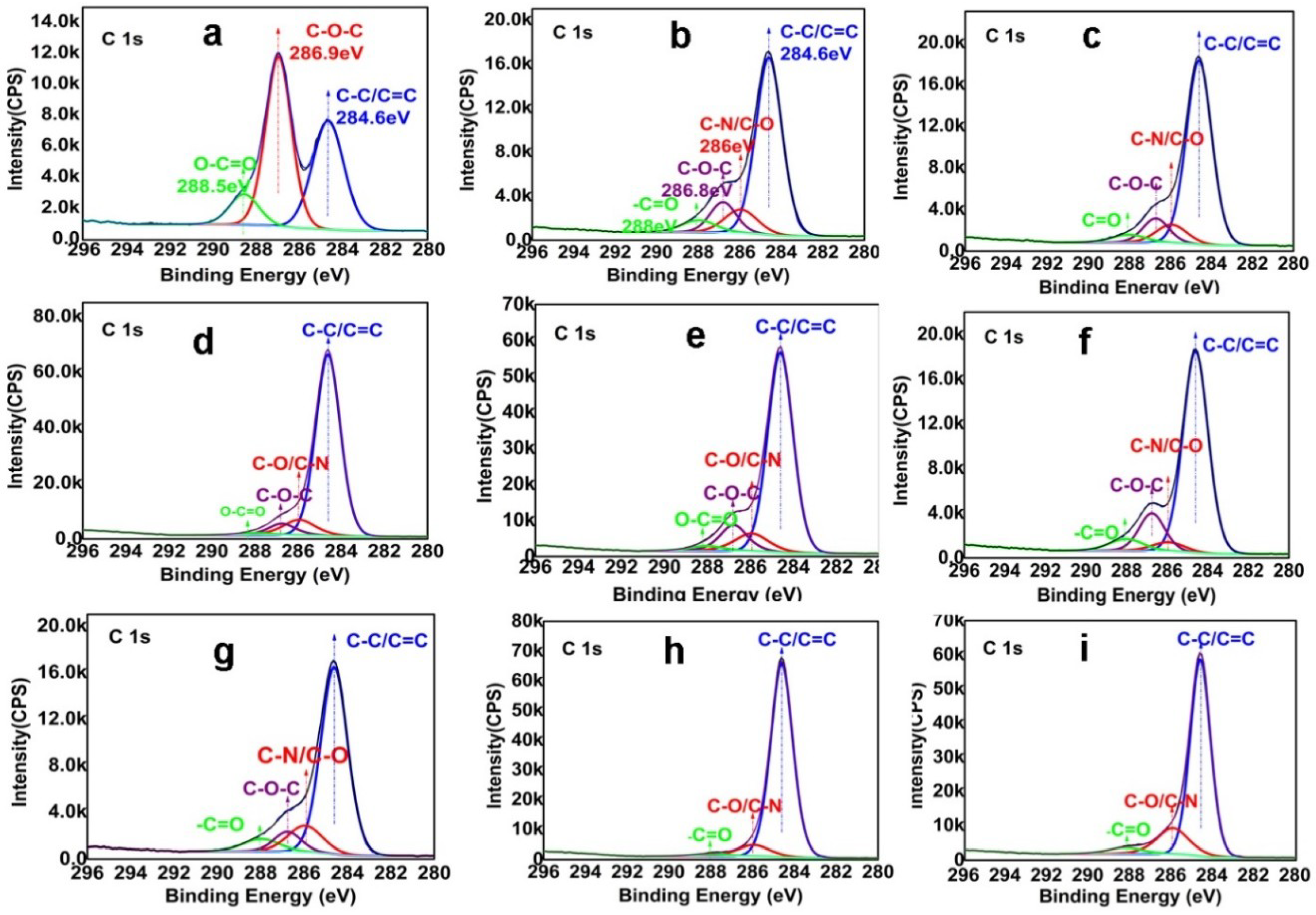
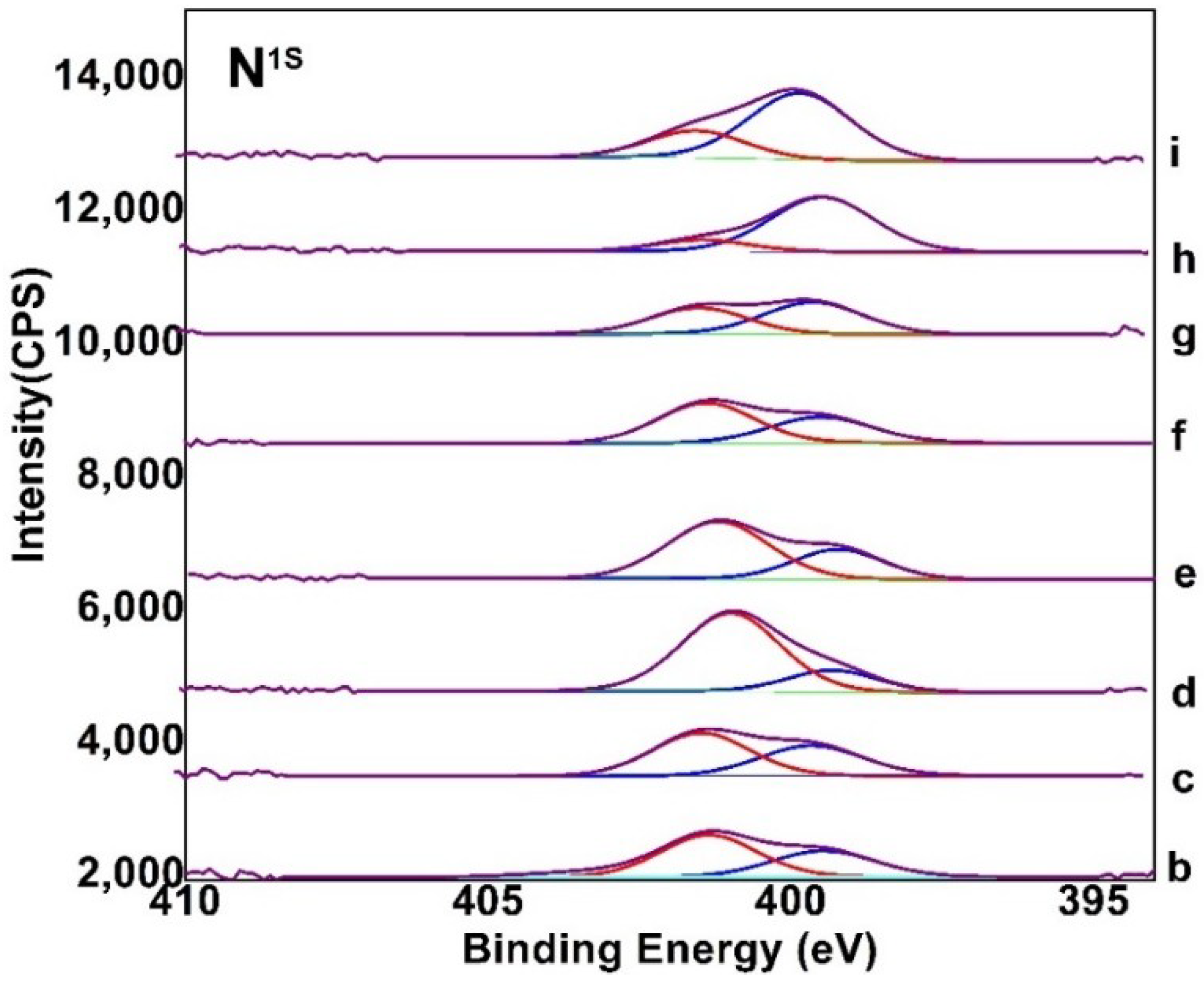
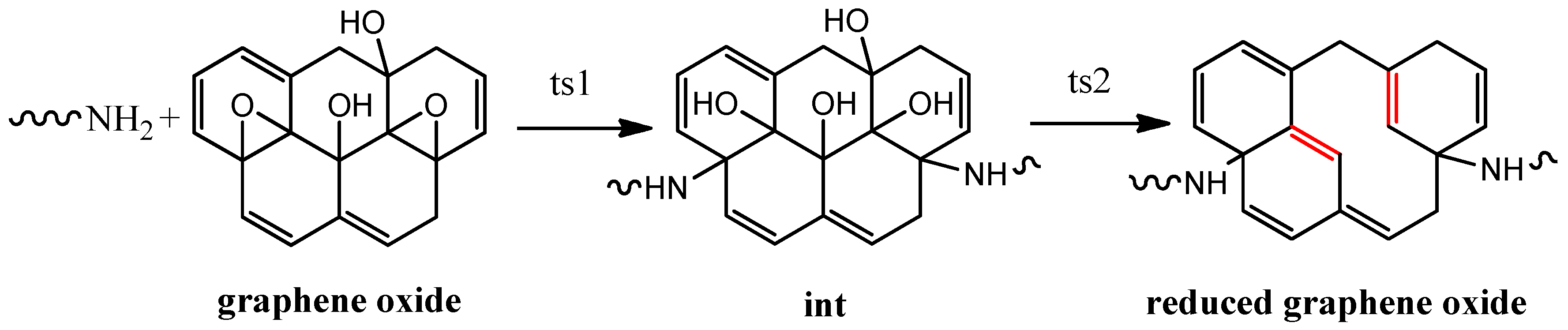
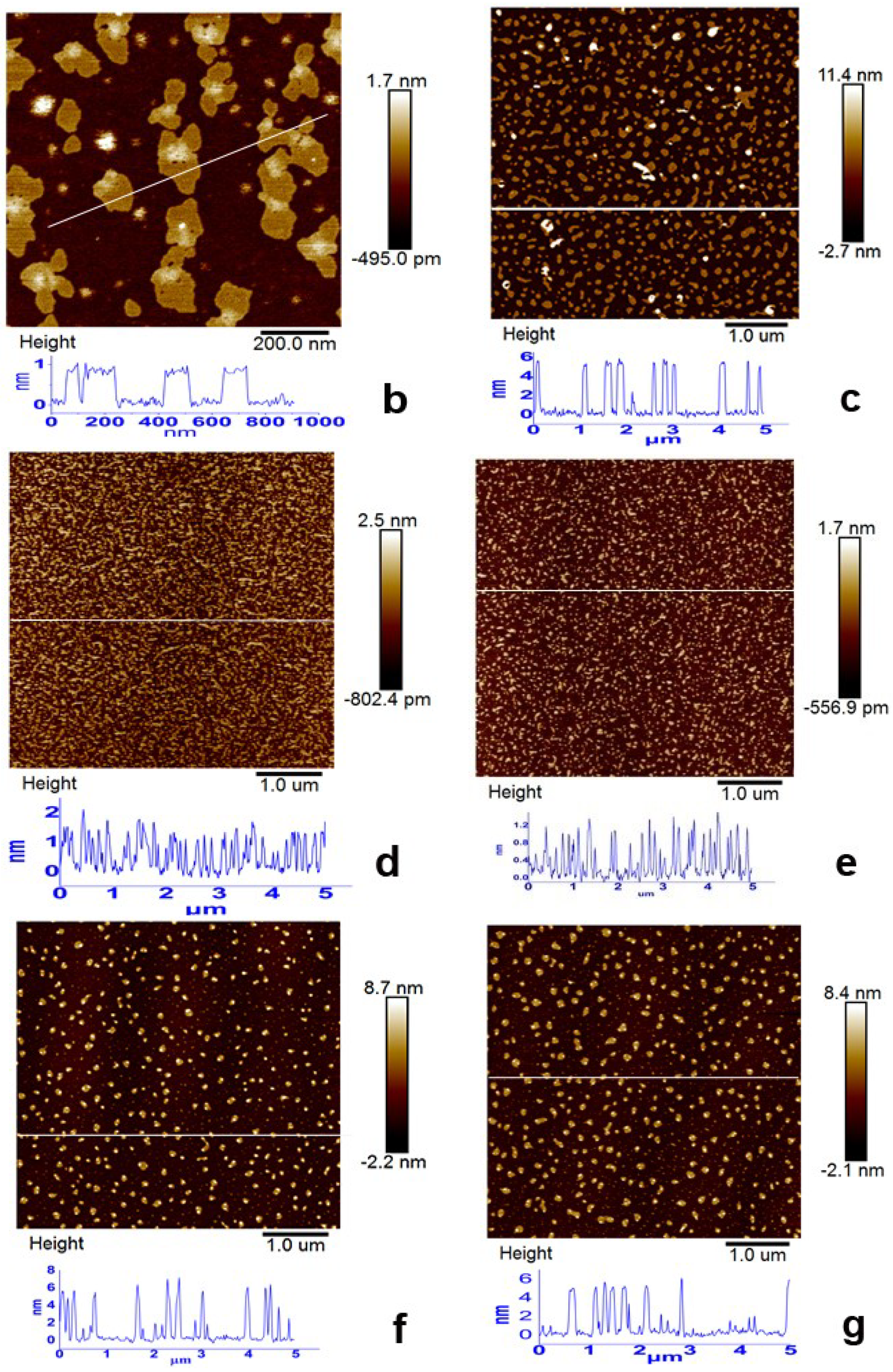
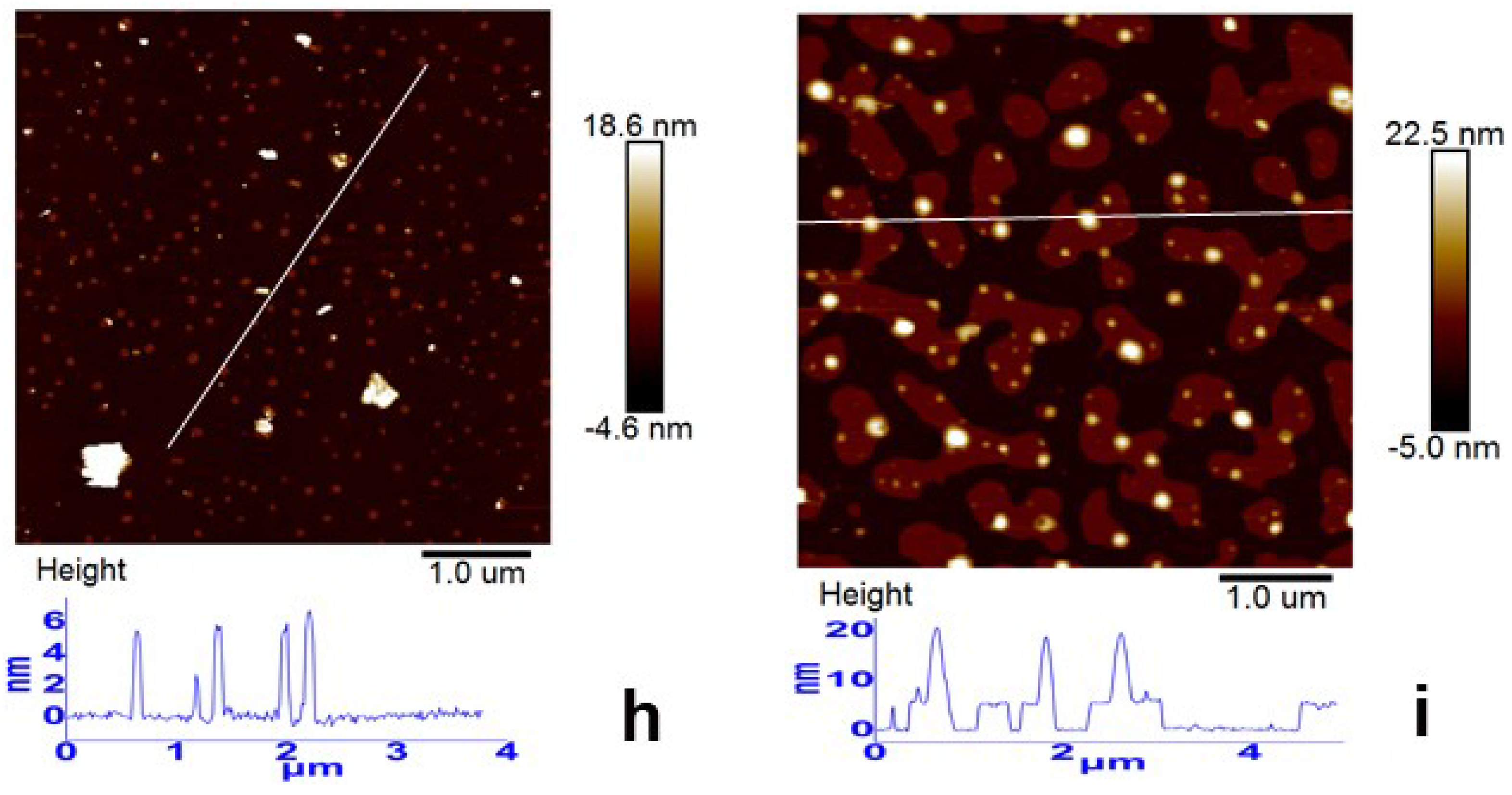
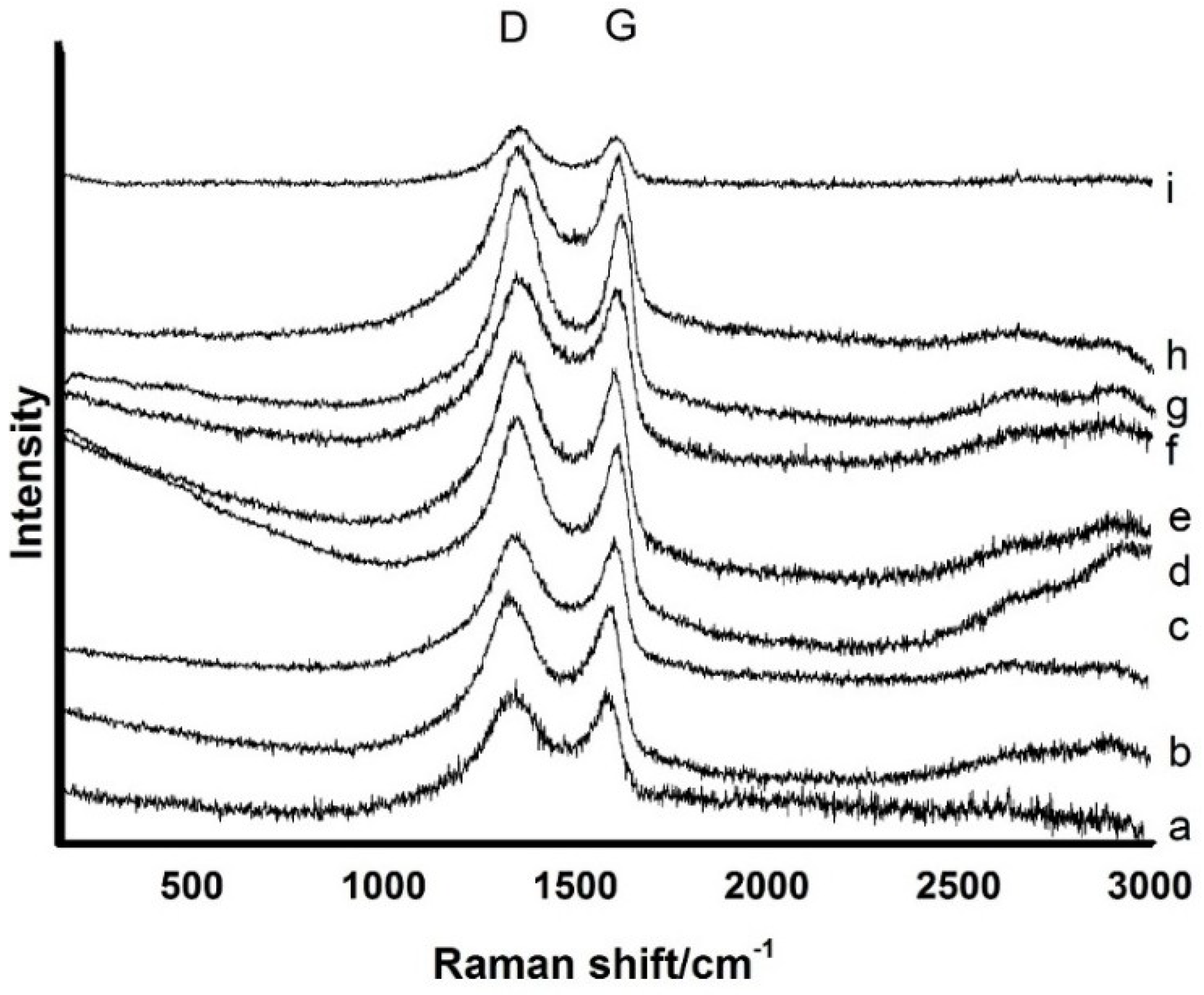
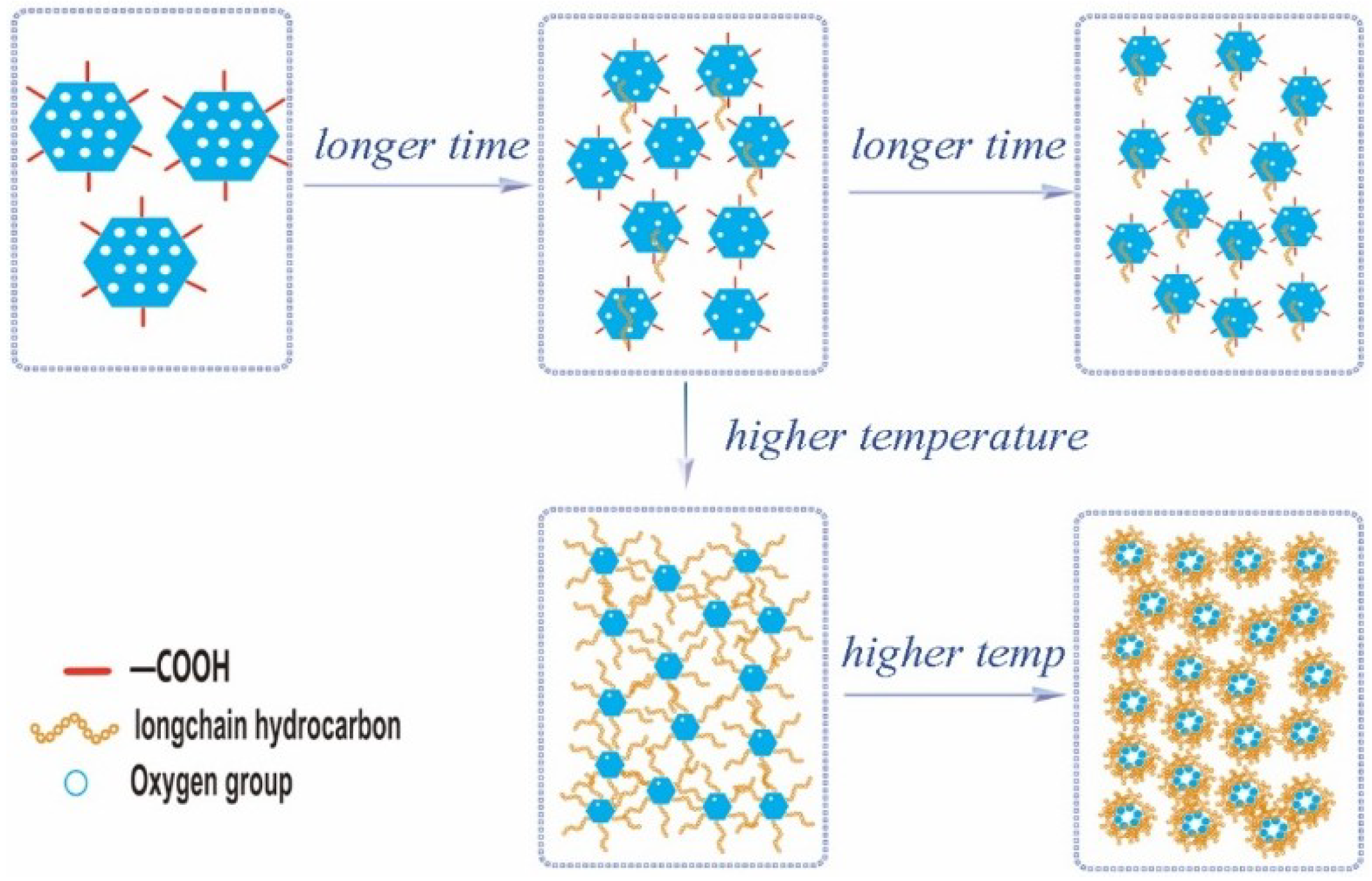


| No. | Notes | C–O/C–N | C=O | C=C/C–C |
|---|---|---|---|---|
| a | GO | 50 | 10 | 39 |
| b | ORGO (35 °C, 0.5 h) | 24 | 6 | 70 |
| c | ORGO (35 °C, 5 h) | 20 | 4 | 76 |
| c-1 | ORGO (35 °C, 24 h) | 18 | 4 | 78 |
| d | ORGO (50 °C, 0.5 h) | 19 | 6 | 75 |
| e | ORGO (50 °C, 5 h) | 21 | 3 | 76 |
| f | ORGO (80 °C, 0.5 h) | 19 | 6 | 75 |
| g | ORGO (80 °C, 5 h) | 17 | 4 | 79 |
| h | ORGO (120 °C, 0.5 h) | 16 | 4 | 80 |
| i | ORGO (120 °C, 5 h) | 8 | 2 | 90 |
| j | HRGO (80 °C, 5 h) | 20 | 5 | 75 |
| No. | Notes | N–C | N–H |
|---|---|---|---|
| b | ORGO (35 °C, 0.5 h) | 36.1 | 63.9 |
| c | ORGO (35 °C, 5 h) | 42.7 | 57.3 |
| c-1 | ORGO (35 °C, 24 h) | 49.5 | 50.5 |
| d | ORGO (50 °C, 0.5 h) | 20.4 | 79.6 |
| e | ORGO (50 °C, 5 h) | 31.9 | 68.1 |
| f | ORGO (80 °C, 0.5 h) | 40.7 | 59.3 |
| g | ORGO (80 °C, 5 h) | 55.7 | 44.3 |
| h | ORGO (120 °C, 0.5 h) | 72 | 28 |
| i | ORGO (120 °C, 5 h) | 84 | 16 |
| No. | a | b | c | d | e | f | g | h | i |
|---|---|---|---|---|---|---|---|---|---|
| ID/IG | 2.26 | 1.94 | 1.77 | 1.95 | 1.80 | 1.79 | 1.92 | 2.13 | 1.75 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, T.; Wang, Z.; Zhao, Y.; Wang, Y.; Yi, X. Composition, Structure and Morphology Evolution of Octadecylamine (ODA)–Reduced Graphene Oxide and Its Dispersion Stability under Different Reaction Conditions. Materials 2018, 11, 1710. https://doi.org/10.3390/ma11091710
Bao T, Wang Z, Zhao Y, Wang Y, Yi X. Composition, Structure and Morphology Evolution of Octadecylamine (ODA)–Reduced Graphene Oxide and Its Dispersion Stability under Different Reaction Conditions. Materials. 2018; 11(9):1710. https://doi.org/10.3390/ma11091710
Chicago/Turabian StyleBao, Tianjiao, Zhiyong Wang, Yan Zhao, Yan Wang, and Xiaosu Yi. 2018. "Composition, Structure and Morphology Evolution of Octadecylamine (ODA)–Reduced Graphene Oxide and Its Dispersion Stability under Different Reaction Conditions" Materials 11, no. 9: 1710. https://doi.org/10.3390/ma11091710
APA StyleBao, T., Wang, Z., Zhao, Y., Wang, Y., & Yi, X. (2018). Composition, Structure and Morphology Evolution of Octadecylamine (ODA)–Reduced Graphene Oxide and Its Dispersion Stability under Different Reaction Conditions. Materials, 11(9), 1710. https://doi.org/10.3390/ma11091710