Preparation and Characterization of Macroalgae Biochar Nanomaterials with Highly Efficient Adsorption and Photodegradation Ability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Activated Kelp Biochar (AKB)
2.3. Fabrication of Bi2MoO6–AKB Composite (BKBC)
2.4. Characterization
2.5. Adsorption and Degradation Experiments
2.6. Main Kinetics Models
- Pseudo-first order modelqe and qt are, respectively, the amounts of MB (mg/g) adsorbed at equilibrium and at time t. K1 is the rate constant of the pseudo-first order (min−1).
- Pseudo-second order modelqe and qt are, respectively, the amounts of MB (mg/g) adsorbed at equilibrium and at time t. K2 is the rate constant of the pseudo-second order (min−1).
2.7. Photodegradation Formula
3. Results and Discussion
3.1. Characterization of Activated Kelp Biochar Nanomaterials
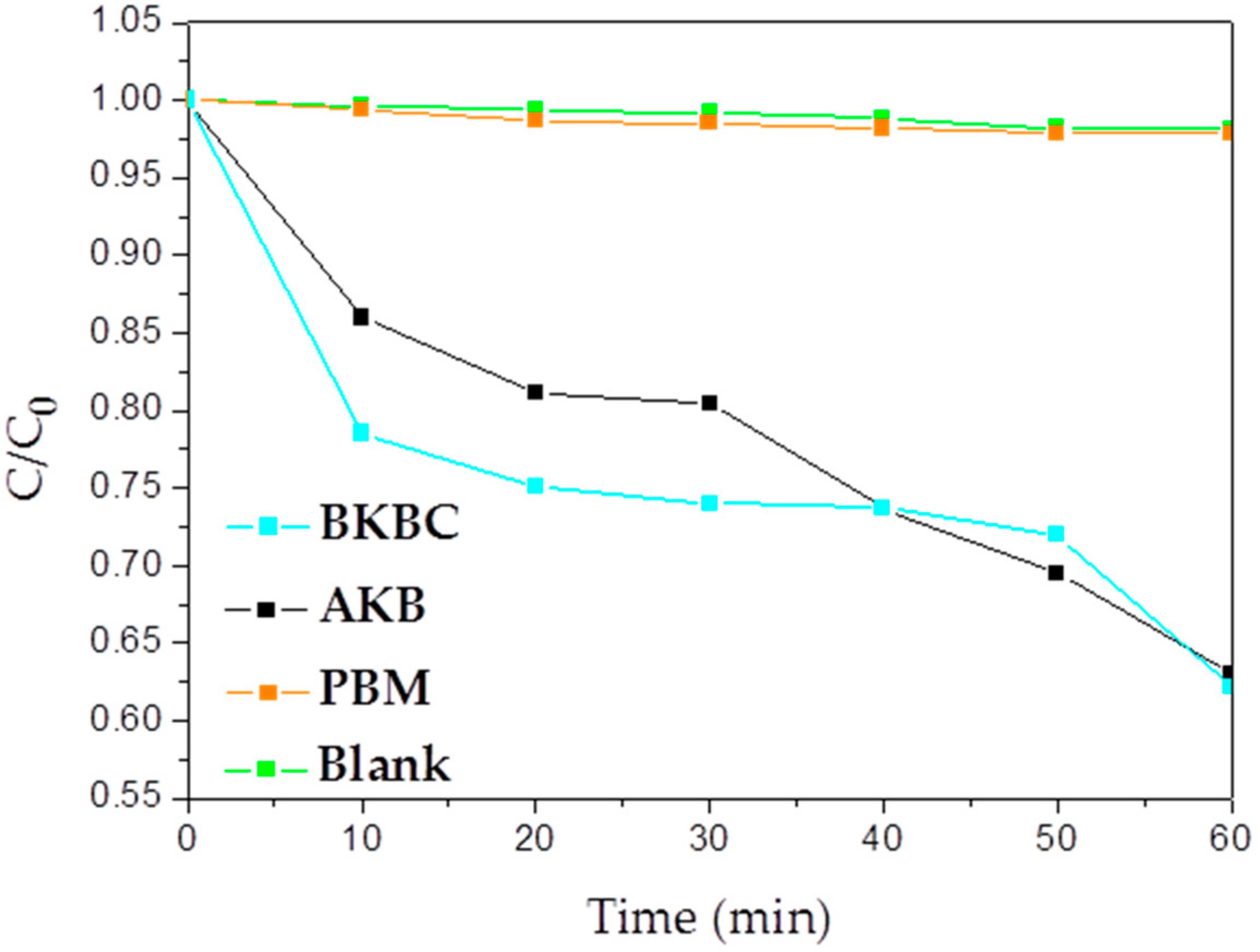
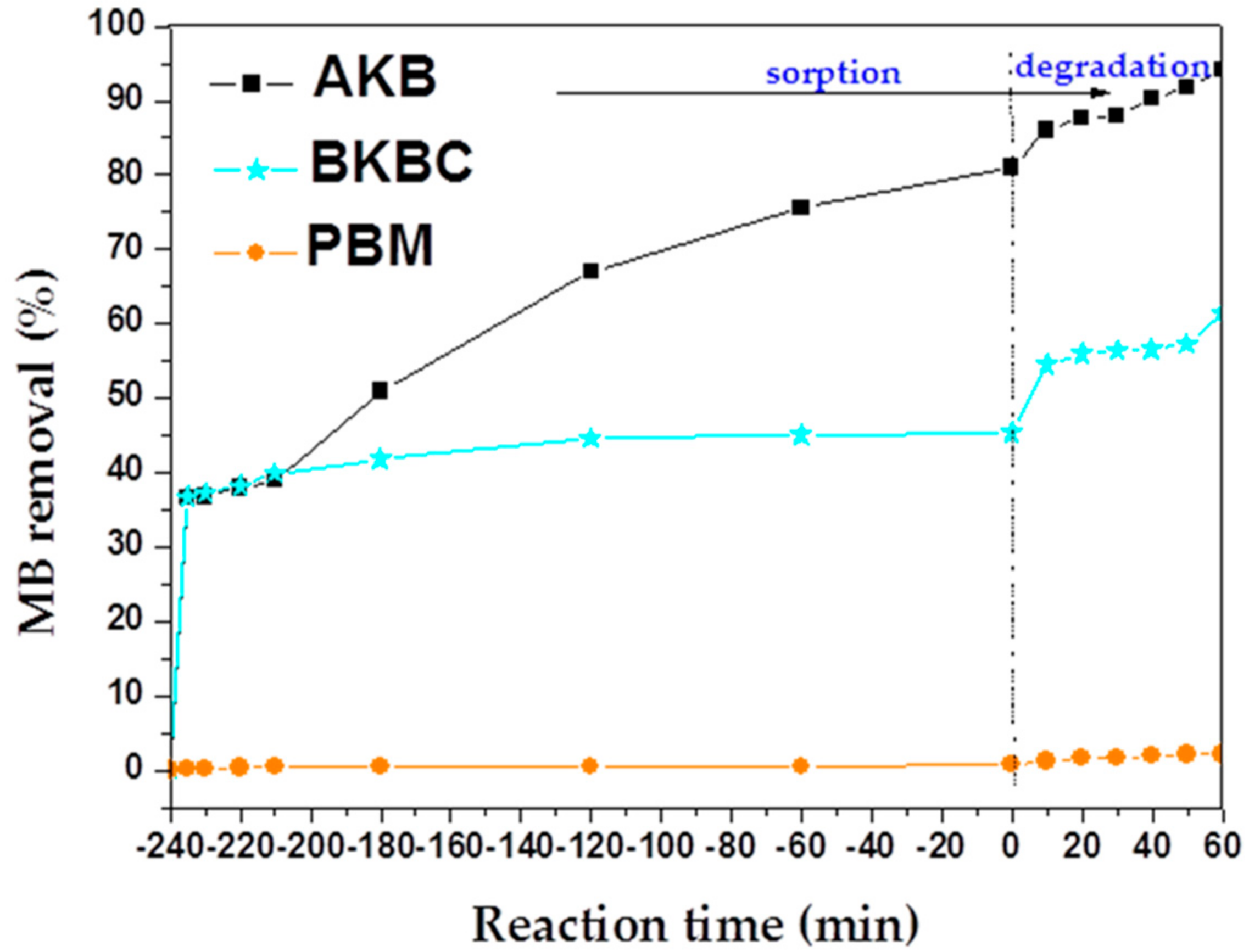
3.2. MB Sorption and Degradation
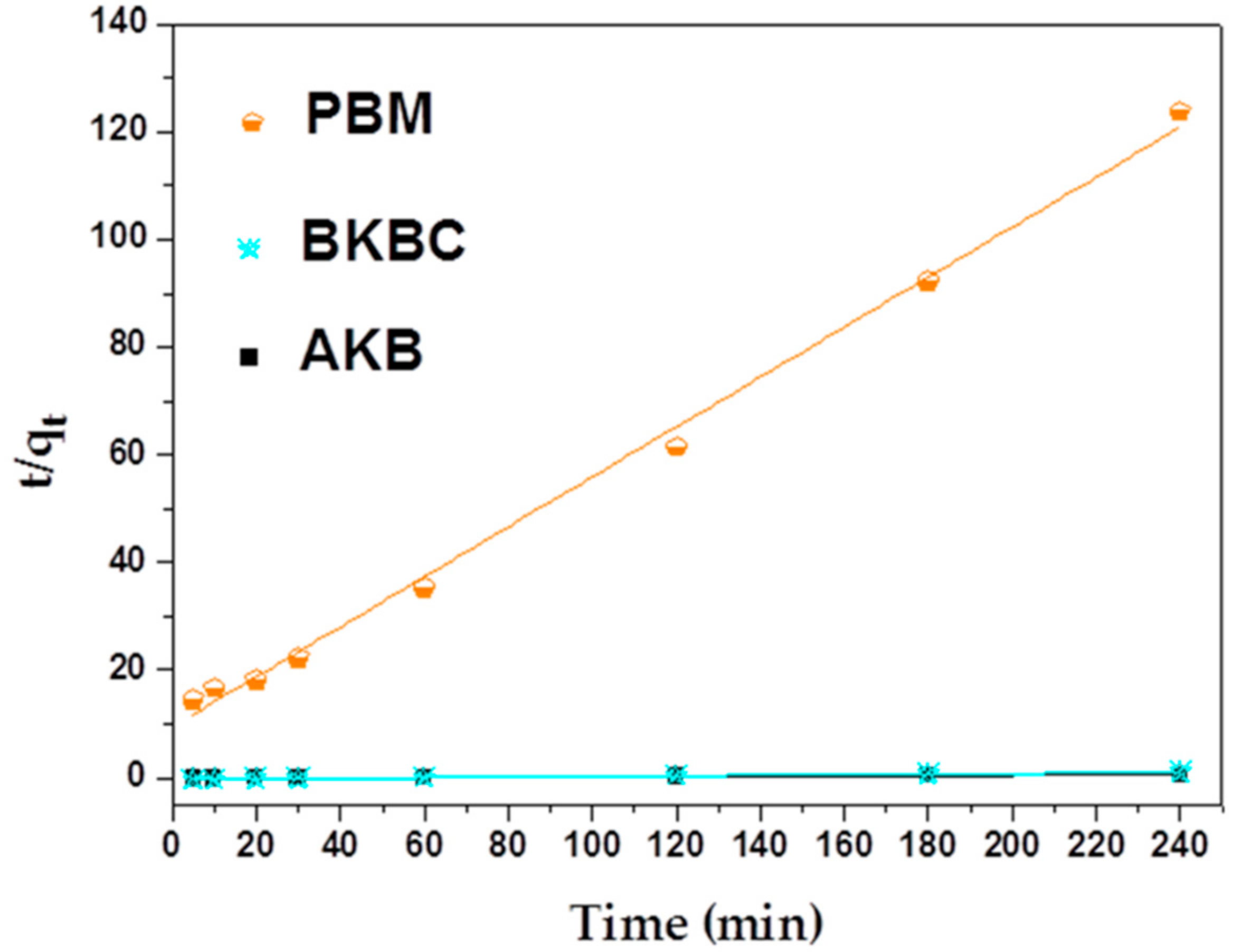
3.2.1. Adsorption Kinetics
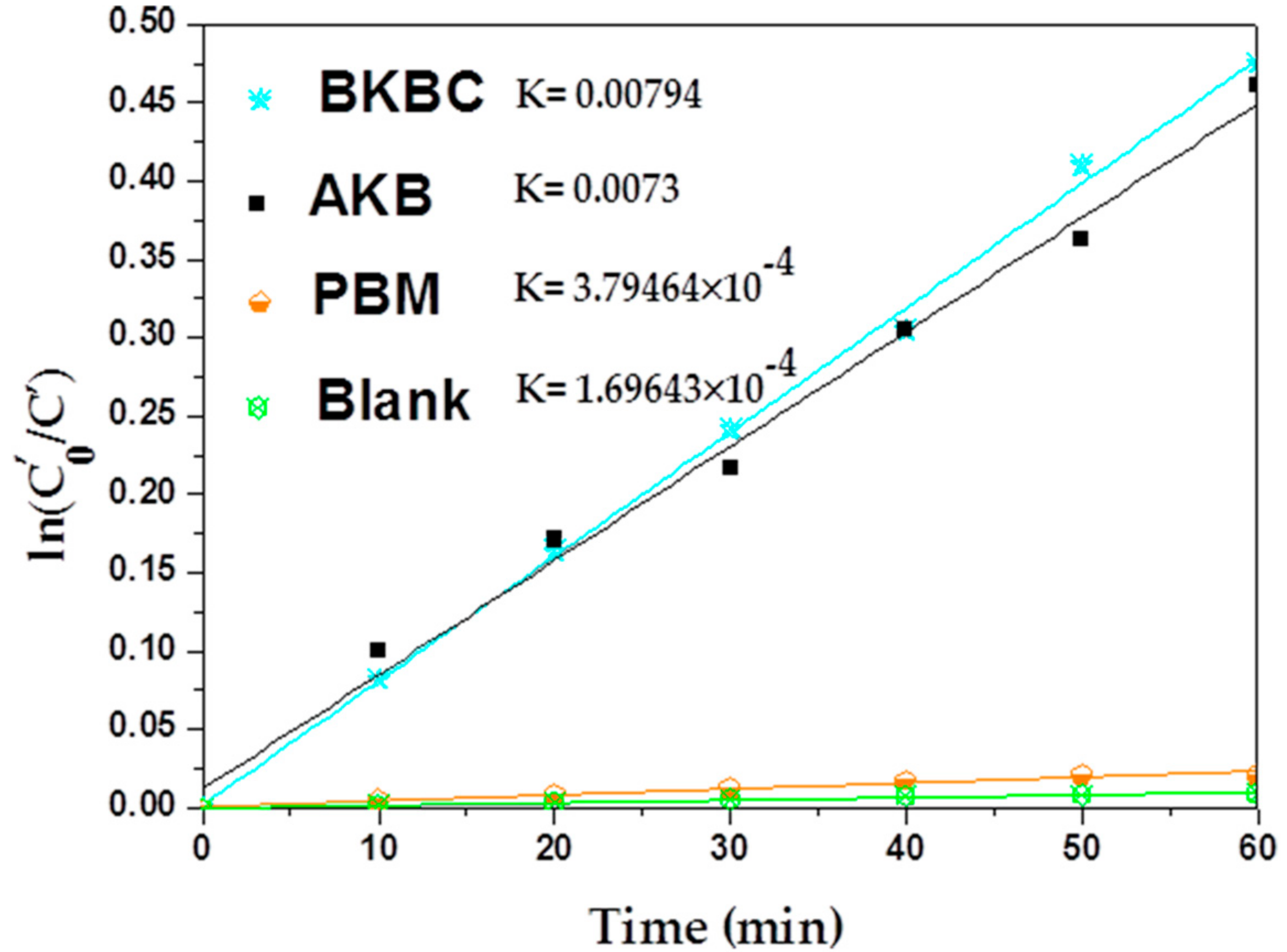
3.2.2. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Su, X.H.; Ling, W.L.; Teng, T.T.; Wong, Y.S. Combination and hybridisation of treatments in dye wastewater treatment: A review. J. Environ. Chem. Eng. 2016, 4, 3618–3631. [Google Scholar] [CrossRef]
- Chen, Y.D.; Lin, Y.C.; Ho, S.H.; Zhou, Y.; Ren, N.Q. Highly efficient adsorption of dyes by biochar derived from pigments-extracted macroalgae pyrolyzed at different temperature. Bioresour. Technol. 2018, 259, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lei, S.; Wan, S.; Luo, W. Biochars prepared from anaerobic digestion residue, palm bark, and eucalyptus for adsorption of cationic methylene blue dye: Characterization, equilibrium, and kinetic studies. Bioresour. Technol. 2013, 140, 406–413. [Google Scholar]
- Abdelwahab, N.A.; Emh, M. Synthesis and characterization of methyl pyrazolone functionalized magnetic chitosan composite for visible light photocatalytic degradation of methylene blue. Int. J. Biol. Macromol. 2018, 108, 1035–1044. [Google Scholar] [CrossRef] [PubMed]
- Shitu, A.M.M.A.; Ibrahim, A. Removal of Methylene Blue Using Low Cost Adsorbent: A Review. J. Hazard. Mater. 2014, 4, 91–102. [Google Scholar]
- Dutta, K.; Mukhopadhyay, S.; Bhattacharjee, S.; Chaudhuri, B. Chemical oxidation of methylene blue using a Fenton-like reaction. J. Hazard. Mater. 2001, 84, 57–71. [Google Scholar] [CrossRef]
- Gupta, V.K.; Pathania, D.; Agarwal, S.; Singh, P. Adsorptional photocatalytic degradation of methylene blue onto pectin-CuS nanocomposite under solar light. J. Hazard. Mater. 2012, 243, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Gao, B. Removal of arsenic, methylene blue, and phosphate by biochar/alooh nanocomposite. Chem. Eng. J. 2013, 226, 286–292. [Google Scholar] [CrossRef]
- Tian, Z.; Qiu, Y.; Zhou, J.; Zhao, X.; Cai, J. The direct carbonization of algae biomass to hierarchical porous carbons and CO2, adsorption properties. Mater. Lett. 2016, 180, 162–165. [Google Scholar] [CrossRef]
- Sewu, D.D.; Boakye, P.; Jung, H.; Woo, S.H. Synergistic dye adsorption by biochar from co-pyrolysis of spent mushroom substrate and Saccharina japonica. Bioresour. Technol. 2017, 244, 1142–1149. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Li, H.; Zhu, W.; Ho, S.H.; Yuan, W.; Chen, J.; Xie, Y. Microalgal-biochar immobilized complex: A novel efficient biosorbent for cadmium removal from aqueous solution. Bioresour. Technol. 2017, 244, 1031–1038. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.D.; Ho, S.H.; Nagarajan, D.; Ren, N.Q.; Chang, J.S. Waste biorefineries—Integrating anaerobic digestion and microalgae cultivation for bioenergy production. Curr. Opin. Biotechnol. 2017, 50, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, A.; Rao, J.R.; Nair, B.U. Adsorption of phenol onto activated carbon from seaweed: Determination of the optimal experimental parameters using factorial design. J. Taiwan Inst. Chem. E 2011, 42, 952–956. [Google Scholar] [CrossRef]
- Aravindhan, R.; Rao, J.R.; Nair, B.U. Kinetic and equilibrium studies on biosorption of basic blue dye by green macro algae Caulerpa scalpelliformis. J. Environ. Sci. Heal. A 2007, 42, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Wang, C.; Guo, X.; Paul, C.J. Modification of carbon derived from Sargassum sp. by lanthanum for enhanced adsorption of fluoride. J. Colloid Interface Sci. 2015, 441, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yang, W.; Xu, W.; Liu, Y. Removal of elemental mercury by bio-chars derived from seaweed impregnated with potassium iodine. Chem. Eng. J. 2018, 339, 468–478. [Google Scholar] [CrossRef]
- Nautiyal, P.; Subramanian, K.A.; Dastidar, M.G. Adsorptive removal of dye using biochar derived from residual algae after in-situ transesterification: Alternate use of waste of biodiesel industry. J. Environ. Manag. 2016, 182, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Guo, W.; Li, S.; Chen, Y.; Wu, Q.; Feng, X.; Yin, R.; Ho, S.H.; Ren, N.; Chang, J.S. Adsorption of p-nitrophenols (pnp) on microalgal biochar: Analysis of high adsorption capacity and mechanism. Bioresour. Technol. 2017, 244, 1456–1464. [Google Scholar] [CrossRef] [PubMed]
- Pintor, M.J.; Jean-Marius, C.; Jeanne-Rose, V.; Taberna, P.L.; Simon, P.; Gamby, J.; Gadiou, R.; Gaspard, S. Preparation of activated carbon from Turbinaria turbinata, seaweeds and its use as supercapacitor electrode materials. C. R. Chim. 2013, 16, 73–79. [Google Scholar] [CrossRef]
- Ross, A.; Jones, J.; Kubacki, M.; Bridgeman, T. Classification of macroalgae as fuel and its thermochemical behaviour. Bioresour. Technol. 2008, 99, 6494–6504. [Google Scholar] [CrossRef] [PubMed]
- Haykiri-Acma, H.; Yaman, S.; Kucukbayrak, S. Production of biobriquettes from carbonized brown seaweed. Fuel Process. Technol. 2013, 106, 33–40. [Google Scholar] [CrossRef]
- Yu, K.L.; Lau, B.F.; Show, P.L.; Ong, H.C.; Ling, T.C.; Chen, W.H.; Ng, E.P.; Chang, J.S. Recent developments on algal biochar production and characterization. Bioresour. Technol. 2017, 246, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Joonhyuk, C.; Jaewook, C.; Dongjin, S.; Jeong-Myeong, H.; Jiwon, H.; Hyunwook, J.; Kwanyoung, L.; Heechul, W. Production of brown algae pyrolysis oils for liquid biofuels depending on the chemical pretreatment methods. Energy Convers. Manag. 2014, 86, 371–378. [Google Scholar]
- Wang, S.; Hu, Y.; Uzoejinwa, B.B.; Cao, B.; He, Z.; Wang, Q.; Xu, S. Pyrolysis Mechanisms of Typical Seaweed Polysaccharides. J. Anal. Appl. Pyrolysis 2016, 124, 373–383. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Z.H.; Song, P.P.; Xu, R.; Wang, H. Facile synthesis of Bi2MoO6/ZnSnO3, heterojunction with enhanced visible light photocatalytic degradation of methylene blue. Appl. Surf. Sci. 2017, 430, 561–570. [Google Scholar]
- Shang, M.; Wang, W.; Ren, J.; Sun, S.; Zhang, L. A novel BiVO4 hierarchical nanostructure: Controllable synthesis, growth mechanism, and application in photocatalysis. Crystengcomm 2010, 12, 1754–1758. [Google Scholar] [CrossRef]
- Li, J.; Liu, X.; Zhuo, S.; Pan, L. Novel Bi2MoO6/TiO2, heterostructure microspheres for degradation of benzene series compound under visible light irradiation. J. Colloid Interface Sci. 2016, 463, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Umapathy, V.; Manikandan, A.; Antony, S.A.; Ramu, P.; Neeraja, P. Structure, morphology and opto-magnetic properties of Bi2MoO6, nano-photocatalyst synthesized by sol–gel method. Trans. Nonferrous Met. Soc. China 2015, 25, 3271–3278. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, C.; Mu, J.; Zhang, Z.; Guo, Z.; Zhang, P. One-dimensional Bi2MoO6/TiO2 hierarchical heterostructures with enhanced photocatalytic activity. Crystengcomm 2011, 14, 605–612. [Google Scholar] [CrossRef]
- Yan, T.; Sun, M.; Liu, H.; Wu, T.; Liu, X.; Yan, Q.; Xu, W.; Du, B. Fabrication of hierarchical BiOI/Bi2MoO6, heterojunction for degradation of bisphenol A and dye under visible light irradiation. J. Alloy. Compd. 2015, 634, 223–231. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.Y.; Chen, X.F.; Song, S.X. Facile synthesis of naon-modified fishbone charcoal (fbc) with remarkable adsorption towards methylene blue. Procedia Eng. 2018, 211, 495–505. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Luo, W.; Sun, J.; Xu, Q.; Chen, F.; Zhao, J.; Wang, S.; Yao, F.; Wang, D.; et al. Effectiveness and mechanisms of phosphate adsorption on iron-modified biochars derived from waste activated sludge. Bioresour. Technol. 2017, 247, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, X.; Zhao, Z.; Li, B.; Gui, J.; Liu, D.; Qiu, J. One-step synthesis of flowerlike C/Fe2O3, nanosheet assembly with superior adsorption capacity and visible light photocatalytic performance for dye removal. Carbon 2017, 116, 59–67. [Google Scholar] [CrossRef]
- Peng, F.; Fu, X.-B.; Tu, H.; Wang, H.J. Preparation of carbon nanotube-supported Fe2O3, catalysts and their catalytic activities for ethylbenzene dehydrogenation. New Carbon Mater. 2007, 22, 213–217. [Google Scholar] [CrossRef]
- Gangaraju, D.; Sridhar, V.; Lee, I.; Park, H. Graphene–carbon nanotube-Mn3O4, mesoporous nano-alloys as high capacity anodes for lithium-ion batteries. J. Alloy. Compd. 2016, 699, 106–111. [Google Scholar] [CrossRef]
- Liu, D.; Zhao, X.; Song, L.; Zhang, S. Synthesis, performance and action mechanism of carbon black/Ag3PO4 photocatalysts. Ceram. Int. 2018, 44, 13712–13719. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, D.P.; Yu, Y.X.; Zhang, W.D. Preparation and photoelectrochemical properties of functional carbon nanotubes and Ti co-doped Fe2O3, thin films. Int. J. Hydrogen Energy 2012, 37, 9566–9575. [Google Scholar] [CrossRef]
- Paduani, C. Structure and electronic properties of a Mn nanowire encapsulated in carbon nanotubes. J. Solid State Chem. 2013, 201, 204–207. [Google Scholar] [CrossRef]
- Enniya, I.; Rghioui, L.; Jourani, A. Adsorption of hexavalent chromium in aqueous solution on activated carbon prepared from apple peels. Sustain. Chem. Pharm. 2018, 7, 9–16. [Google Scholar] [CrossRef]
- Lo, I.M.C.; Zhang, W. EDTA-enhanced washing for remediation of Pb- and/or Zn-contaminated soils. J. Environ. Eng. 2005, 132, 1282–1288. [Google Scholar]
- Zhou, F.; Shi, R.; Zhu, Y. Significant enhancement of the visible photocatalytic degradation performances of γ-Bi2MoO6, nanoplate by graphene hybridization. J. Mol. Catal. A Chem. 2011, 340, 77–82. [Google Scholar] [CrossRef]
- Cai, L.; Gong, J.; Liu, J.; Zhang, H.; Song, W.; Ji, L. Facile preparation of nano-Bi2MoO6/diatomite composite for enhancing photocatalytic performance under visible light irradiation. Materials 2018, 11, 267. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Hu, S.; Zhang, J.; Jiang, W.; Liu, J. Facile synthesis of Fe2O3 nanoparticles anchored on Bi2MoO6 microflowers with improved visible light photocatalytic activity. J. Colloid Interface Sci. 2017, 497, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Zhang, M.; Yao, C.; Liu, C.; Xu, S.; Li, Z. High performance visible-light driven photocatalysts of Bi2MoO6-g-C3N4 with controllable solvothermal fabrication. J. Photochem. Photobiol. A 2017, 332, 357–363. [Google Scholar] [CrossRef]
- Ma, D.; Wu, J.; Gao, M.; Xin, Y.; Chai, C. Enhanced debromination and degradation of 2,4-dibromophenol by an z-scheme Bi2MoO6/cnts/g-C3N4 visible light photocatalyst. Chem. Eng. J. 2017, 316, 461–470. [Google Scholar] [CrossRef]
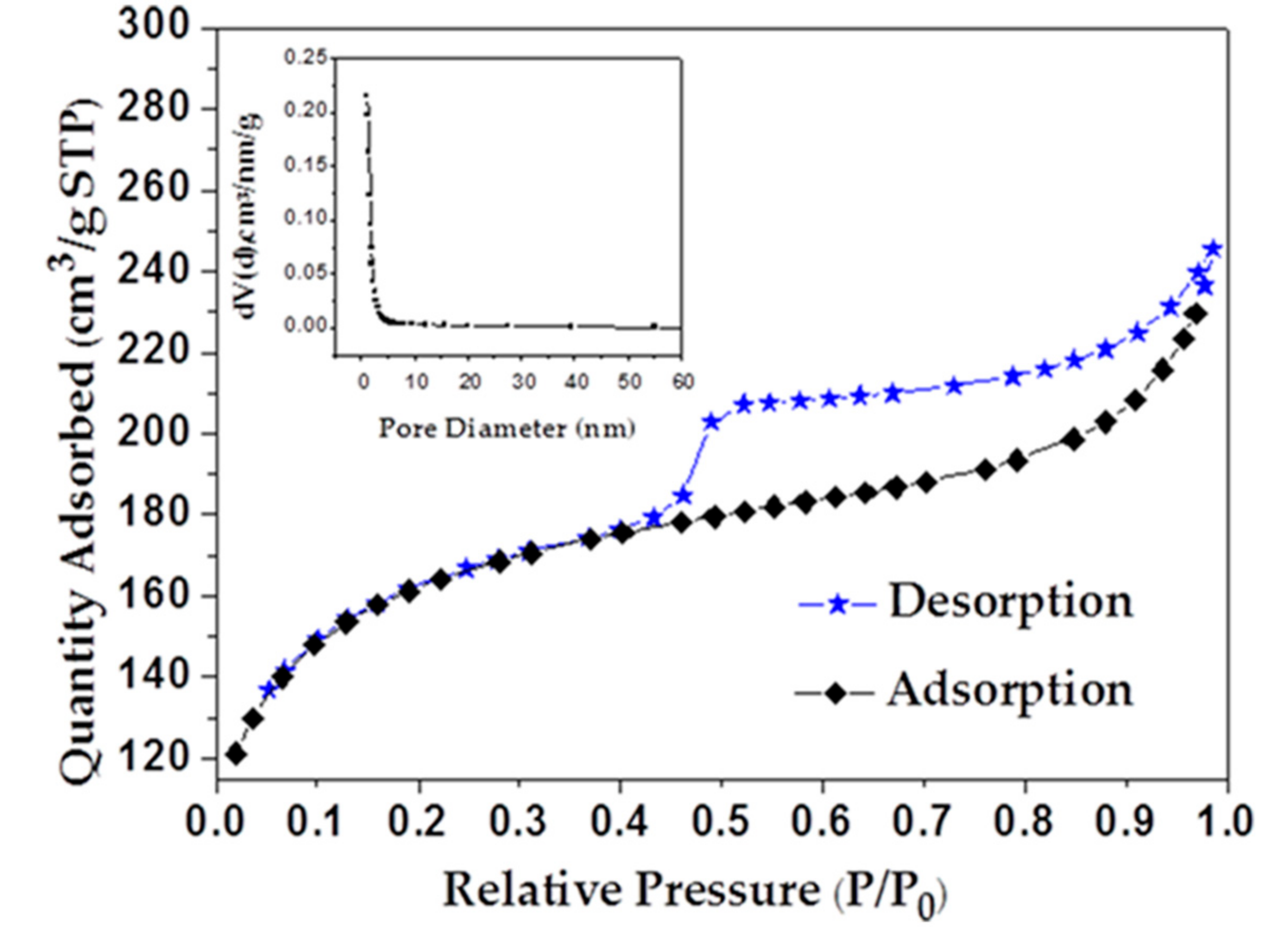


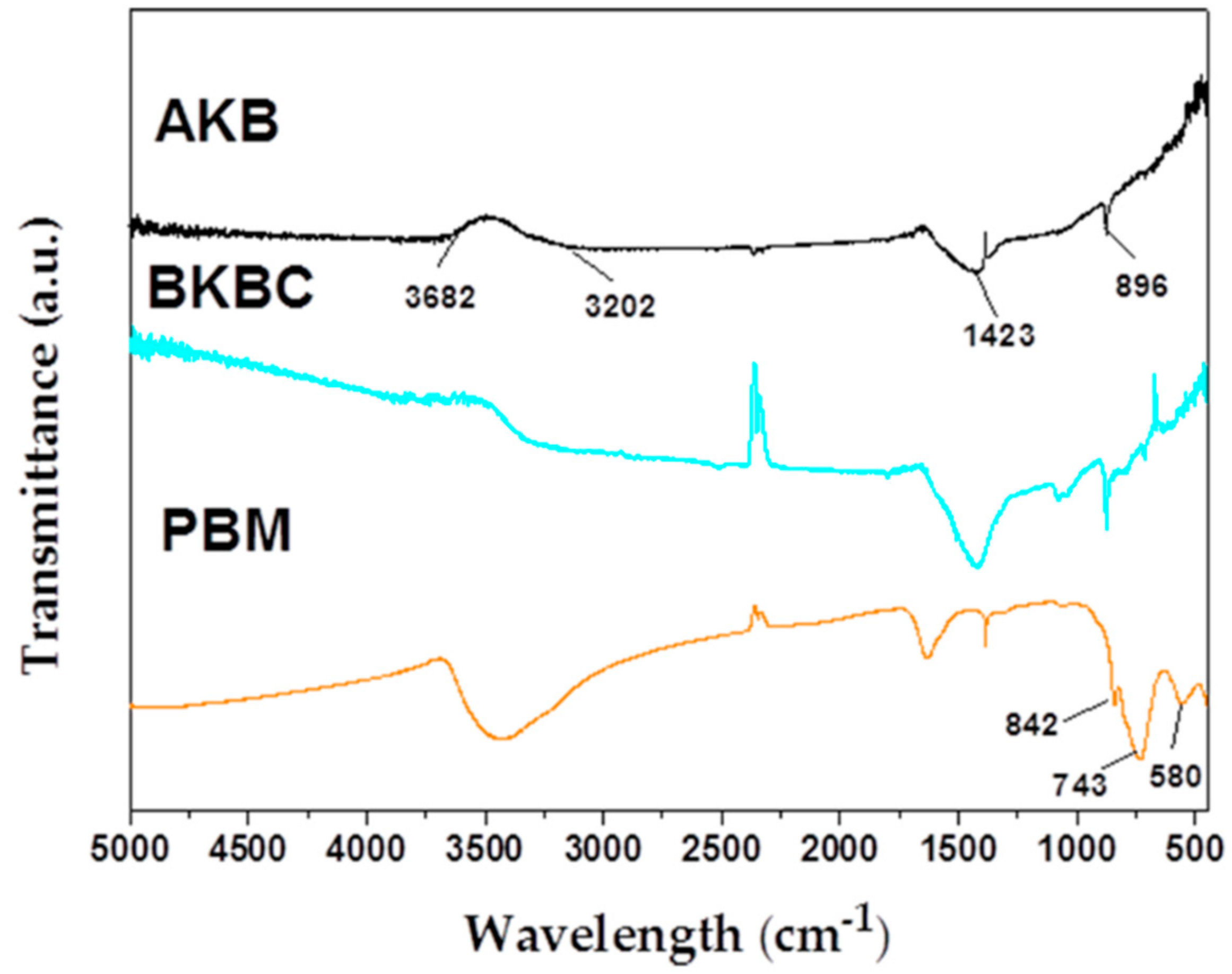
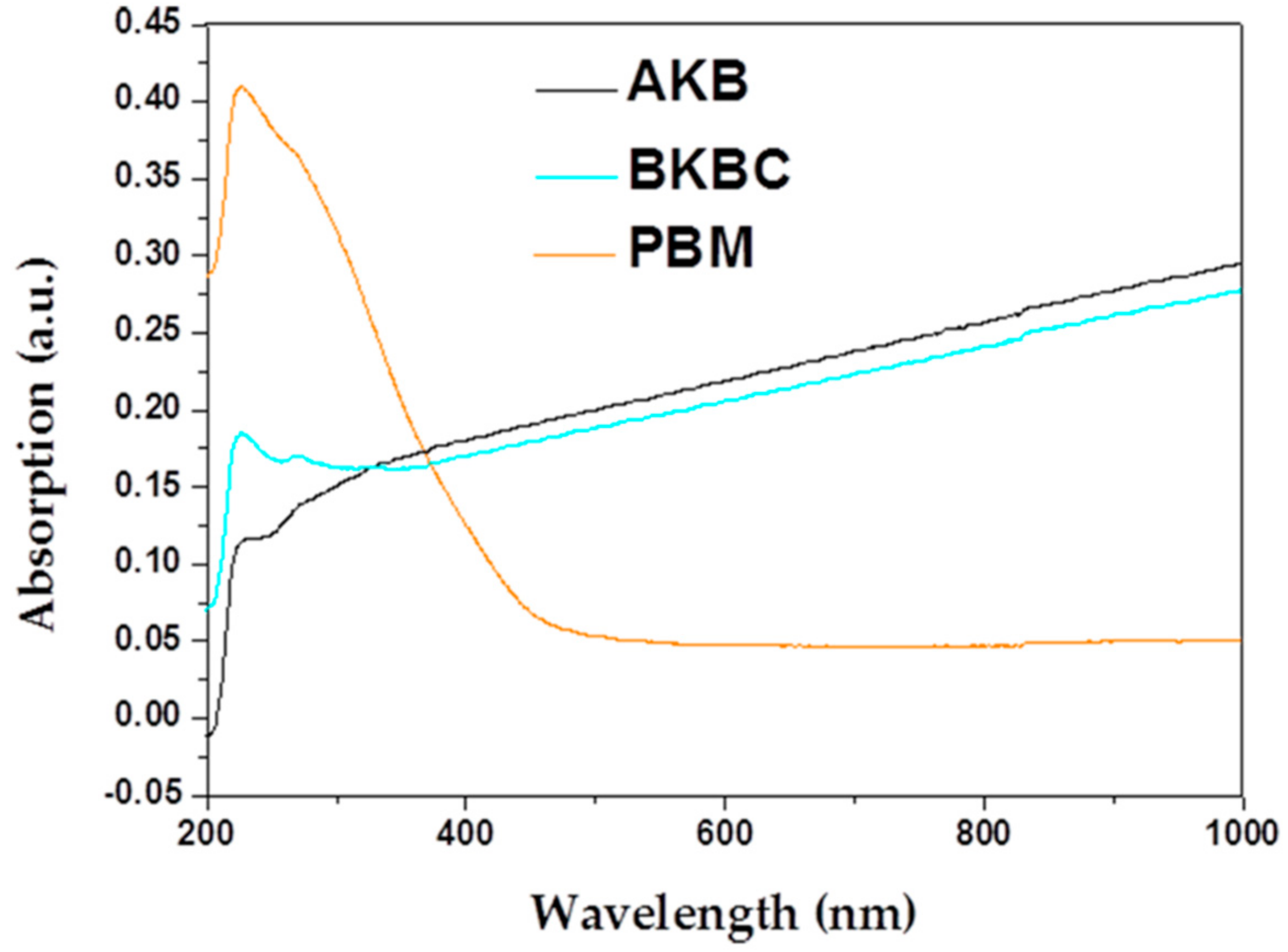

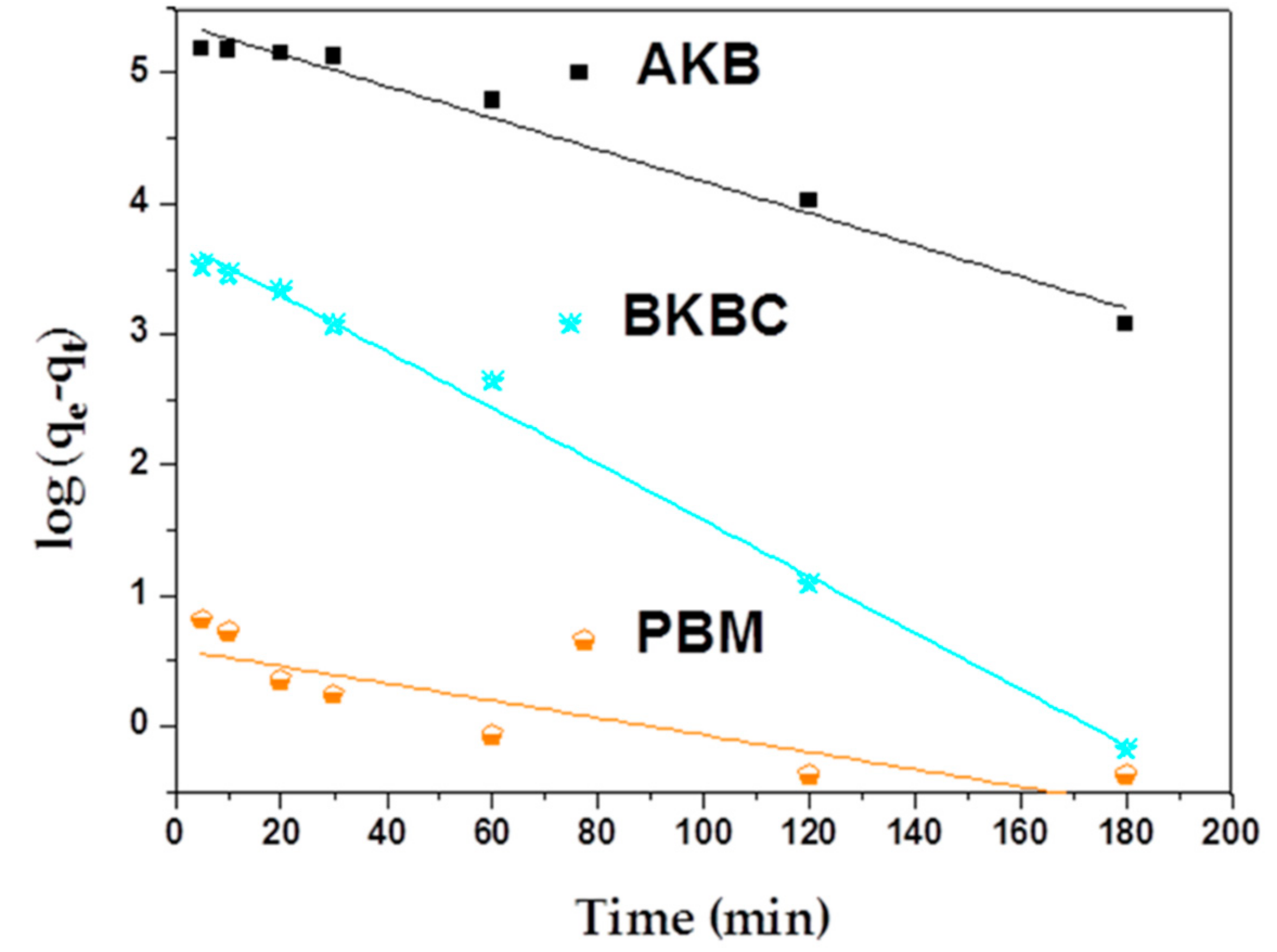
| Metal Elements | Cr | Er | Fe | Mn | Ni | Ti | Y |
|---|---|---|---|---|---|---|---|
| AKB | 37.9 | 17.9 | 510 | 72.1 | 3.4 | 8.8 | 15.3 |
| Metal elements | Sr | Al | Ca | K | Mg | Zn | Cu |
| AKB | 2.38 × 103 | 6.15 × 104 | 1.07 × 105 | 2.61 × 103 | 1.49 × 104 | 37.8 | 11.1 |
| Non-Metallic Elements | C | H | N |
|---|---|---|---|
| AKB | 59.81 | 2.30 | 2.41 |
| Adsorbents | qe (mg/g) |
|---|---|
| PBM | 2.65 |
| BKBC | 181.1 |
| AKB | 324.1 |
| Adsorbents | Pseudo-First Model | Pseudo-Second Model | ||||
|---|---|---|---|---|---|---|
| qe.Cal (mg/g) | k1 (min−1 ) | R1 | qe.Cal (mg/g) | k2 (g mg−1 min−1) | R2 | |
| AKB | 217.58 | 0.01214 | 0.97497 | 343.64 | 1.18603 × 10−4 | 0.97796 |
| BKBC | 42.11 | 0.02153 | 0.99412 | 183.49 | 1.57566 × 10−3 | 0.9997 |
| PBM | 1.83 | 0.00657 | 0.75445 | 2.15 | 0.02290 | 0.99582 |
| Catalyst | Dye | Dye (mL) | Dye Conc. (mg/L) | Catalyst Content (g) | Reaction Time (min) | K0 * (min−1) | K * (min−1) | Ref. |
|---|---|---|---|---|---|---|---|---|
| Bi2MoO6/graphene | MB | 100 | 0.01 | 0.05 | 120 | 0.0037 | 0.014 | [41] |
| Bi2MoO6/diatomite | RhB | 40 | 4 | 0.015 | 60 | 0.019 | 0.059 | [42] |
| Bi2MoO6/Fe2O3 | RhB | 50 | 5 | 0.03 | 60 | 0.015 | 0.086 | [43] |
| Bi2MoO6/C3N4 | RhB | 50 | 10 | 0.02 | 50 | 0.0027 | 0.079 | [44] |
| Bi2MoO6/CNTs/g-C3N4 | 2,4–DBP | 250 | 20 | 0.25 | 120 | 0.0013 | 0.0078 | [45] |
| AKB | MB | 50 | 80 | 0.01 | 60 | 0.00038 | 0.0073 | This study |
| BKBC | MB | 50 | 80 | 0.01 | 60 | 0.00038 | 0.0079 | This study |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Zhang, H.; Cai, L.; Guo, J.; Wang, Y.; Ji, L.; Song, W. Preparation and Characterization of Macroalgae Biochar Nanomaterials with Highly Efficient Adsorption and Photodegradation Ability. Materials 2018, 11, 1709. https://doi.org/10.3390/ma11091709
Zhou Y, Zhang H, Cai L, Guo J, Wang Y, Ji L, Song W. Preparation and Characterization of Macroalgae Biochar Nanomaterials with Highly Efficient Adsorption and Photodegradation Ability. Materials. 2018; 11(9):1709. https://doi.org/10.3390/ma11091709
Chicago/Turabian StyleZhou, Yarui, Hailong Zhang, Lu Cai, Jian Guo, Yaning Wang, Lili Ji, and Wendong Song. 2018. "Preparation and Characterization of Macroalgae Biochar Nanomaterials with Highly Efficient Adsorption and Photodegradation Ability" Materials 11, no. 9: 1709. https://doi.org/10.3390/ma11091709
APA StyleZhou, Y., Zhang, H., Cai, L., Guo, J., Wang, Y., Ji, L., & Song, W. (2018). Preparation and Characterization of Macroalgae Biochar Nanomaterials with Highly Efficient Adsorption and Photodegradation Ability. Materials, 11(9), 1709. https://doi.org/10.3390/ma11091709






