Measurement and Analysis of Thermal Conductivity of Ti3C2Tx MXene Films
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
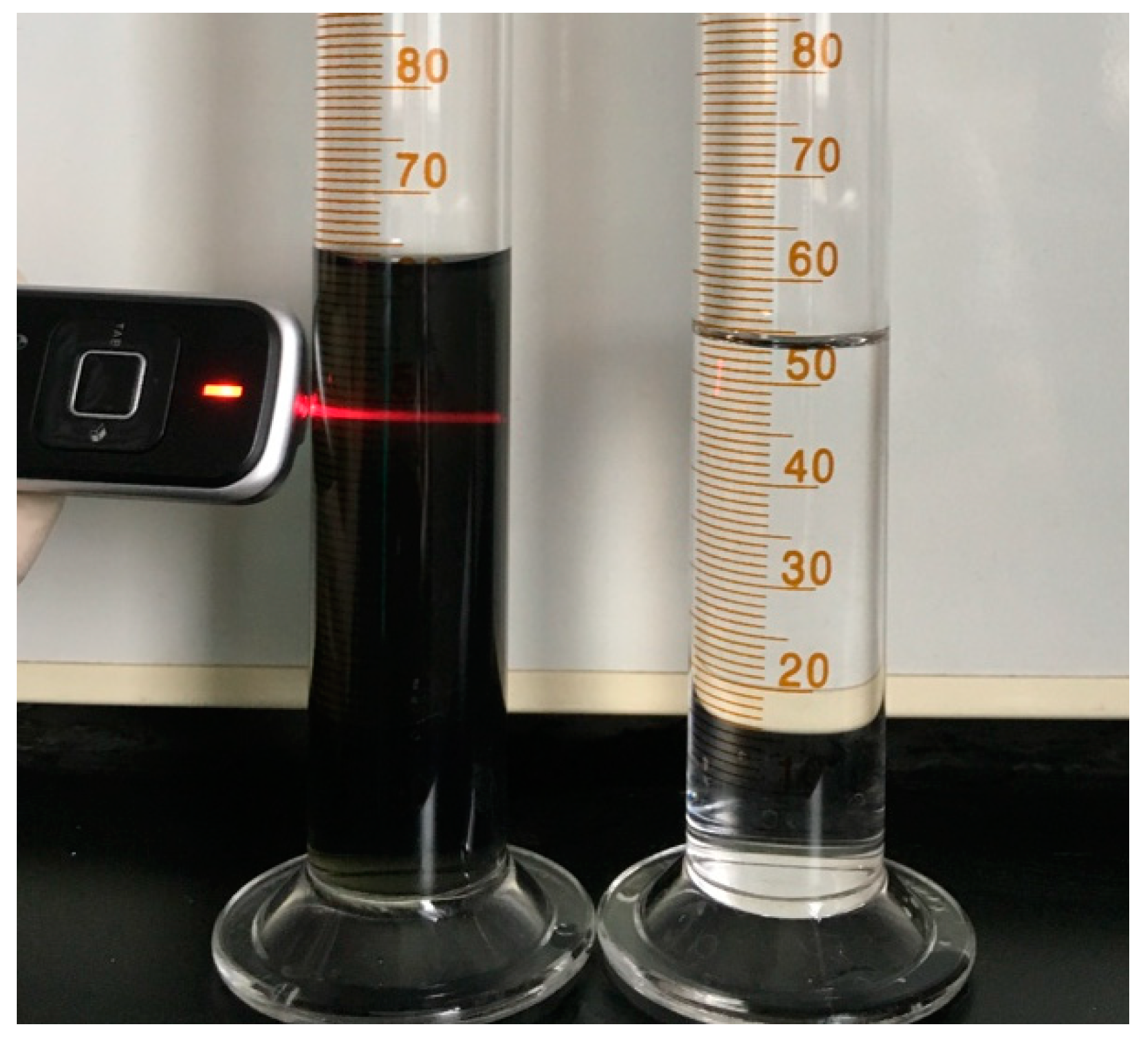
2.2. Preparation of Ti3C2Tx Powders
2.3. Preparation of Ti3C2Tx Film
2.4. Characterization
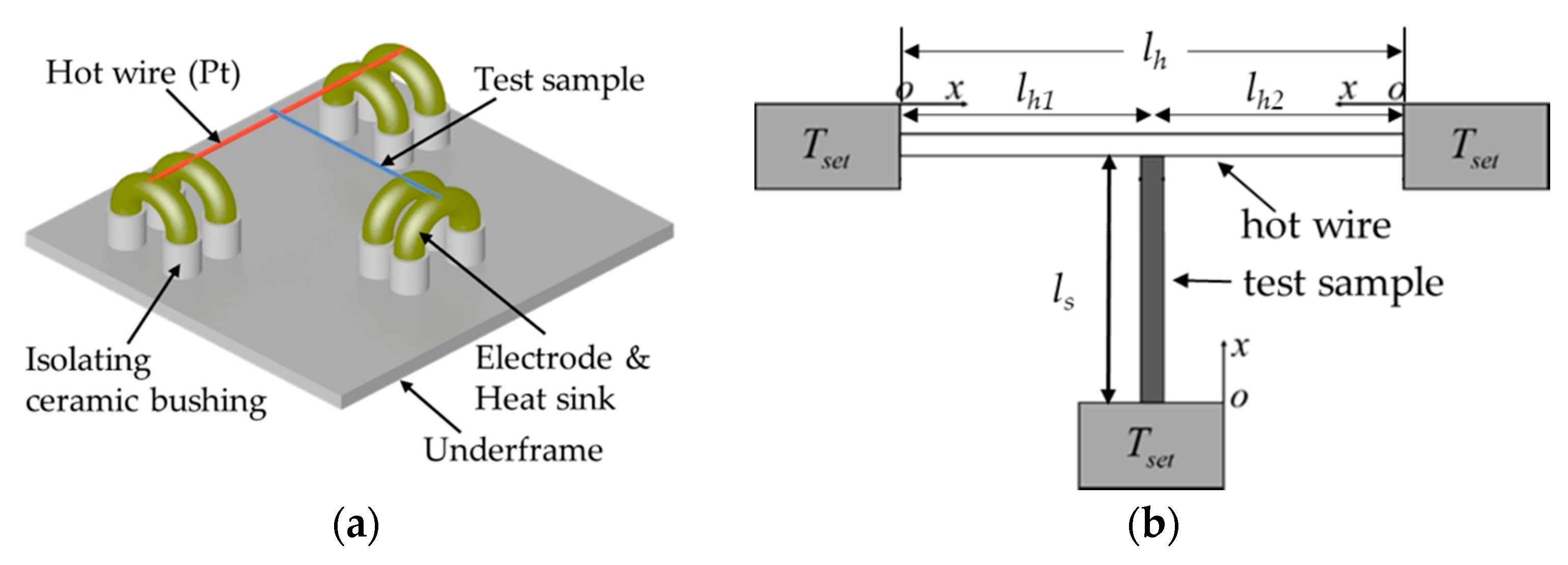
2.5. Measurements
3. Results and Discussion
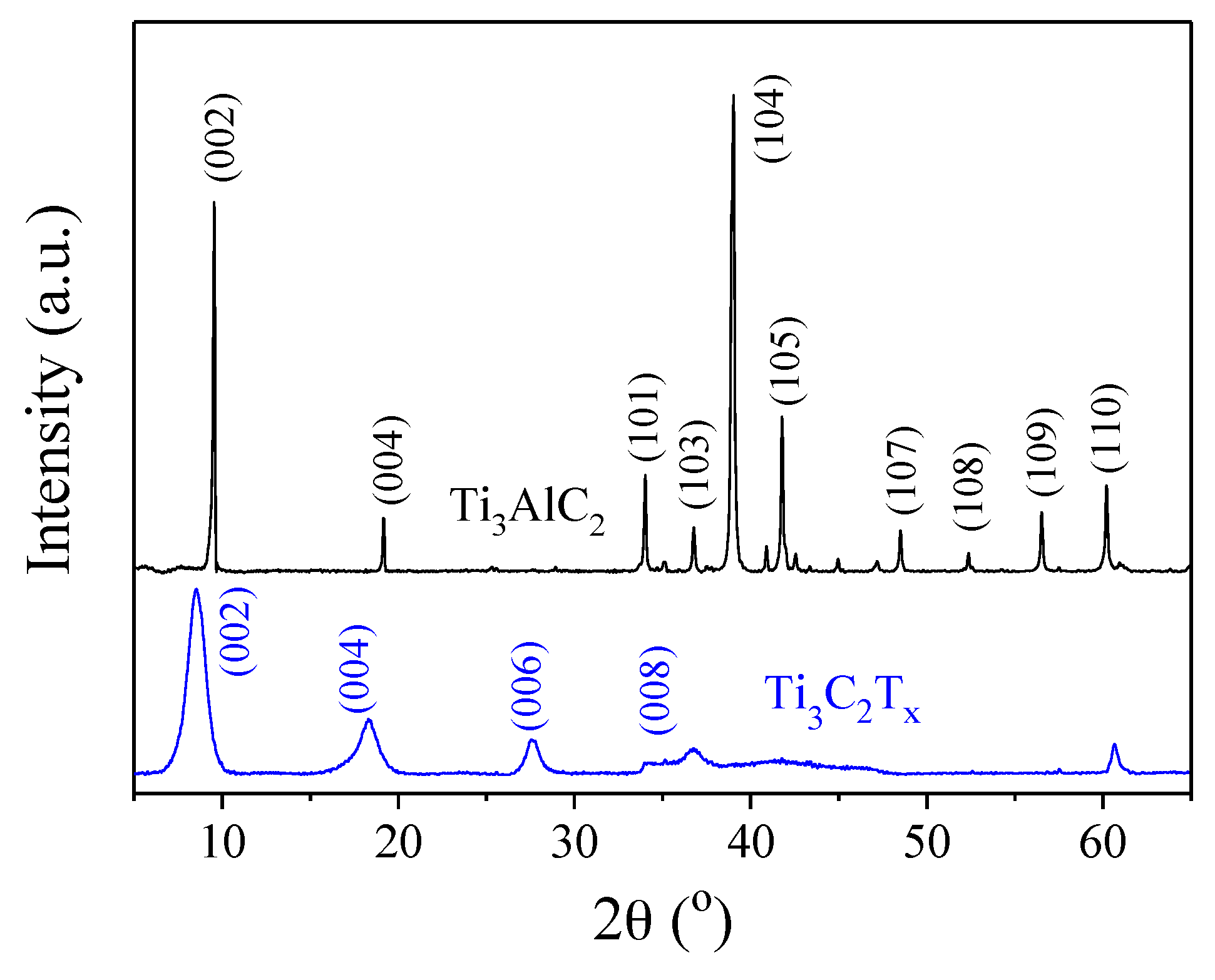
3.1. Characterization of Ti3C2Tx
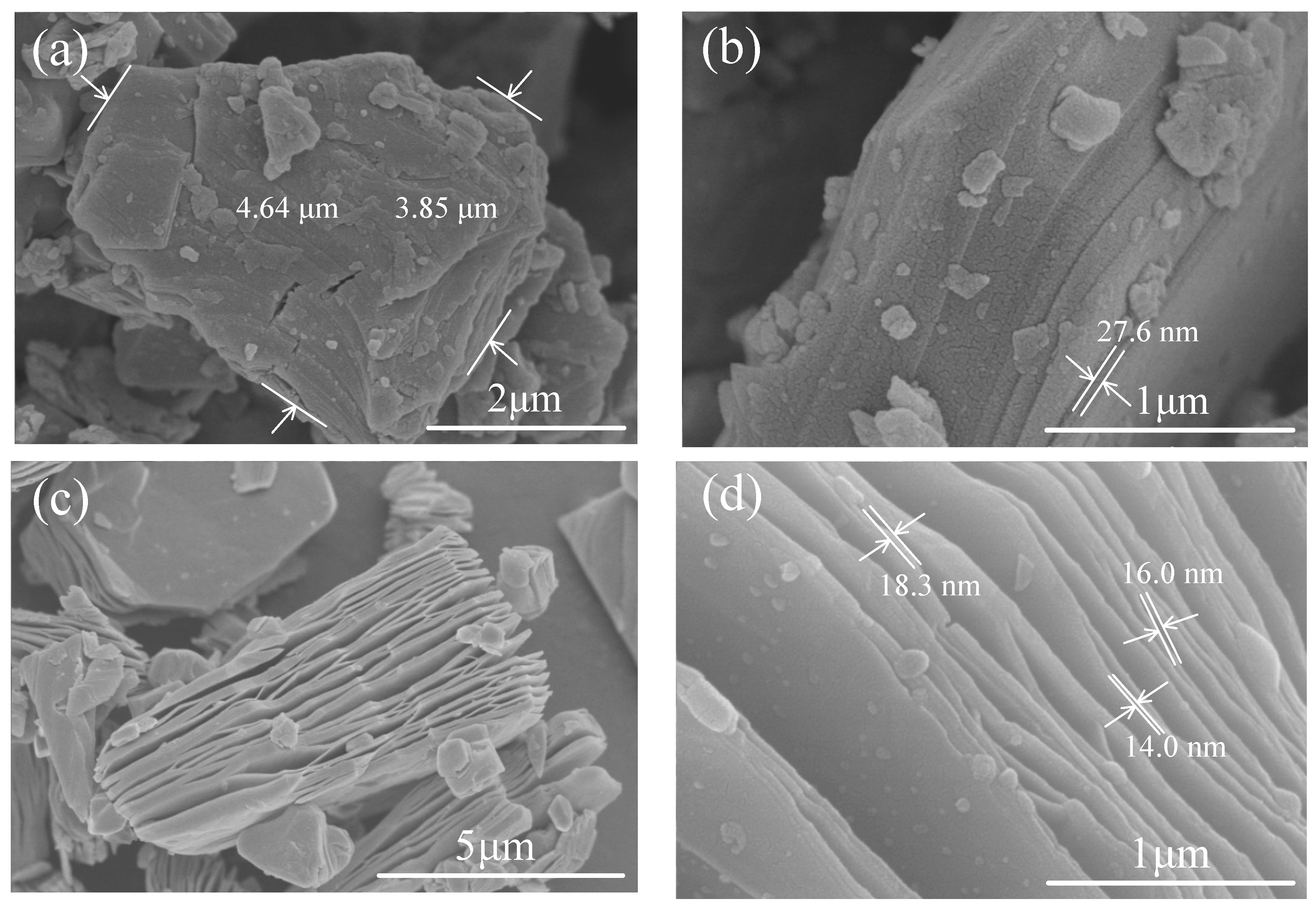
3.1.1. Ti3AlC2 and Ti3C2Tx Powders
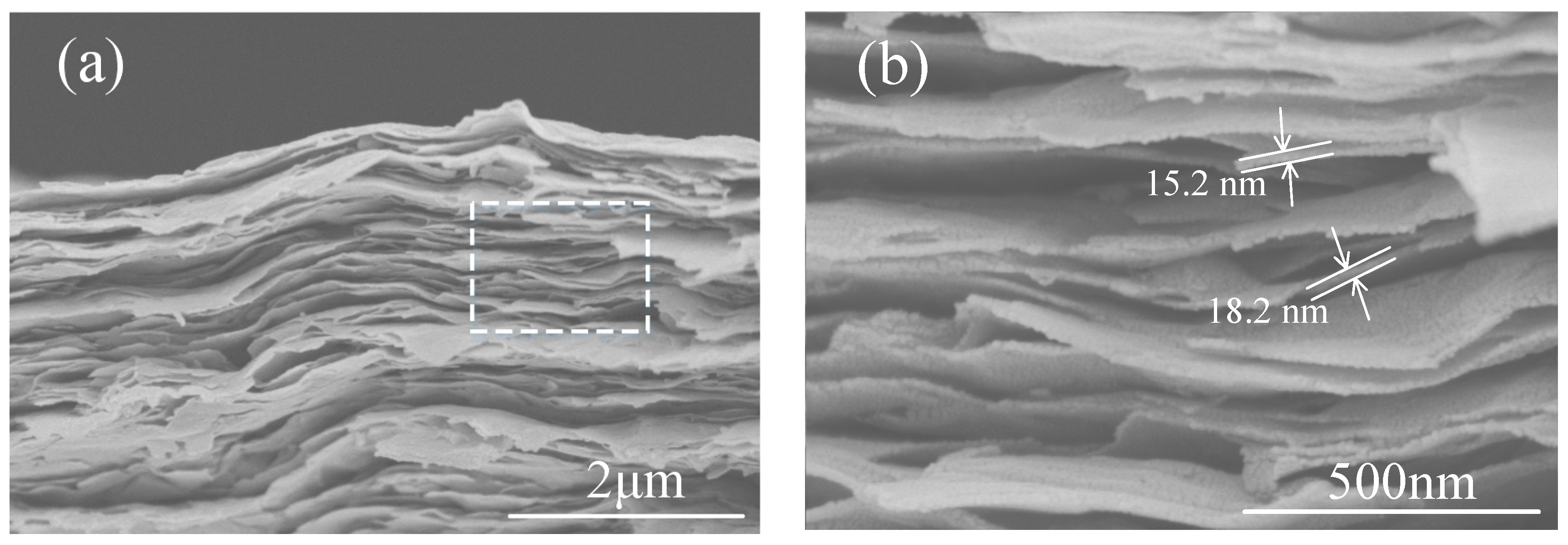
3.1.2. Ti3C2Tx Film
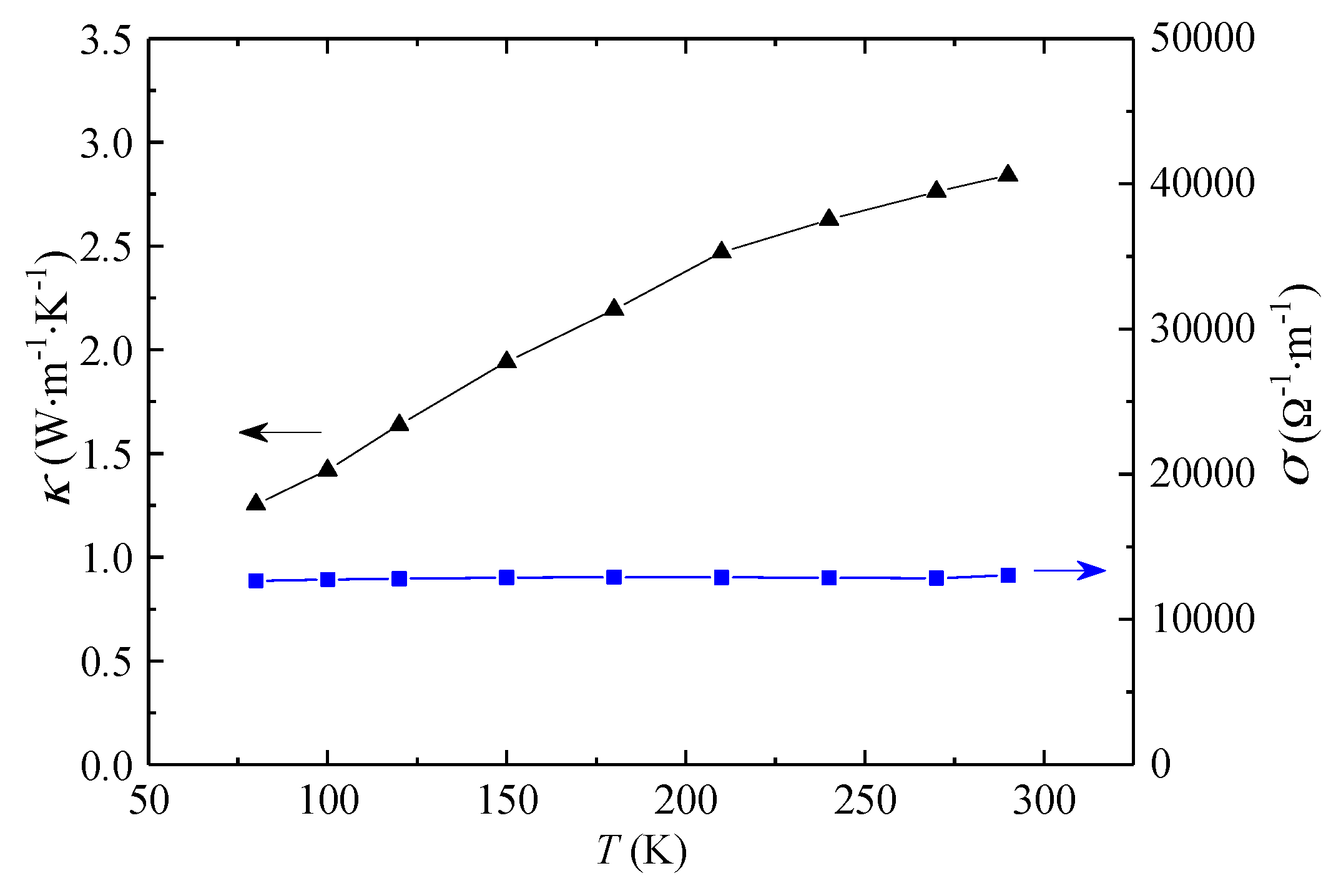
3.2. Thermal and Electrical Conductivities of Ti3C2Tx Films
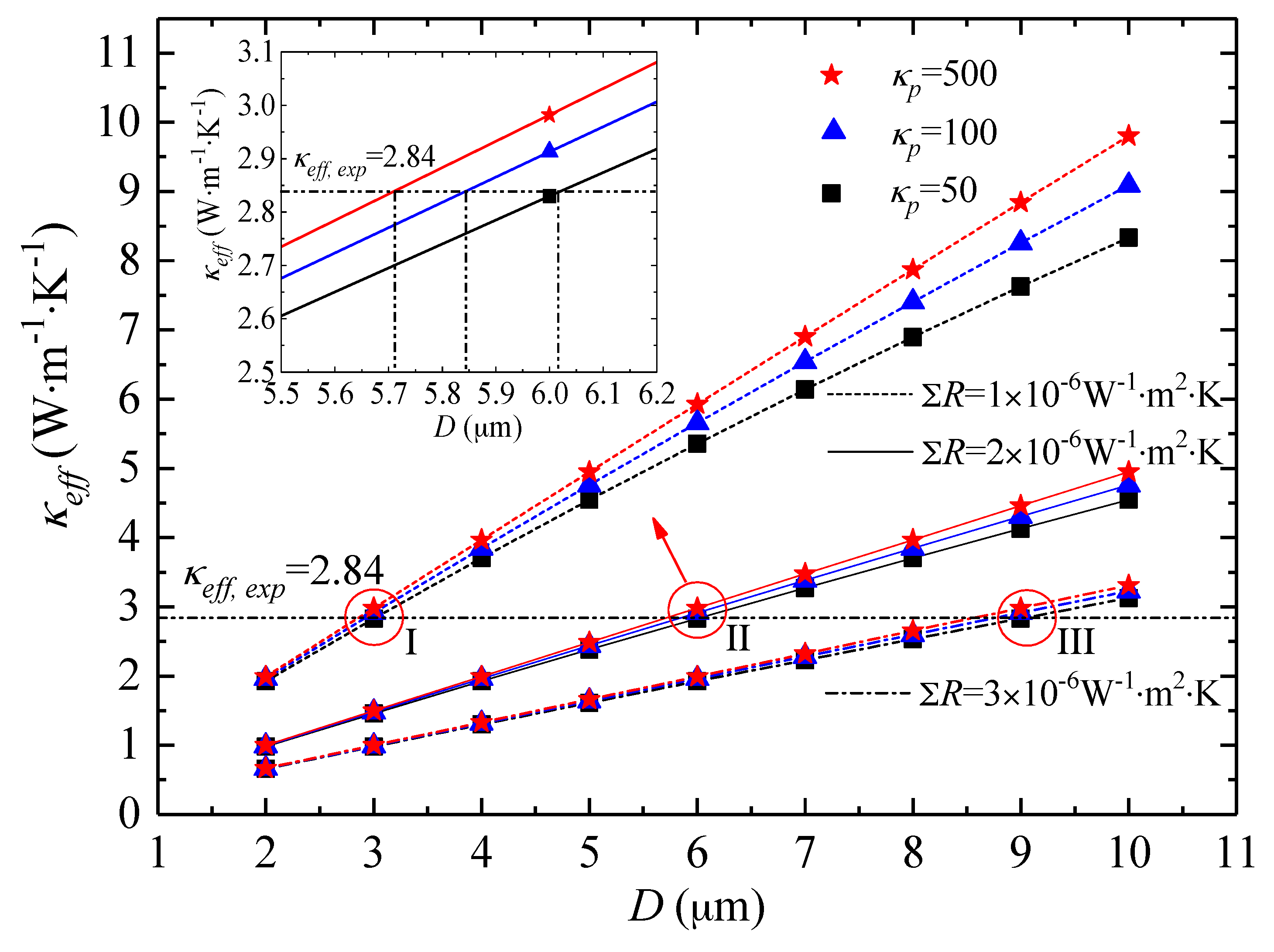
3.3. Thermal Conductivity Analysis of Ti3C2Tx Films
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3 AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Mashtalir, O.; Carle, J.; Presser, V.; Lu, J.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional transition metal carbides. ACS Nano 2012, 6, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Lukatskaya, M.R.; Mashtalir, O.; Ren, C.E.; Dall’Agnese, Y.; Rozier, P.; Taberna, P.L.; Naguib, M.; Simon, P.; Barsoum, M.W.; Gogotsi, Y. Cation intercalation and high volumetric capacitance of two-dimensional titanium carbide. Science 2013, 341, 1502–1505. [Google Scholar] [CrossRef] [PubMed]
- Mashtalir, O.; Naguib, M.; Mochalin, V.N.; Dall’Agnese, Y.; Heon, M.; Barsoum, M.W.; Gogotsi, Y. Intercalation and delamination of layered carbides and carbonitrides. Nat. Commun. 2013, 4, 1716. [Google Scholar] [CrossRef] [PubMed]
- Ghidiu, M.; Lukatskaya, M.R.; Zhao, M.Q.; Gogotsi, Y.; Barsoum, M.W. Conductive two-dimensional titanium carbide ‘clay’ with high volumetric capacitance. Nature 2014, 516, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Ren, C.E.; Zhao, M.Q.; Yang, J.; Giammarco, J.M.; Qiu, J.S.; Barsoum, M.W.; Gogotsi, Y. Flexible and conductive MXene films and nanocomposites with high capacitance. Proc. Natl. Acad. Sci. USA 2014, 111, 16676–16681. [Google Scholar] [CrossRef] [PubMed]
- Maleki, H.; Selman, J.R.; Dinwiddie, R.B.; Wang, H. High thermal conductivity negative electrode material for lithium-ion batteries. J. Power Sour. 2001, 94, 26–35. [Google Scholar] [CrossRef]
- Qin, S.; Ho, J.; Rabuffi, M.; Borelli, G.; Jow, T.R. Implications of the anisotropic thermal conductivity of capacitor windings. IEEE Electr. Insul. Mag. 2011, 27, 7–13. [Google Scholar] [CrossRef]
- Khazaei, M.; Ranjbar, A.; Arai, M.; Sasaki, T.; Yunoki, S. Electronic properties and applications of MXenes: A theoretical review. J. Mater. Chem. C 2017, 5, 2488–2503. [Google Scholar] [CrossRef]
- Liu, R.; Li, W. High-thermal-stability and high-thermal-conductivity Ti3C2Tx MXene/Poly(vinyl alcohol) (PVA) composites. ACS Omega 2018, 3, 2609–2617. [Google Scholar] [CrossRef]
- Zhang, X.; Fujiwara, S.; Fujii, M. Measurements of thermal conductivity and electrical conductivity of a single carbon fiber. Int. J. Thermophys. 2000, 21, 965–980. [Google Scholar] [CrossRef]
- Fujii, M.; Zhang, X.; Xie, H.Q.; Ago, H.; Takahashi, K.; Ikuta, T.; Abe, H.; Shimizu, T. Measuring the thermal conductivity of a single carbon nanotube. Phys. Rev. Lett. 2005, 95, 065502. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gu, M.; Zhang, X.; Wu, G. Measurements of thermal effusivity of a fine wire and contact resistance of a junction using a T type probe. Rev. Sci. Instrum. 2009, 80, 076107. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhang, X. Measurement methods and applications for thermophysical properties at micro/nanoscales. Jpn. J. Appl. Phys. 2011, 50, 11RC01. [Google Scholar] [CrossRef]
- Wang, H.; Kurata, K.; Fukunaga, T.; Ago, H.; Takamatsu, H.; Zhang, X.; Ikuta, T.; Takahashi, K.; Nishiyama, T.; Takata, Y. Simultaneous measurement of electrical and thermal conductivities of suspended monolayer graphene. J. Appl. Phys. 2016, 119, 244306. [Google Scholar] [CrossRef]
- Shi, X.; Cheng, S.; Ma, W.; Zhang, X.; Liu, G.; Pan, M.; Wang, W. Experimental research on thermal transport properties of palladium-based amorphous alloys. J. Non-Cryst. Solids 2017, 458, 157–161. [Google Scholar] [CrossRef]
- Chang, F.; Li, C.; Yang, J.; Tang, H.; Xue, M. Synthesis of a new graphene-like transition metal carbide by de-intercalating Ti3AlC2. Mater. Lett. 2013, 109, 295–298. [Google Scholar] [CrossRef]
- Zhao, M.Q.; Ren, C.E.; Ling, Z.; Lukatskaya, M.R.; Zhang, C.; Van Aken, K.L.; Barsoum, M.W.; Gogotsi, Y. Flexible MXene/carbon nanotube composite paper with high volumetric capacitance. Adv. Mater. 2015, 27, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wu, Y.; Zhang, J.; Li, G.; Huang, H.; Zhang, X.; Jiang, Q. Enhancement of the electrical properties of MXene Ti3C2 nanosheets by post-treatments of alkalization and calcination. Mater. Lett. 2015, 160, 537–540. [Google Scholar] [CrossRef]
- Kittel, C. Introduction to Solid State Physics, 8th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Chen, L.; Sun, Y.Y.; Lin, J.; Du, X.Z.; Wei, G.S.; He, S.J.; Nazarenko, S. Modeling and analysis of synergistic effect in thermal conductivity enhancement of polymer composites with hybrid filler. Int. J. Heat Mass Transf. 2015, 81, 457–464. [Google Scholar] [CrossRef]
- Chen, L.; Sun, Y.Y.; Xu, H.F.; He, S.J.; Wei, G.S.; Du, X.Z.; Lin, J. Analytic modeling for the anisotropic thermal conductivity of polymer composites containing aligned hexagonal boron nitride. Compos. Sci. Technol. 2016, 122, 42–49. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Shi, X.; Yu, N.; Zhang, X.; Du, X.; Lin, J. Measurement and Analysis of Thermal Conductivity of Ti3C2Tx MXene Films. Materials 2018, 11, 1701. https://doi.org/10.3390/ma11091701
Chen L, Shi X, Yu N, Zhang X, Du X, Lin J. Measurement and Analysis of Thermal Conductivity of Ti3C2Tx MXene Films. Materials. 2018; 11(9):1701. https://doi.org/10.3390/ma11091701
Chicago/Turabian StyleChen, Lin, Xuguo Shi, Nanjie Yu, Xing Zhang, Xiaoze Du, and Jun Lin. 2018. "Measurement and Analysis of Thermal Conductivity of Ti3C2Tx MXene Films" Materials 11, no. 9: 1701. https://doi.org/10.3390/ma11091701
APA StyleChen, L., Shi, X., Yu, N., Zhang, X., Du, X., & Lin, J. (2018). Measurement and Analysis of Thermal Conductivity of Ti3C2Tx MXene Films. Materials, 11(9), 1701. https://doi.org/10.3390/ma11091701





