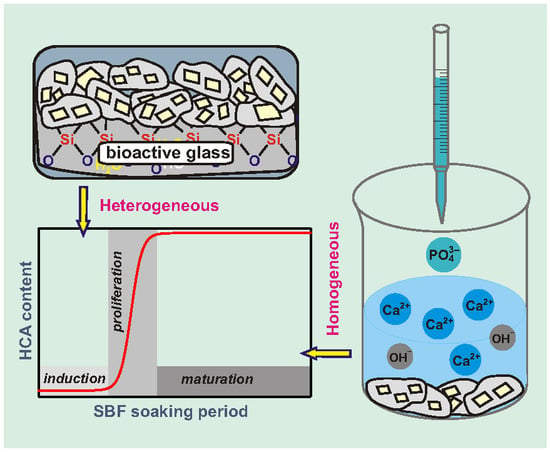
Contrasting In Vitro Apatite Growth from Bioactive Glass Surfaces with that of Spontaneous Precipitation
Abstract
1. Introduction
2. Materials and Methods
2.1. Glass Preparation and SBF Testing
2.2. Measurements of pH-Values and Ion Concentrations
2.3. Powder XRD and FTIR Experiments
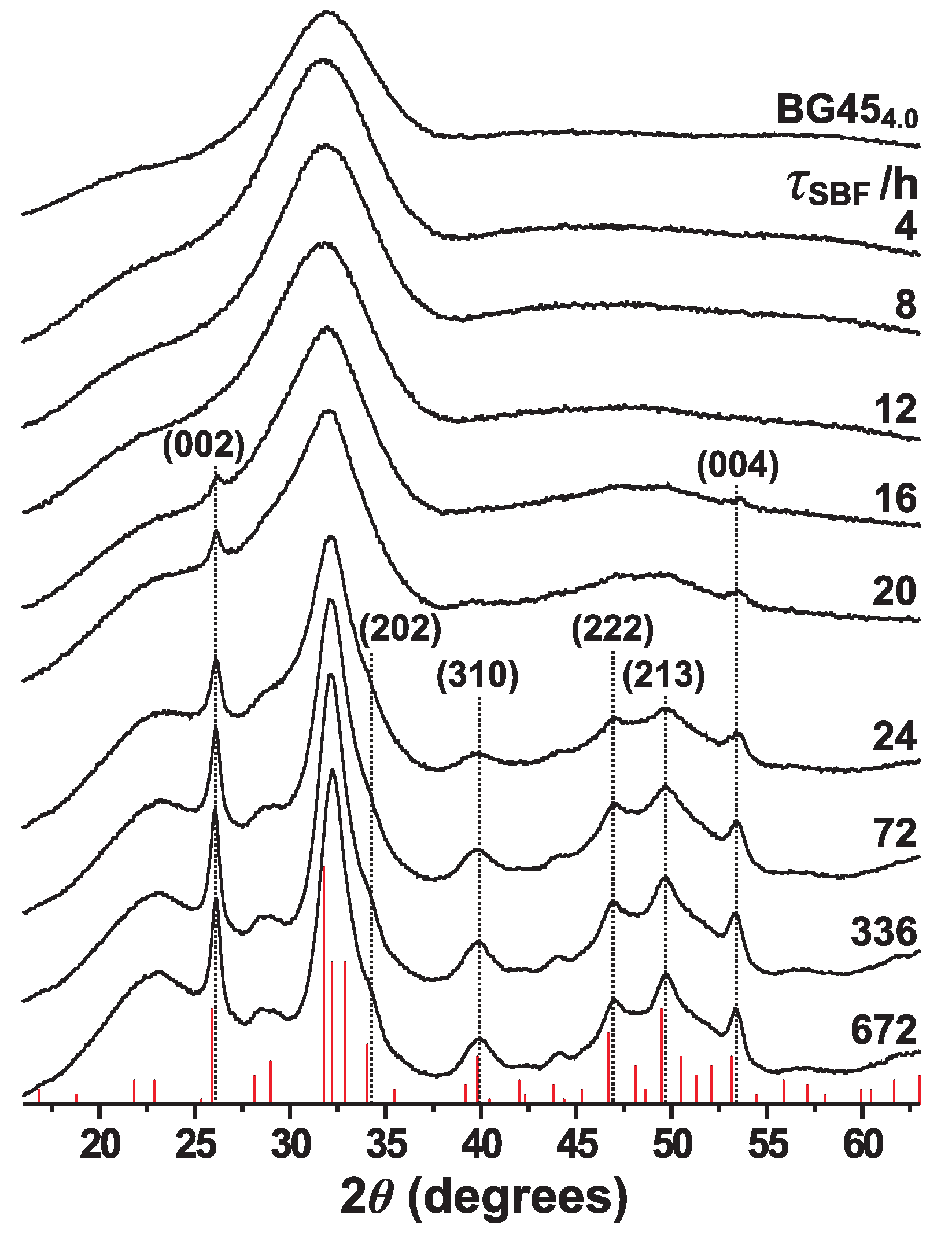
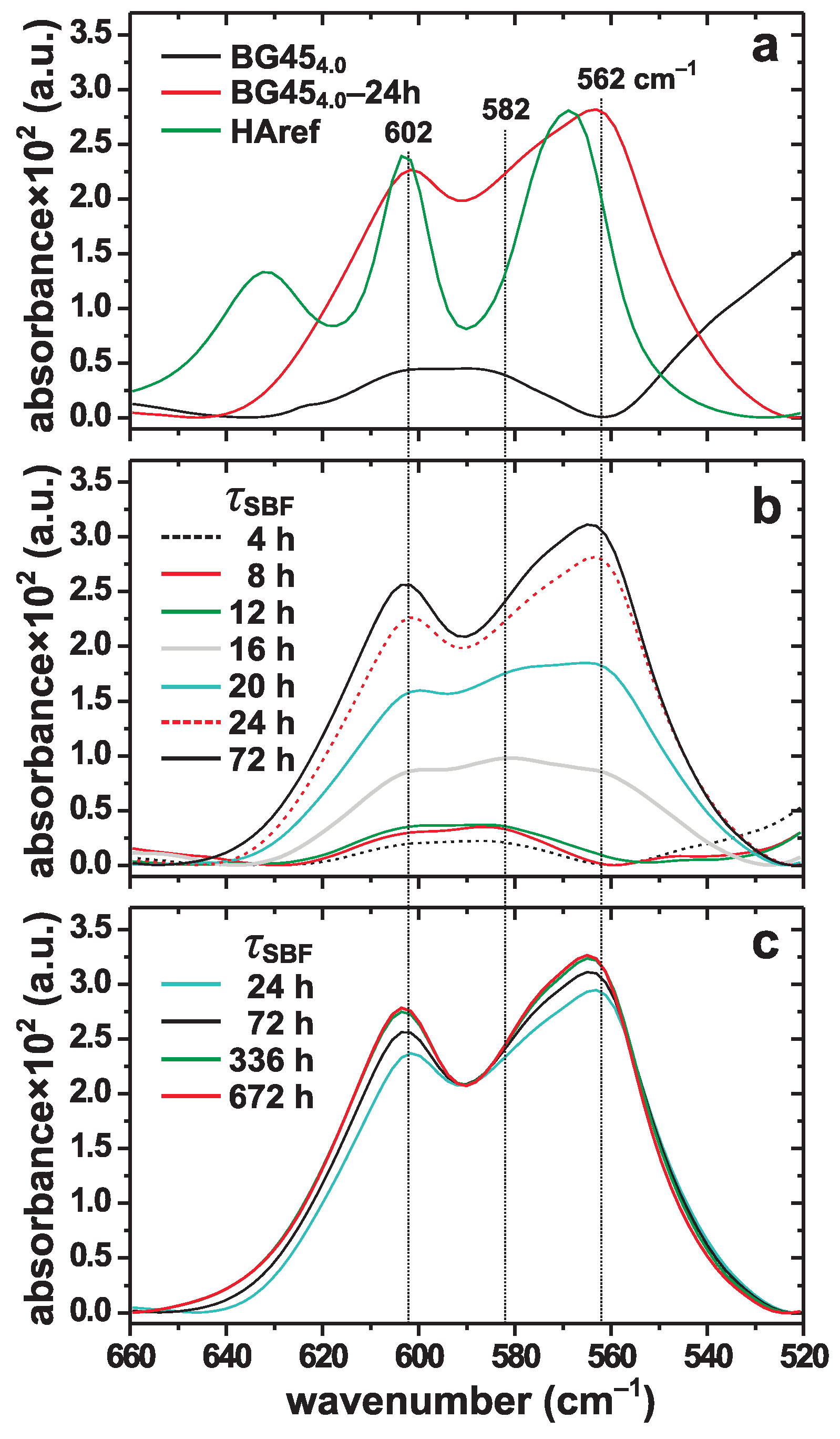
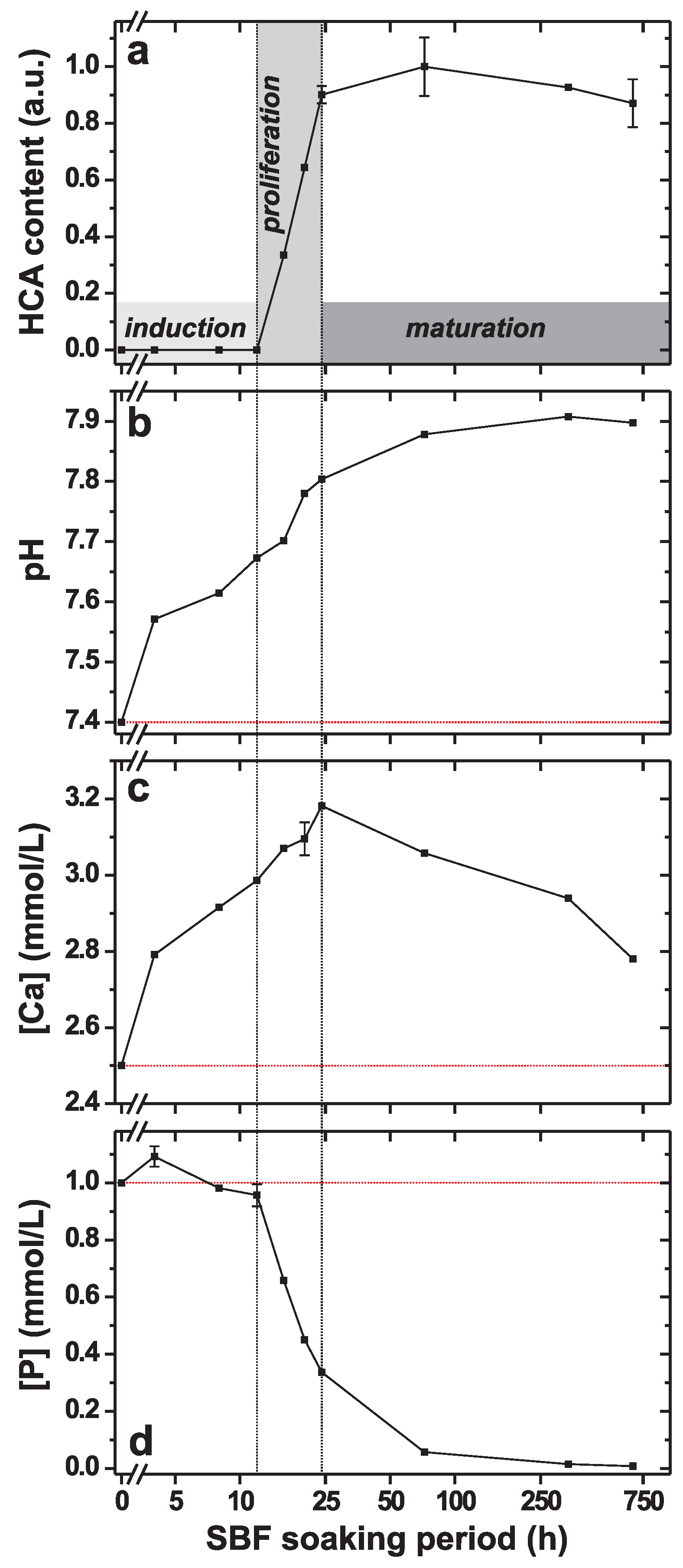
3. Results
3.1. Apatite Formation Probed by XRD and FTIR
3.2. Trends of pH, [Ca] and [P] Solution Parameters
4. Discussion
4.1. Similarities between Apatite Formation by Direct Precipitation and from Glass Surfaces
4.1.1. Induction Period
4.1.2. Proliferation Period
4.1.3. Maturation Period
4.2. Further Support for Coincident Net ACP/HCA Growth
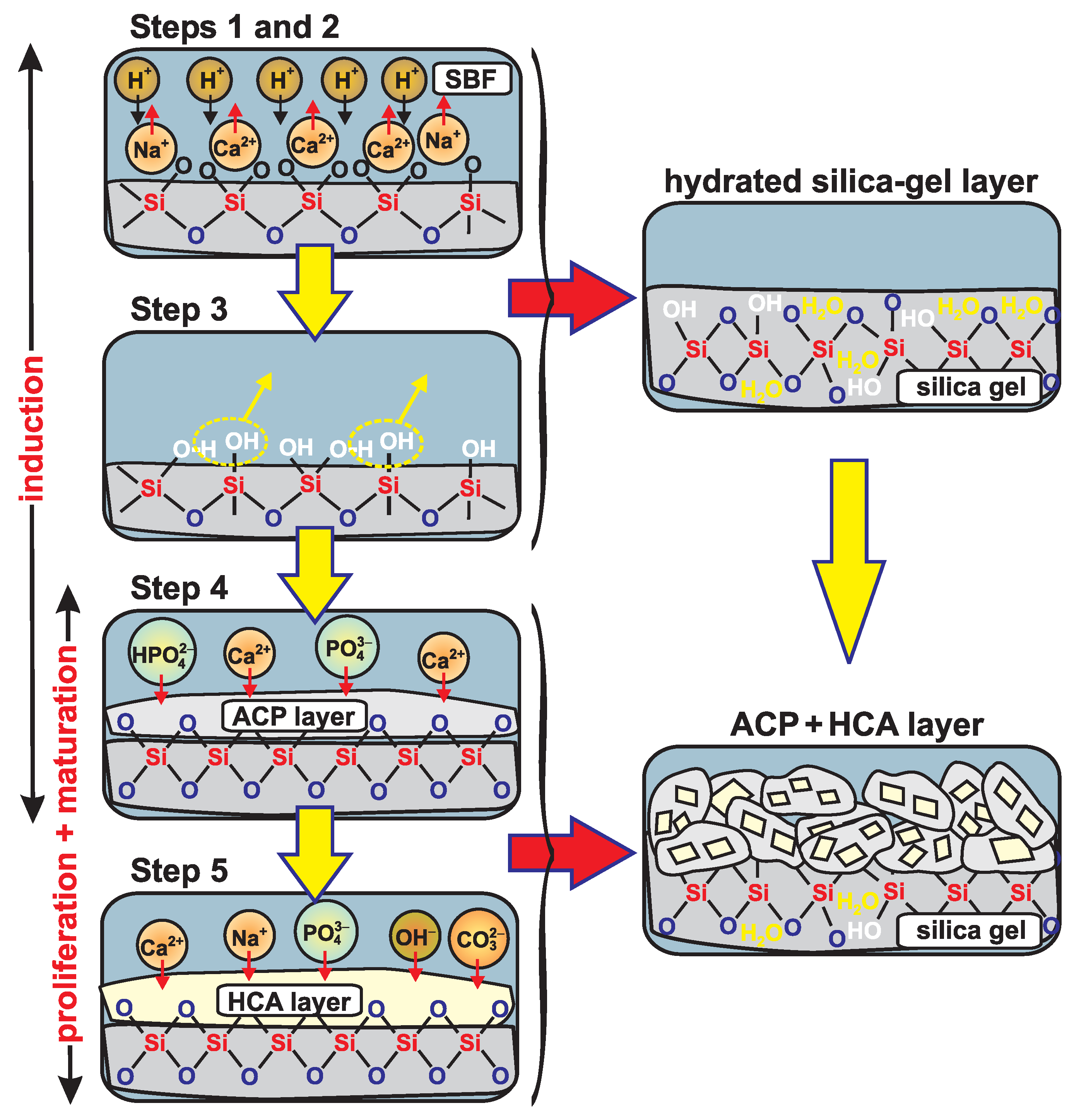
4.3. Discussion on the Validity of the Hench Mechanism
- (1)
- First, a rapid exchange occurs of the Ca and (particularly) Na cations with protons from the solution, where the glass-modifier leaching is accompanied by a rise in the pH of the solution, and creation of SiOH (“silanol”) groups at the glass surface.
- (2)
- The silicate network at the surface fragments due to hydrolysis of Si–O–Si bonds according to Si–O–Si + HO→2SiOH; this results in a further increased silanol concentration, but also in loss of Si species, which enter the solution as Si(OH).
- (3)
- This step is essentially a reversal of stage (2), implying that Si–O–Si bonds form via condensation of neighboring SiOH moieties (see Figure 4).
- (4)
- “Migration of Ca and PO groups to the surface through the SiO-rich layer forming a CaO–PO-rich film on top of the SiO-rich layer, followed by growth of the amorphous CaO–PO-rich film by incorporation of soluble calcium and phosphates from solution” [2]; we remind that the “amorphous CaO–PO-rich film” is generally to be equated with “ACP”.
- (5)
- According to Hench, the subsequent ACP→HCA transformation proceeds as “crystallization of the amorphous CaO–PO film by incorporation of OH, CO or F anions from solution to form a mixed hydroxyl, carbonate, fluoroapatite layer” [2].
5. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone Mineral: Update on Chemical Composition and Structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Hench, L.L. Bioceramics: From Concept to Clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Jones, J.R. Review of Bioactive Glass: From Hench to Hybrids. Acta Biomater. 2013, 9, 4457–4486. [Google Scholar] [CrossRef] [PubMed]
- Eanes, E.D.; Posner, A.S. Kinetics and Mechanism of Conversion of Noncrystalline Calcium Phosphate to Crystalline Hydroxyapatite. Trans. N. Y. Acad. Sci. 1965, 28, 233–241. [Google Scholar] [CrossRef]
- Boskey, A.L.; Posner, A.S. Conversion of Amorphous Calcium Phosphate to Microcrystalline Hydroxyapatite. A pH-Dependent, Solution-Mediated, Solid-Solid Conversion. J. Phys. Chem. 1973, 77, 2313–2317. [Google Scholar] [CrossRef]
- Eanes, E.D.; Meyer, J.L. The Maturation of Crystalline Calcium Phosphates in Aqueous Suspensions at Physiologic pH. Calcif. Tissue Res. 1977, 23, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.L.; Eanes, E.D. A Thermodynamic Analysis of the Amorphous to Crystalline Calcium Phosphate Transformation. Calcif. Tissue Res. 1978, 25, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Posner, A.S. Formation of Hydroxyapatite at Low Supersaturation. J. Phys. Chem. 1976, 80, 40–45. [Google Scholar] [CrossRef]
- Nancollas, G.H.; Tomažiǒ, B. Growth of Calcium Phosphate on Hydroxyapatite Crystals. Effect of Supersaturation and Ionic Medium. J. Phys. Chem. 1974, 78, 2218–2225. [Google Scholar] [CrossRef]
- Blumenthal, N.C.; Posner, A.S. Hydroxyapatite: Mechanism of Formation and Properties. Calcif. Tissue Res. 1973, 13, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.L.; Weatherall, C.C. Amorphous to Crystalline Calcium Phosphate Phase Transformation at Elevated pH. J. Colloid Interface Sci. 1982, 89, 257–267. [Google Scholar] [CrossRef]
- Lazić, S. Microcrystalline Hydroxyapatite Formation from Alkaline Solutions. J. Cryst. Growth 1995, 317, 147–154. [Google Scholar] [CrossRef]
- Kim, S.; Ryu, H.S.; Shin, H.; Jung, H.S.; Hong, K.S. In Situ Observation of Hydroxyapatite Nanocrystal Formation from Amorphous Calcium Phosphate in Calcium-Rich Solutions. Mater. Chem. Phys. 2005, 91, 500–506. [Google Scholar] [CrossRef]
- Tao, J.; Pan, H.; Zeng, Y.; Xu, X.; Tang, R. Roles of Amorphous Calcium Phosphate and Biological Additives in the Assembly of Hydroxyapatite Nanoparticles. J. Phys. Chem. B 2008, 111, 13410–13418. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.G.; Liao, J.W.; Gou, B.D.; Huang, J.; Tang, R.K.; Tao, J.H.; Zhang, T.L.; Wang, K. Crystallization at Multiple Sites Inside Particles of Amorphous Calcium Phosphate. Cryst. Growth Des. 2009, 9, 2620–2626. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Tao, J.; Brylka, L.J.; Friedrich, H.; Bertinetti, L.; Schenk, A.S.; Verch, A.; Dmitrovich, V.; Bomans, P.H.H.; Frederik, P.M.; et al. Ion-Association Complexes Unite Classical and Non-Classical Theories for the Biomimetic Nucleation of Calcium Phosphate. Nat. Commun. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Xie, B.; Halter, T.J.; Borah, B.M.; Nancollas, G.H. Tracking Amorphous Precursor Formation and Transformation During Induction States of Nucleation. Cryst. Growth Des. 2014, 14, 1659–1665. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Liu, X.Y.; Tang, R.; Xu, H.Y. Mystery of the Transformation from Amorphous Calcium Phosphate to Hydroxyapatite. Chem. Commun. 2010, 46, 7415–7417. [Google Scholar] [CrossRef] [PubMed]
- Carino, A.; Ludwig, C.; Cervellino, A.; Müller, E.; Testino, A. Formation and Transformation of Calcium Phosphate Phases Under Biologically Relevant Conditions: Experiments and Modelling. Acta Biomater. 2018, 74, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.E.; Heughebaert, M.; Heughebaert, J.C.; Bonar, L.C.; Glimcher, M.J.; Griffin, R.G. Solid State 31NMR Studies of the Conversion of Amorphous Tricalcium Phosphate to Apatitic Tricalcium Phosphate. Calcif. Tissue Int. 1991, 49, 378–382. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, C.; Maltsev, S.; Karrasch, A. Progress of Structural Elucidation of Amorphous Calcium Phosphate (ACP) and Hydroxyapatite (HAp): Disorder and Surfaces as Seen by Solid State NMR. Key Eng. Mater. 2006, 309–311, 69–72. [Google Scholar] [CrossRef]
- Wang, Y.; von Euw, S.; Fernandes, F.M.; Cassaignon, S.; Selmane, M.; Laurant, G.; Pehau-Arnaudet, G.; Coelho, C.; Bonhomme-Coury, L.; Giraud-Guille, M.M.; et al. Water-Mediated Structuring of Bone Apatite. Nat. Mater. 2013, 12, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- West, V.C. Observations on Phase Transformation of a Precipitated Calcium Phosphate. Calcif. Tissue Res. 1971, 7, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Harries, J.E.; Hukins, D.W.L.; Holt, C.; Hasnain, S.S. Conversion of Amorphous Calcium Phosphate into Hydroxyapatite Investigated by EXAFS Spectroscopy. J. Cryst. Growth 1987, 84, 563–570. [Google Scholar] [CrossRef]
- Tao, J.; Pan, H.; Wang, J.; Wu, J.; Wang, B.; Xu, X.; Tang, R. Evolution of Amorphous Calcium Phosphate to Hydroxyapatite Probed by Gold Nanoparticles. J. Phys. Chem. C 2008, 112, 14929–14933. [Google Scholar] [CrossRef]
- Yang, X.; Xie, B.; Wang, L.; Qin, Y.; Henneman, Z.J.; Nancollas, G.H. Influence of Magnesium Ions and Amino Acids on the Nucleation and Growth of Hydroxyapatite. Cryst. Eng. Comm. 2011, 13, 1153–1158. [Google Scholar] [CrossRef]
- Hench, L.L. Bioactive Ceramics. Ann. N. Y. Acad. Sci. 1988, 523, 54–71. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.E.; Hench, L.L.; Paschall, H.A. The Influence of Surface Chemistry on Implant Interface Histology: A Theoretical Basis for Implant Materials Selection. J. Biomed. Mater. Res. 1976, 10, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Andersson, O.H.; Karlsson, K.H. On the Bioactivity of Silicate Glass. J. Non-Cryst. Solids 1991, 129, 145–151. [Google Scholar] [CrossRef]
- Hayakawa, S.; Tsuru, K.; Ohtsuki, C.; Osaka, A. Mechanism of Apatite Formation on a Sodium Silicate Glass in a Simulated Body Fluid. J. Am. Ceram. Soc. 1999, 82, 2155–2160. [Google Scholar] [CrossRef]
- Banchet, V.; Jallot, E.; Michel, J.; Wortham, L.; Laurent-Maquin, D.; Balossier, G. X-ray Microanalysis in STEM of Short-Term Physicochemical Reactions at Bioactive Glass Particle/Biological Fluid Interface. Determination of O/Si Atomic Ratios. Surf. Interface Anal. 2004, 36, 658–665. [Google Scholar] [CrossRef]
- Cerruti, M.; Perardi, A.; Cerrato, G.; Morterra, C. Formation of a Nanostructured Layer on Bioglass Particles of Different Sizes Immersed in Tris-Buffered Solution. N2 Adsorption and HR-TEM/EDS Analysis. Langmuir 2005, 21, 9327–9333. [Google Scholar] [CrossRef] [PubMed]
- Berbecaru, C.; Stan, G.E.; Pina, S.; Tulyaganov, D.U.; Ferreira, J.M.F. The Bioactivity Mechanism of Magnetron Sputtered Bioglass Thin Films. Appl. Surf. Sci. 2012, 258, 9840–9848. [Google Scholar] [CrossRef]
- West, J.H.; Hench, L.L. Reaction Kinetics of Bioactive Ceramics Part V: Molecular Orbital Modeling of Bioactive Glass Surface Reactions. In Bioceramics; Yamamuro, T., Kokubo, T., Nakamura, T., Eds.; Kobunshi Kankokai: Kyoto, Japan, 1992; pp. 75–86. [Google Scholar]
- Sahai, N.; Tossell, J.A. Molecular Orbital Study of Apatite (Ca5(PO4)3OH) Nucleation at Silica Bioceramic Surfaces. J. Phys. Chem. B 2000, 104, 4322–4341. [Google Scholar] [CrossRef]
- Lin, K.S.K.; Tseng, Y.H.; Mou, Y.; Hsu, Y.C.; Yang, C.M.; Chan, J.C.C. Mechanistic Study of Apatite Formation on Bioactive Glass Surface using 31P Solid-State NMR Spectroscopy. Chem. Mater. 2005, 17, 4493–4501. [Google Scholar] [CrossRef]
- Gunawidjaja, P.N.; Lo, A.Y.H.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Stevensson, B.; Grins, J.; Vallet-Regí, M.; Edén, M. Biomimetic Apatite Mineralization Mechanisms of Mesoporous Bioactive Glasses as Probed by Multinuclear 31P, 29Si, 23Na and 13C Solid State NMR. J. Phys. Chem. C 2010, 114, 19345–19356. [Google Scholar] [CrossRef]
- Mathew, R.; Gunawidjaja, P.N.; Izquierdo-Barba, I.; Jansson, K.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Solid State 31P and 1H NMR Investigations of Amorphous and Crystalline Calcium Phosphates Grown Biomimetically from a Mesoporous Bioactive Glass. J. Phys. Chem. C 2011, 115, 20572–20582. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Turdean-Ionescu, C.; Yu, Y.; Stevensson, B.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Proton Environments in Biomimetic Calcium Phosphates Formed from Mesoporous Bioactive CaO–SiO2–P2O5 Glasses In Vitro: Insights from Solid-State NMR. J. Phys. Chem. C 2017, 121, 13223–13238. [Google Scholar] [CrossRef] [PubMed]
- Turdean-Ionescu, C.; Stevensson, B.; Grins, J.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Composition-Dependent In Vitro Apatite Formation at Mesoporous Bioactive Glass-Surfaces Quantified by Solid-State NMR and Powder XRD. RSC Adv. 2015, 5, 86061–86071. [Google Scholar] [CrossRef]
- Takadama, H.; Kim, H.M.; Kokubo, T.; Nakamura, T. Mechanism of Biomineralization of Apatite on a Sodium Silicate Glass: TEM-EDX Study In Vitro. Chem. Mater. 2001, 13, 1108–1113. [Google Scholar] [CrossRef]
- Martin, R.A.; Twyman, H.; Qiu, D.; Knowles, J.C.; Newport, R.J. A Study of the Formation of Amorphous Calcium Phosphate and Hydroxyapatite on Melt Quenched Bioglass® Using Surface Sensitive Shallow Angle X-ray Diffraction. J. Mater. Sci. Mater. Med. 2009, 20, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Turdean-Ionescu, C.; Stevensson, B.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Surface Reactions of Mesoporous Bioactive Glasses Monitored by Solid-State NMR: Concentration Effects in Simulated Body Fluid. J. Phys. Chem. C 2016, 120, 4961–4974. [Google Scholar] [CrossRef]
- Souza, M.T.; Crovace, M.C.; Schröder, C.; Eckert, H.; Peitl, O.; Zanotto, E.D. Effect of Magnesium Ion Incorporation on the Thermal Stability, Dissolution Behavior and Bioactivity in Bioglass-Derived Glasses. J. Non-Cryst. Solids 2013, 382, 57–65. [Google Scholar] [CrossRef]
- Salinas, A.J.; Martin, A.I.; Vallet-Regí, M. Bioactivity of Three CaO–P2O5–SiO2 Sol-Gel Glasses. J. Biomed. Mater. Res. 2002, 61, 524–532. [Google Scholar] [CrossRef] [PubMed]
- López-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regí, M. Ordered Mesoporous Bioactive Glasses for Bone Tissue Regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar] [CrossRef]
- Gunawidjaja, P.N.; Mathew, R.; Lo, A.Y.H.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Local Structures of Mesoporous Bioactive Glasses and their Surface Alterations In Vitro: Inferences from Solid State Nuclear Magnetic Resonance. Philos. Trans. R. Soc. A 2012, 370, 1376–1399. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Miyaji, F.; Kokubo, T.; Ohtsuki, C.; Nakamura, T. Bioactivity of Na2O–CaO–SiO2 Glasses. J. Am. Ceram. Soc. 1995, 78, 2405–2411. [Google Scholar] [CrossRef]
- Mathew, R.; Stevensson, B.; Tilocca, A.; Edén, M. Toward a Rational Design of Bioactive Glasses with Optimal Structural Features: Composition-Structure Correlations Unveiled by Solid-State NMR and MD Simulations. J. Phys. Chem. B 2014, 118, 833–844. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Stevensson, B.; Edén, M. Na/Ca Intermixing around Silicate and Phosphate Groups in Bioactive Phosphosilicate Glasses Revealed by Heteronuclear Solid-State NMR and Molecular Dynamics Simulations. J. Phys. Chem. B 2015, 119, 5701–5715. [Google Scholar] [CrossRef] [PubMed]
- Edén, M. The Split Network Analysis for Exploring Composition-Structure Correlations in Multi-Component Glasses: I. Rationalizing Bioactivity-Composition Trends of Bioglasses. J. Non-Cryst. Solids 2011, 357, 1595–1602. [Google Scholar] [CrossRef]
- O’Donnell, M.D.; Watts, S.J.; Hill, R.G.; Law, R.V. The Effect of Phosphate Content on the Bioactivity of Soda-Lime Phosphosilicate Glasses. J. Mater. Sci.: Mater. Med. 2009, 20, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Mathew, R.; Edén, M. Quantitative Composition-Bioactivity Relationships of Phosphosilicate Glasses: Bearings from the Phosphorus Content and Network Polymerization. J. Non-Cryst. Solids 2018. [Google Scholar] [CrossRef]
- Lebecq, I.; Désanglois, F.; Leriche, A.; Follet-Houttemane, C. Compositional Dependence on the In Vitro Bioactivity of Invert or Conventional Bioglasses in the Si–Ca–Na–P System. J. Biomed. Mater. Res. 2007, 83A, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.X.; Yu, C.Z.; Zhou, X.F.; Tang, J.W.; Zhao, D.Y. Highly Ordered Mesoporous Bioactive Glasses with Superior In Vitro Bone-Forming Bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T. Bioactive Glass Ceramics: Properties and Applications. Biomaterials 1991, 12, 155–163. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How Useful is SBF in Predicting In Vivo Bone Bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Relationship Between in vitro Apatite-Forming Ability Measured Using Simulated Body Fluid and In Vivo Bioactivity of Biomaterials. Mater. Sci. Eng. C 2014, 35, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Tropp, J.; Blumenthal, N.C.; Waugh, J.S. Phosphorus NMR Study of Solid Amorphous Calcium Phosphate. J. Am. Chem. Soc. 1983, 105, 22–26. [Google Scholar] [CrossRef]
- Belton, P.S.; Harris, R.K.; Wilkes, P.J. Solid-State Phosphorus-31 NMR Studies of Synthetic Inorganic Calcium Phosphates. J. Phys. Chem. Solids 1988, 49, 21–27. [Google Scholar] [CrossRef]
- Jones, J.R.; Sepulveda, P.; Hench, L.L. Dose-Dependent Behavior of Bioactive Glass Dissolution. J. Biomed. Mater. Res. 2001, 58, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Mathew, R.; Turdean-Ionescu, C.; Stevensson, B.; Izquierdo-Barba, I.; García, A.; Arcos, D.; Vallet-Regí, M.; Edén, M. Direct Probing of the Phosphate-Ion Distribution in Bioactive Silicate Glasses by Solid-State NMR: Evidence for Transitions between Random/Clustered Scenarios. Chem. Mater. 2013, 25, 1877–1885. [Google Scholar] [CrossRef]
- Drouet, C. Apatite Formation: Why It May Not Work as Planned, and How to Conclusively Identify Apatite Compounds. BioMed Res. Int. 2013, 2013. [Google Scholar] [CrossRef] [PubMed]
- Clesceri, L.S.; Greenberg, A.E.; Eaton, A.D. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Ciavatta, C.; Antisari, L.V.; Sequi, P. Interference of Soluble Silica in the Determination of Orthophosphate-Phosphorus. J. Environ. Qual. 1990, 19, 761–764. [Google Scholar] [CrossRef]
- Reig, F.B.; Adelantado, J.V.G.; Moreno, M.C.M.M. FTIR Quantitative Analysis of Calcium Carbonate (Calcite) and Silica (Quartz) Mixtures Using the Constant Ratio Method. Application to Geological Samples. Talanta 2002, 58, 811–821. [Google Scholar] [CrossRef]
- Kim, C.Y.; Clark, A.E.; Hench, L.L. Early Stages of Calcium-Phosphate Layer Formation in Bioglasses. J. Non-Cryst. Solids 1989, 113, 195–202. [Google Scholar] [CrossRef]
- Cerruti, M.G.; Greenspan, D.; Powers, K. An Analytical Model for the Dissolution of Different Particle Size Samples of Bioglass® in TRIS-Buffered Solution. Biomaterials 2005, 26, 4903–4911. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, H.; Serra, J.; González, P.; León, B. Structural Study of Sol–Gel Silicate Glasses by IR and Raman Spectroscopies. J. Non-Cryst. Solids 2009, 355, 475–480. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, K.; Blanford, C.F.; Francis, L.F.; Stein, A. In Vitro Hydroxycarbonate Apatite Mineralization of CaO–SiO2 Sol–Gel Glasses with a Three-Dimensionally Ordered Macroporous Structure. Chem. Mater. 2001, 13, 1374–1382. [Google Scholar] [CrossRef]
- Gunawidjaja, P.N.; Izquierdo-Barba, I.; Mathew, R.; Jansson, K.; García, A.; Grins, J.; Arcos, D.; Vallet-Regí, M.; Edén, M. Quantifying Apatite Formation and Cation Leaching from Mesoporous Bioactive Glasses In Vitro: A SEM, Solid-State NMR and Powder XRD Study. J. Mater. Chem. 2012, 22, 7214–7223. [Google Scholar] [CrossRef]
- Ohtsuki, C.; Kokubo, T.; Yamamuro, T. Mechanism of Apatite Formation on CaO–SiO2–P2O5 Glasses in a Simulated Body Fluid. J. Non-Cryst. Solids 1992, 143, 84–92. [Google Scholar] [CrossRef]
- Greenspan, D.C.; Zhong, J.P.; LaTorre, G.P. Effect of Surface Area to Volume Ratio on In Vitro Surface Reactions of Bioactive Glass Particulates. In Bioceramics; Andersson, O.H., Happonen, R.P., Yli-Urpo, A., Eds.; Pergamon: Oxford, UK, 1994; pp. 55–60. [Google Scholar]
- Li, P.; Kangasniemi, I.; de Groot, K.; Kokubo, T.; Yli-Urpo, A.U. Apatite Crystallization from Metastable Calcium Phosphate Solution on Sol-Gel-Prepared Silica. J. Non-Cryst. Solids 1994, 168, 281–286. [Google Scholar] [CrossRef]
- Pereira, M.M.; Clark, A.E.; Hench, L.L. Effect of Texture on the Rate of Hydroxyapatite Formation on Gel-Silica Surface. J. Am. Ceram. Soc. 1995, 78, 2463–2468. [Google Scholar] [CrossRef]
- Finney, E.E.; Finke, R.G. Nanocluster Nucleation and Growth Kinetic and Mechanistic Studies: A Review Emphasizing Transition-Metal Nanoclusters. J. Colloid Interface Sci. 2008, 317, 351–374. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Nakanishi, K.; Kokubo, T.; de Groot, K. Induction and Morphology of Hydroxyapatite, Precipitated from Metastable Simulated Body Fluids on Sol-Gel Prepared Silica. Biomaterials 1993, 14, 963–968. [Google Scholar] [CrossRef]
- Bingel, L.; Groh, D.; Karpukhina, N.; Brauer, D.S. Influence of Dissolution Medium pH on Ion Release and Apatite Formation of Bioglass® 45S5. Mater. Lett. 2015, 143, 279–282. [Google Scholar] [CrossRef]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; Nakamura, T.; Yamamuro, T. Process of Formation of Bone-Like Apatite Layer on Silica Gel. J. Mater. Sci. Mater. Med. 1993, 4, 127–131. [Google Scholar] [CrossRef]
- Martínez, A.; Izquierdo-Barba, I.; Vallet-Regí, M. Bioactivity of a CaO–SiO2 Binary Glasses System. Chem. Mater. 2000, 2, 3080–3088. [Google Scholar] [CrossRef]
- Li, P.; Ohtsuki, C.; Kokubo, T.; Nakanishi, K.; Soga, N.; Nakamura, T.; Yamamuro, T. Apatite Formation Induced by Silica Gel in a Simulated Body Fluid. J. Am. Ceram. Soc. 1992, 75, 2094–2097. [Google Scholar] [CrossRef]
- Branda, F.; Fresa, R.; Costantini, A.; Buri, A. Bioactivity of 1.25CaO·SiO2 Glass: An FTIR and X-ray Study of Powdered Samples. Biomaterials 1996, 17, 2247–2251. [Google Scholar] [CrossRef]
- Jones, J.R.; Kemp, T.F.; Smith, M.E. Effect of OH Content on the Bioactivity of Sol-Gel Derived Glass Foam Scaffolds. Key Eng. Mater. 2006, 309–311, 1031–1034. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, Y.; Bacsik, Z.; Edén, M. Contrasting In Vitro Apatite Growth from Bioactive Glass Surfaces with that of Spontaneous Precipitation. Materials 2018, 11, 1690. https://doi.org/10.3390/ma11091690
Yu Y, Bacsik Z, Edén M. Contrasting In Vitro Apatite Growth from Bioactive Glass Surfaces with that of Spontaneous Precipitation. Materials. 2018; 11(9):1690. https://doi.org/10.3390/ma11091690
Chicago/Turabian StyleYu, Yang, Zoltán Bacsik, and Mattias Edén. 2018. "Contrasting In Vitro Apatite Growth from Bioactive Glass Surfaces with that of Spontaneous Precipitation" Materials 11, no. 9: 1690. https://doi.org/10.3390/ma11091690
APA StyleYu, Y., Bacsik, Z., & Edén, M. (2018). Contrasting In Vitro Apatite Growth from Bioactive Glass Surfaces with that of Spontaneous Precipitation. Materials, 11(9), 1690. https://doi.org/10.3390/ma11091690