Push-Out Bond Strength and SEM Evaluation in Roots Filled with Two Different Techniques Using New and Conventional Sealers
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Selection and Sample Size Calculation
2.2. Root Canal Preparation
2.3. Root Filling
2.4. Sample Preparation for Push-Out Test and Scanning Electron Microscope (SEM) Analysis
2.5. Push-Out Test
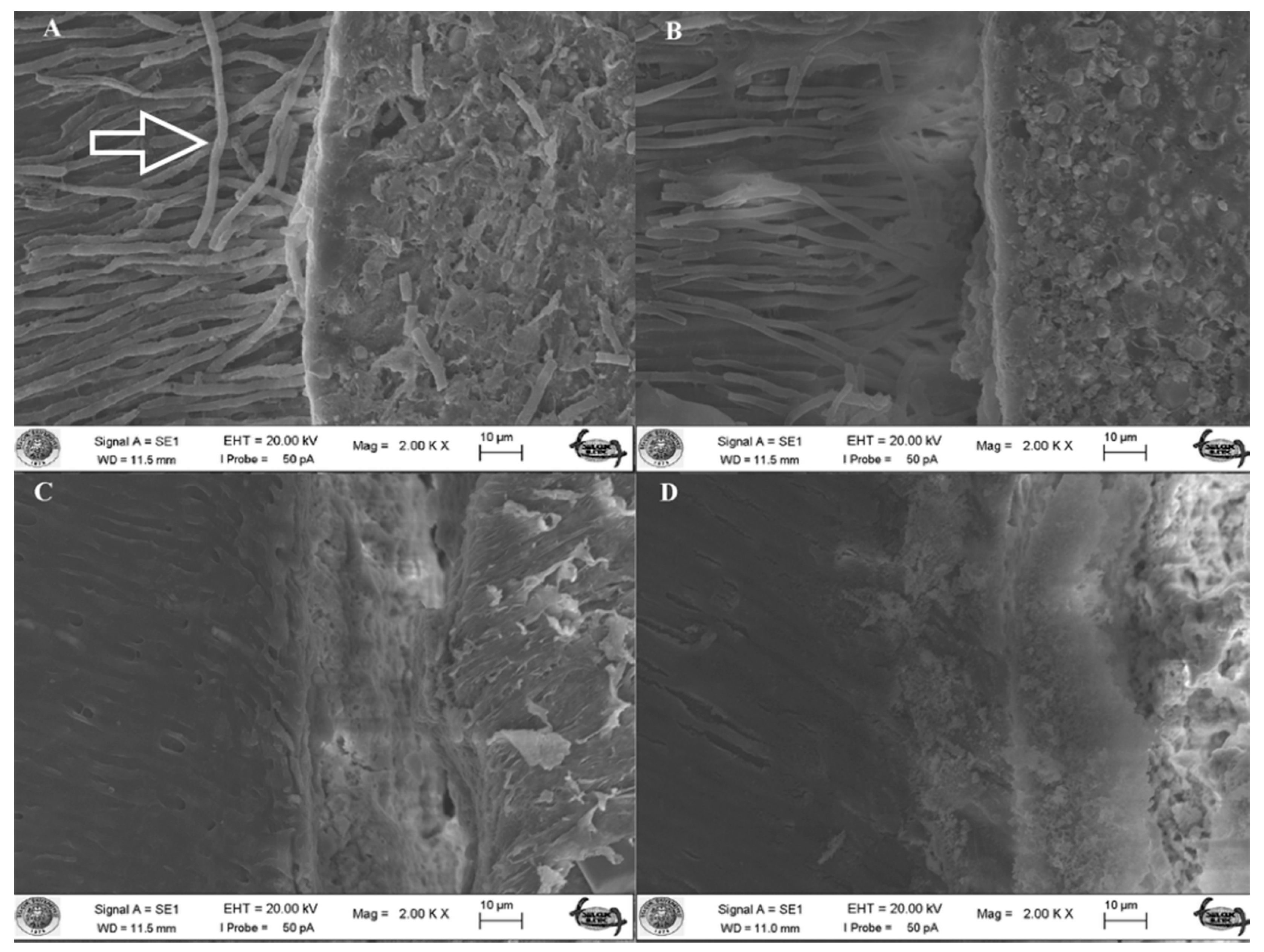
2.6. SEM Analysis
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- (1)
- AH Plus showed significantly higher push-out bonding strength amongst all experimental groups, regardless of the obturation techniques used.
- (2)
- AH Plus, when used especially in cold lateral compaction technique, significantly proved to show the highest push-out bonding strength values amongst all experimental groups.
- (3)
- Endosequence BC Sealer proved to be weaker in bonding strength when compared to AH Plus sealer, regardless of the obturation techniques used.
- (4)
- The bonding strength of Endosequence BC Sealer is even more weakened in-conjuction-with thermo-plasticized injectable technique with Calamus Flow Delivery System—however, there was no significant difference between the two Endosequence BC Sealer groups.
Author Contributions
Funding
Conflicts of Interest
References
- Shipper, G.; Ørstavik, D.; Teixeira, F.B.; Trope, M. An evaluation of microbial leakage in roots filled with a thermoplastic synthetic polymer-based root canal filling material (Resilon). J. Endod. 2004, 30, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Nagas, E.; Cehreli, Z.; Uyanik, M.O.; Durmaz, V. Bond strength of a calcium silicate-based sealer tested in bulk or with different main core materials. Bras. Oral Res. 2014, 28, 1–7. [Google Scholar] [CrossRef]
- Lee, K.W.; Williams, M.C.; Camps, J.J.; Pashley, D.H. Adhesion of endodontic sealers to dentin and gutta-percha. J. Endod. 2002, 28, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Shokouhinejad, N.; Gorjestani, H.; Nasseh, A.A.; Hoseini, A.; Mohammadi, M.; Shamshiri, A.R. Push-out bond strength of gutta-percha with a new bioceramic sealer in the presence or absence of smear layer. Aust. Endod. J. 2013, 39, 102–106. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.R.; Guinesi, A.S.; De Camargo, E.J.; Pizzolitto, A.J.; Filho, I.B. Bacterial leakage evaluation of root canals filled with different endodontic sealers. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, e56–e60. [Google Scholar] [CrossRef] [PubMed]
- Tagger, M.; Tagger, E.; Tjan, A.H.L.; Bakland, L.K. Measurement of adhesion of endodontic sealers to dentin. J. Endod. 2002, 28, 351–354. [Google Scholar] [CrossRef] [PubMed]
- Naser, S.H.; Al-Zaka, I.M. Push-out bond strength of different root canal obturation materials. J. Baghdad. Coll. Dent. 2013, 25, 14–20. [Google Scholar]
- Dawood, A.E.; Parashos, P.; Wong, R.H.K.; Reynolds, E.C.; Manton, D.J. Calcium silicate-based cements: Composition, properties, and clinical applications. J. Investig. Clin. Dent. 2017, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Topcuoglu, H.S.; Tuncay, O.; Karatas, E.; Arslan, H.; Yeter, K. In vitro fracture resistance of roots obturated with epoxy resin-based, mineral trioxide aggregate-based, and bioceramic root canal sealers. J. Endod. 2013, 39, 1630–1633. [Google Scholar] [CrossRef] [PubMed]
- Clark-Holke, D.; Drake, D.; Walton, R.; Rivera, R.; Guthmiller, J.M. Bacterial penetration through canals of endodontically treated teeth in the presence or absence of the smear layer. J. Dent. 2003, 31, 275–281. [Google Scholar] [CrossRef]
- Cobankara, F.K.; Adanir, N.; Belli, S. Evaluation of the influence of smear layer on the apical and coronal sealing ability of two sealers. J. Endod. 2004, 30, 406–409. [Google Scholar] [CrossRef] [PubMed]
- Aminsobhani, M.; Ghorbanzadeh, A.; Sharifian, M.R.; Namjou, S.; Kharazifard, M.J. Comparison of obturation quality in modified continuous wave compaction, continuous wave compaction. Lateral compaction and warm vertical compaction techniques. J. Dent. (Tehran) 2015, 12, 99–108. [Google Scholar]
- Schneider, S.W. A comparison of canal preparations in straight and curved root canals. Oral Surg. Oral Med. Oral Pathol. 1971, 32, 271–275. [Google Scholar] [CrossRef]
- Huffman, B.P.; Mai, S.; Pinna, L.; Weller, R.N.; Primus, C.M.; Gutmann, J.L.; Pashley, D.H.; Tay, F.R. Dislocation resistance of proroot endo sealer, a calcium silicate-based root canal sealer, from radicular dentine. Int. Endod. J. 2009, 42, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Ørstavik, D.; Eriksen, H.M.; Beyer-Olsen, E.M. Adhesive properties and leakage of root canal sealers in vitro. Int. Endod. J. 1983, 16, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Ungor, M.; Onay, E.O.; Orucoglu, H. Push-out bond strengths: The epiphany-resilon endodontic obturation system compared with different pairings of epiphany, resilon, ah plus and gutta-percha. Int. Endod. J. 2006, 39, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Gogos, C.; Economides, N.; Stavrianos, C.; Kolokouris, I.; Kokorikos, I. Adhesion of a new methacrylate resin-based sealer to human dentin. J. Endod. 2004, 30, 238–240. [Google Scholar] [CrossRef] [PubMed]
- Pane, E.S.; Palamara, J.E.A.; Messer, H.H. Critical evaluation of the push-out test for root canal filling materials. J. Endod. 2013, 39, 669–673. [Google Scholar] [CrossRef] [PubMed]
- Delong, C.; He, J.; Woodmansey, K.F. The effect of obturation technique on the push-out bond strength of calcium silicate sealers. J. Endod. 2015, 41, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Madhuri, G.V.; Varri, S.; Bolla, N.; Mandava, P.; Akkala, L.S.; Shaik, J. Comparison of bond strength of different endodontic sealers to root dentin: An in vitro push-out test. J. Conserv. Dent. 2016, 19, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.M.; Pawar, S.; Kfir, A.; Pawar, M.; Kokate, S. Push-out bond strength of root fillings made with C-Point and BC sealer versus gutta-percha and AH Plus after the instrumentation of oval canals with the Self-Adjusting File versus WaveOne. Int. Endod. J. 2016, 49, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Gurgel-Filho, E.D.; Martins, F. Comparative evaluation of push-out bond strength of a MTA based root canal sealer. Braz. J. Oral Sci. 2014, 13, 114–117. [Google Scholar] [CrossRef]
- Neelakantan, P.; Sharma, S.; Shemesh, H.; Wesselink, P.R. Influence of ırrigation sequence on the adhesion of root canal sealers to dentin: A fourier transform ınfrared spectroscopy and push-out bond strength analysis. J. Endod. 2015, 41, 1108–1111. [Google Scholar] [CrossRef] [PubMed]
- Flores, D.S.H.; Rached-Junior, F.J.A.; Versiani, M.A.; Guedes, D.F.; Sousa-Neto, M.D.; Pecora, J.D. Evaluation of physicochemical properties of four root canal sealers. Int. Endod. J. 2011, 44, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mobarak, A.; Mousa, S.; Zaazou, A.; Abdelfattah, H. Comparison of bacterial coronal leakage between different obturation materials (An In Vitro Study). Alex. Dent. J. 2015, 40, 1–7. [Google Scholar]
- Sayin, T.C.; Serper, A.; Cehreli, Z.C.; Kalayci, S. Calcium loss from root canal dentin following EDTA, EGTA, EDTAC, and tetracycline-hcl treatment with or without subsequent naocl irrigation. J. Endod. 2007, 33, 581–584. [Google Scholar] [CrossRef] [PubMed]
- Eldeniz, A.U.; Erdemir, A.; Belli, S. Shear bond strength of three resin based sealers to dentin with and without the smear layer. J. Endod. 2005, 31, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Lalh, M.S.; Titley, K.; Torneck, C.D.; Friedman, S. The shear bond strength of glass ionomer cement sealers to bovine dentine conditioned with common endodontic irrigants. Int. Endod. J. 1999, 32, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, T.; Orucoglu, H.; Cobankara, F.K. Long-term evaluation of the influence of smear layer on the apical sealing ability of MTA. J. Endod. 2008, 34, 1537–1540. [Google Scholar] [CrossRef] [PubMed]
- Shokouhinejad, N.; Hoseini, A.; Gorjestani, H.; Raoof, M.; Assadian, H.; Shamshiri, A.R. Effect of phosphate-buffered saline on push-out bond strength of a new bioceramic sealer to root canal dentin. Dent. Res. J. (Isfahan) 2012, 9, 595–599. [Google Scholar] [CrossRef]
- Yildirim, T.; Er, K.; Taşdemir, T.; Tahan, E.; Buruk, K.; Serper, A. Effect of smear layer and root-end cavity thickness on apical sealing ability of mta as a root-end filling material: A bacterial leakage study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Razmi, H.; Bolhari, B.; Dashti, N.K.; Fazlyab, M. The effect of canal dryness on bond strength of bioceramic and epoxy-resin sealers after irrigation with sodium hypochlorite or chlorhexidine. Iran. Endod. J. 2016, 11, 129–133. [Google Scholar] [PubMed]
- Saleh, I.; Ruyter, I.; Haapasalo, M.; Ørstavik, D. Adhesion of endodontic sealers: Scanning electron microscopy and energy dispersive spectroscopy. J. Endod. 2003, 29, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, N.K.; Prado, M.C.; Senna, P.M.; Neves, A.A.; Souza, E.M.; Fıdel, S.R.; Sassone, L.M.; Sılva, E.J.N.L. Do smear-layer removal agents affect the push-out bond strength of calcium silicate-based endodontic sealers? Int. Endod. J. 2017, 50, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, S.M.B.S.; Sousa-Neto, M.D.; Rached-Junior, F.A.; Miranda, C.E.S.; Silva, S.R.C.; Silva-Sousa, Y.T.C. Push-out strength of root fillings with or without thermomechanical compaction. Int. Endod. J. 2012, 45, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Nagas, E.; Cehreli, Z.C.; Durmaz, V. Effect of light-emitting diode photopolymerization modes on the push-out bond strength of a methacrylate-based sealer. J. Endod. 2011, 37, 832–835. [Google Scholar] [CrossRef] [PubMed]
- Kaya, B.U.; Kececi, A.D.; Orhan, H.; Belli, S. Micropush-out bond strengths of gutta-percha versus thermoplastic synthetic polymer-based systems- an ex vivo study. Int. Endod. J. 2008, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Qu, W.; Bai, W.; Liang, Y.H.; Gao, X.J. Influence of warm verticsal compaction technique on physical properties of root canal sealers. J. Endod. 2016, 42, 1829–1833. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J. Sealers and warm gutta-percha obturation techniques. J. Endod. 2015, 41, 72–78. [Google Scholar] [CrossRef] [PubMed]
| Group | Formulation | Mean Bond Strength | Standard Deviation |
|---|---|---|---|
| 1 | CLC-AH | 17,48 | 7,131 |
| 2 | CLC-ES | 3,56 | 1,703 |
| 3 | C-AH | 9,573 | 7,291 |
| 4 | C-ES | 3,127 | 2,286 |
| Group | Level | Number | Mean Bond Strength | Standard Deviation |
|---|---|---|---|---|
| 1 (CLC-AH) | Coronal | 20 | 20,500 | 2,121 |
| Middle | 20 | 19,467 | 10,619 | |
| Apical | 20 | 15,480 | 6,070 | |
| 2 (CLC-ES) | Coronal | 20 | 4,350 | 1,974 |
| Middle | 20 | 3,250 | 1,768 | |
| Apical | 20 | 2,888 | 1,037 | |
| 3 (C-AH) | Coronal | 20 | 13,800 | 10,391 |
| Middle | 20 | 13,400 | 4,243 | |
| Apical | 20 | 5,975 | 3,208 | |
| 4 (C-ES) | Coronal | 20 | 3,460 | 2,862 |
| Middle | 20 | 3,186 | 1,508 | |
| Apical | 20 | 2,500 | 0,707 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dabaj, P.; Kalender, A.; Unverdi Eldeniz, A. Push-Out Bond Strength and SEM Evaluation in Roots Filled with Two Different Techniques Using New and Conventional Sealers. Materials 2018, 11, 1620. https://doi.org/10.3390/ma11091620
Dabaj P, Kalender A, Unverdi Eldeniz A. Push-Out Bond Strength and SEM Evaluation in Roots Filled with Two Different Techniques Using New and Conventional Sealers. Materials. 2018; 11(9):1620. https://doi.org/10.3390/ma11091620
Chicago/Turabian StyleDabaj, Pervin, Atakan Kalender, and Ayce Unverdi Eldeniz. 2018. "Push-Out Bond Strength and SEM Evaluation in Roots Filled with Two Different Techniques Using New and Conventional Sealers" Materials 11, no. 9: 1620. https://doi.org/10.3390/ma11091620
APA StyleDabaj, P., Kalender, A., & Unverdi Eldeniz, A. (2018). Push-Out Bond Strength and SEM Evaluation in Roots Filled with Two Different Techniques Using New and Conventional Sealers. Materials, 11(9), 1620. https://doi.org/10.3390/ma11091620





