Production and Physicochemical Characterization of Cu-Doped Silicate Bioceramic Scaffolds
Abstract
1. Introduction
2. Materials and Methods
2.1. First Strategy: Preparation of Scaffolds Based on Cu-Containing Melt-Derived Glasses Ubsection
2.2. Second Strategy: Scaffolds Coated with xCu-MBGs
2.3. Characterizations
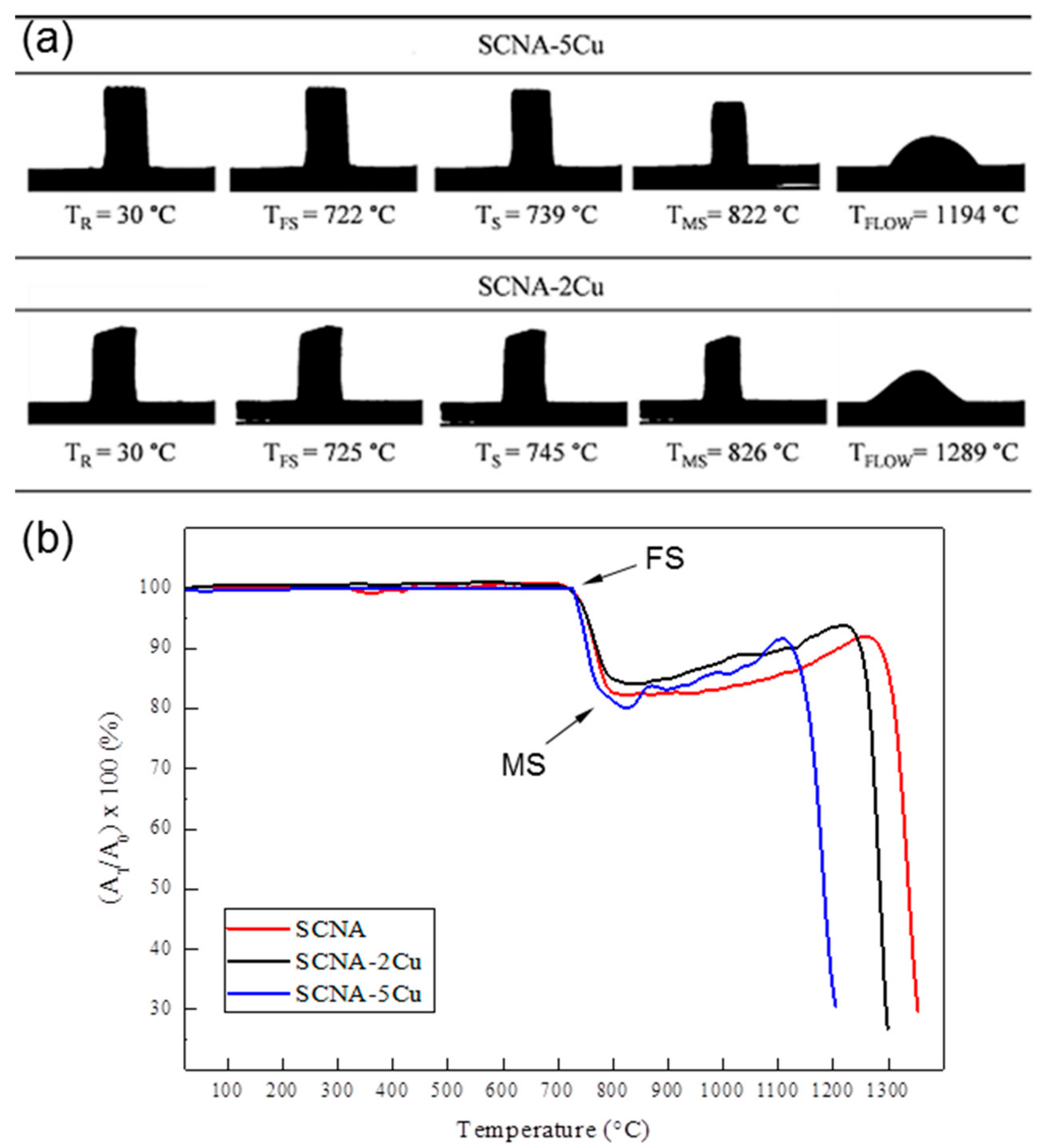
2.3.1. Hot-Stage Microscopy
2.3.2. Microstructural Analysis
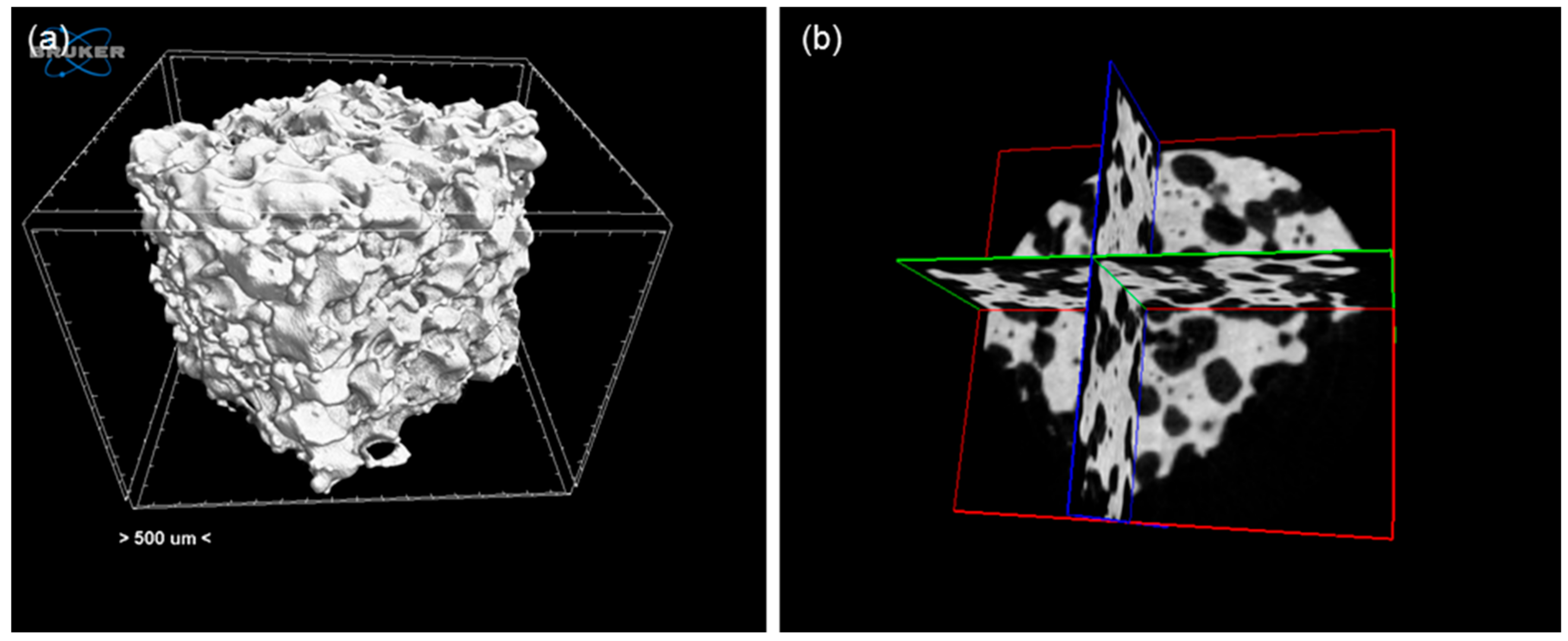
2.3.3. Morphology, Composition and Porosity
2.3.4. Mechanical Testing
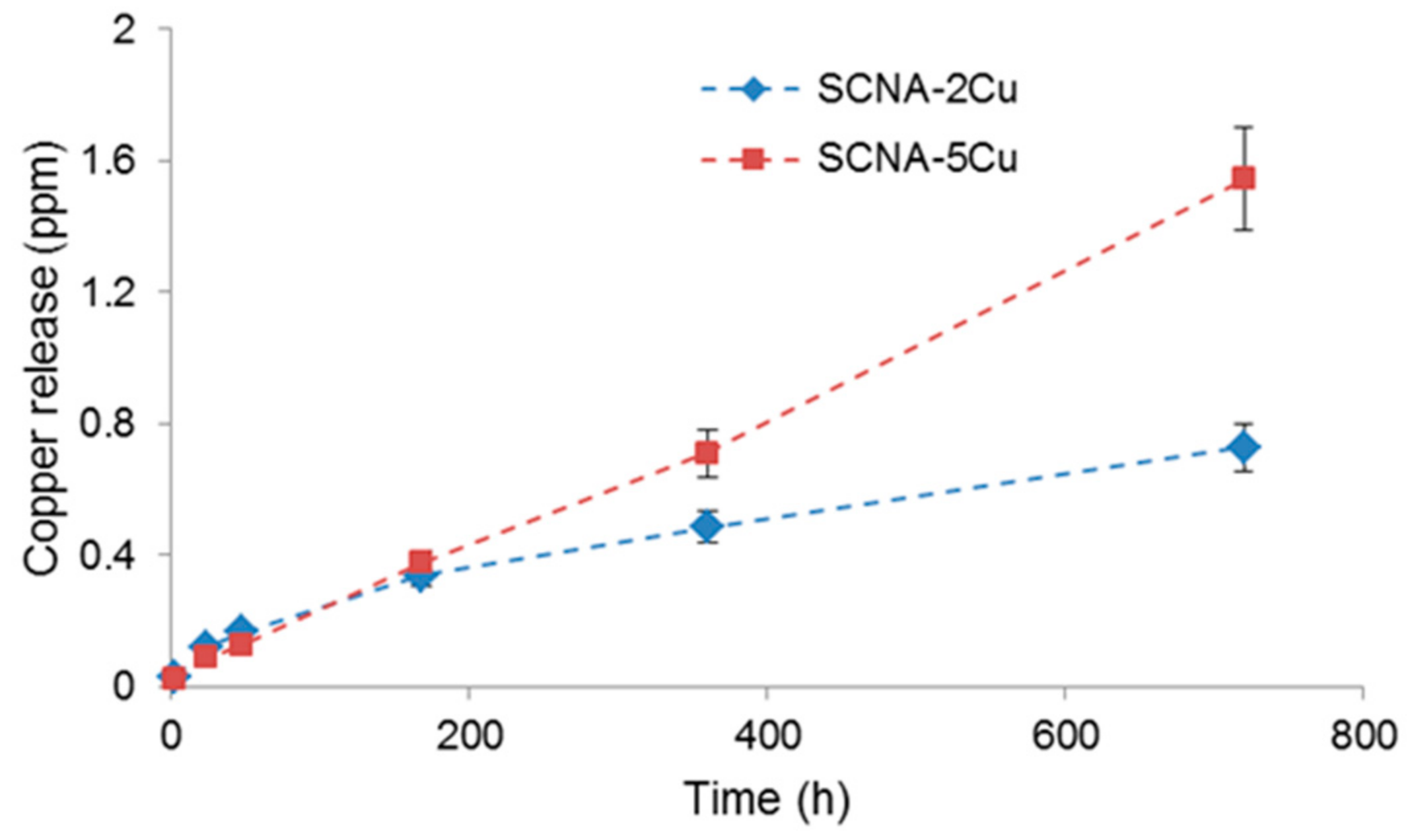
2.3.5. Copper Release Studies
3. Results and Discussion
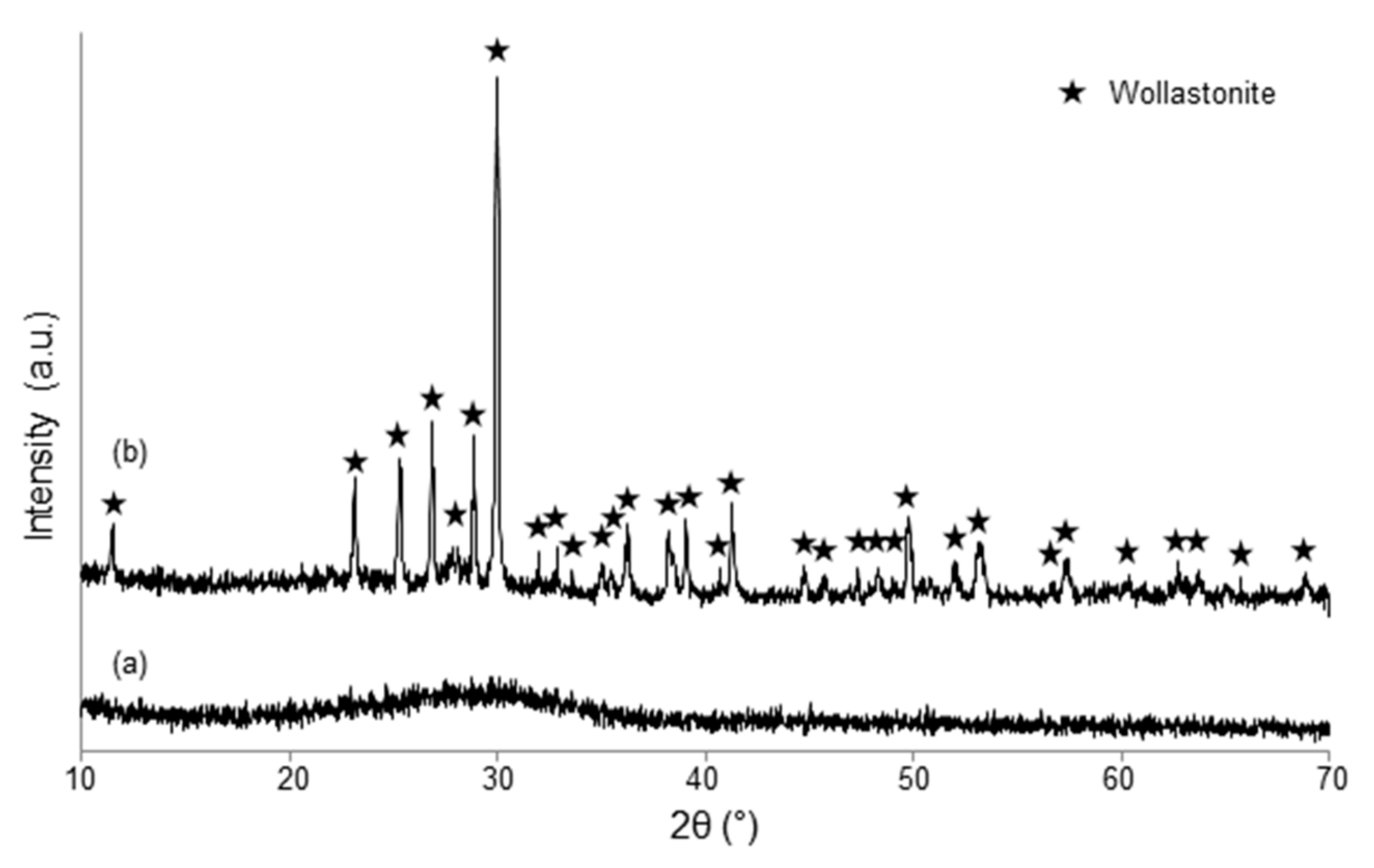
3.1. Scaffolds Based on Cu-Containing Melt-Derived Glasses
3.2. Scaffolds Coated with Cu-Doped MBGs
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Habibovic, P.; Barralet, J.E. Bioinorganics and biomaterials: Bone repair. Acta Biomater. 2014, 7, 3013–3026. [Google Scholar] [CrossRef] [PubMed]
- Glenske, K.; Donkiewicz, P.; Köwitsch, A.; Milosevic-Oljaca, N.; Rider, P.; Rofall, S.; Franke, J.; Jung, O.; Smeets, R.; Schnettler, R.; et al. Applications of metals for bone regeneration. Int. J. Mol. Sci. 2018, 19, 826. [Google Scholar] [CrossRef] [PubMed]
- Xynos, I.D.; Edgar, A.J.; Buttery, L.D.K.; Hench, L.L.; Polak, J.M. Gene-expression profiling of human osteoblasts following treatment with the ionic products of Bioglass® 45S5 dissolution. J. Biomed. Mater. Res. 2001, 55, 151–157. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Miola, M.; Verné, E.; Vitale-Brovarone, C.; Baino, F. Antibacterial bioglass-derived scaffolds: Innovative synthesis approach and characterization. Int. J. Appl. Glass Sci. 2016, 7, 238–247. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Chang, J. Multifunctional mesoporous bioactive glasses for effective delivery of therapeutic ions and drug/growth factors. J. Control. Release 2014, 193, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, M.; Nazparvar, N.; Azizian, M.; Vashaee, D.; Tayebi, L. Effect of ion substitution on properties of bioactive glasses: A review. Ceram. Int. 2015, 41, 7241–7251. [Google Scholar] [CrossRef]
- Kargozar, S.; Baino, F.; Hamzehlou, S.; Hill, R.G.; Mozafari, M. Bioactive glasses: Sprouting angiogenesis in tissue engineering. Trends Biotechnol. 2018, 36, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.K.; Chakraborty, R.; Basu, T. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology 2014, 25, 135101. [Google Scholar] [CrossRef] [PubMed]
- Giacomelli, C.; Trincavelli, M.L.; Satriano, C.; Hansson, O.; La Mendola, D.; Rizzarelli, E.; Martini, C. Copper (II) ions modulate angiogenin activity in human endothelial cells. Int. J. Biochem. Cell Biol. 2015, 60, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Milkovic, L.; Hoppe, A.; Detsch, R.; Boccaccini, A.R.; Zarkovic, N. Effects of Cu-doped 45S5 bioactive glass on the lipid peroxidation-associated growth of human osteoblast-like cells in vitro. J. Biomed. Mater. Res. A 2014, 102, 3556–3561. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef] [PubMed]
- Moshfeghi, D.M.; Moshfeghi, A.A.; Finger, P.T. Enucleation. Surv. Ophthalmol. 2000, 44, 277–301. [Google Scholar] [CrossRef]
- Baino, F.; Potestio, I. Orbital implants: State-of-the-art review with emphasis on biomaterials and recent advances. Mater. Sci. Eng. C 2016, 69, 1410–1428. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, R.; Poole-Warren, L.; Conway, R.M.; Ben-Nissan, B. Porous orbital implants in enucleation: A systematic review. Surv. Ophthalmol. 2007, 52, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Mourits, D.L.; Hartong, D.T.; Bosscha, M.I.; Kloos, R.J.H.M.; Moll, A.C. Worldwide enucleation techniques and materials for treatment of retinoblastoma: An international survey. PLoS ONE 2015, 10, e0121292. [Google Scholar] [CrossRef] [PubMed]
- Vitale-Brovarone, C.; Baino, F.; Tallia, F.; Gervasio, C.; Verné, E. Bioactive glass-derived trabecular coating: A smart solution for enhancing osteointegration of prosthetic elements. J. Mater. Sci. Mater. Med. 2012, 23, 2369–2380. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. Engl. 2004, 43, 5980–5984. [Google Scholar] [CrossRef] [PubMed]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of gases in multimolecular layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Landers, L.; Gor, G.Y.; Neimark, A.V. Density functional theory methods for characterization of porous materials. Colloids Surf. A 2013, 437, 3–32. [Google Scholar] [CrossRef]
- Kokubo, T.; Takadama, H. How useful is SBF in predicting in vivo bone bioactivity? Biomaterials 2006, 27, 2907–2915. [Google Scholar] [CrossRef] [PubMed]
- Nommeots-Nomm, A.; Labbaf, S.; Devlin, A.; Todd, N.; Geng, H.; Solanki, A.K.; Tang, H.M.; Perdika, P.; Pinna, A.; Ejeian, F.; et al. Highly degradable porous melt-derived bioactive glass foam scaffolds for bone regeneration. Acta Biomater. 2017, 57, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Wers, E.; Oudadesse, H.; Lefeuvre, B.; Lucas-Girot, A.; Rocherullé, J.; Lebullenger, R. Excess entropy and thermal behavior of Cu- and Ti-doped bioactive glasses. J. Therm. Anal. Calorim. 2014, 117, 579–588. [Google Scholar] [CrossRef]
- Bejarano, J.; Caviedes, P.; Palza, H. Sol-gel synthesis and in vitro bioactivity of copper and zinc-doped silicate bioactive glasses and glass-ceramics. Biomed. Mater. 2015, 10, 025001. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Baino, F.; Fiorilli, S.; Vitale-Brovarone, C.; Onida, B. Al-MCM-41 inside a glass-ceramic scaffold: A meso-macroporous system for acid catalysis. J. Eur. Ceram. Soc. 2013, 33, 1535–1543. [Google Scholar] [CrossRef]
- Pérez, J.M.; Teixeira, S.R.; Rincõn, J.M.; Romero, M. Understanding the crystallization mechanism of a wollastonite base glass using isoconversional, IKP methods and master plots. J. Am. Ceram. Soc. 2012, 95, 3441–3447. [Google Scholar] [CrossRef]
- Nakamura, T.; Yamamuro, T.; Higashi, S.; Kokubo, T.; Ito, S. A new glass-ceramic for bone replacement: Evaluation of its bonding to bone tissue. J. Biomed. Mater. Res. 1985, 19, 685–698. [Google Scholar] [CrossRef] [PubMed]
- Kokubo, T.; Ito, S.; Sakka, S.; Yamamuro, T. Formation of a high-strength bioactive glass-ceramic in the system MgO-CaO-SiO2-P2O5. J. Mater. Sci. 1986, 21, 536–540. [Google Scholar] [CrossRef]
- Eckstein, F.; Matsuura, M.; Kuhn, V.; Priemel, M.; Müller, R.; Link, T.M.; Lochmüller, E. Sex differences of human trabecular bone microstructure in aging are site-dependent. J. Bone Miner. Res. 2007, 22, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhou, X.; Shoumura, S.; Emura, S.; Bunai, Y. Age- and gender-dependent changes in three-dimensional microstructure of cortical and trabecular bone at the human femoral neck. Osteoporos. Int. 2010, 21, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Baino, F.; Vitale-Brovarone, C. Bioceramics in ophthalmology. Acta Biomater. 2014, 10, 3372–3397. [Google Scholar] [CrossRef] [PubMed]
- Abou Neel, E.A.; Ahmed, I.; Pratten, J.; Nazhat, S.N.; Knowles, J.C. Characterisation of antibacterial copper releasing degradable phosphate glass fibres. Biomaterials 2005, 26, 2247–2254. [Google Scholar] [CrossRef] [PubMed]
- Palza, H.; Escobar, B.; Bejarano, J.; Bravo, D.; Diaz-Dosque, M.; Perez, J. Designing antimicrobial bioactive glass materials with embedded metal ions synthesized by the sol-gel method. Mater. Sci. Eng. C 2013, 33, 3795–3801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, H.; Zhang, Y.; Huang, W.; Rahaman, M.N.; Liu, Z.; Wang, D.; Zhang, C. Copper-doped borosilicate bioactive glass scaffolds with improved angiogenic and osteogenic capacity for repairing osseous defects. Acta Biomater. 2015, 14, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Barba, I.; Vallet-Regi, M. Mesoporous bioactive glasses: Relevance of their porous structure compared to that of classical bioglasses. Biomed. Glassses 2015, 1, 140–150. [Google Scholar] [CrossRef]
- Rouquerol, J.; Avnir, D.; Fairbridge, C.W.; Everett, D.H.; Haynes, J.M.; Pernicone, N.; Ramsay, J.D.F.; Sing, K.S.W.; Unger, K.K. Recommendations for the characterization of porous solids. Pure Appl. Chem. 1994, 66, 1739–1758. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Everett, D.H.; Haul, R.A.V.; Moscou, I.; Pierotti, R.A.; Rouquerol, J.; Siemieniewska, T. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl. Chem. 1985, 57, 603–619. [Google Scholar] [CrossRef]
- Sing, K.S.W.; Williams, R.T. Physisorption hysteresis loops and the characterization of nanoporous materials. Adsorpt. Sci. Technol. 2004, 22, 773–782. [Google Scholar] [CrossRef]
- Lopez-Noriega, A.; Arcos, D.; Izquierdo-Barba, I.; Sakamoto, Y.; Terasaki, O.; Vallet-Regi, M. Ordered mesoporous bioactive glasses for bone tissue regeneration. Chem. Mater. 2006, 18, 3137–3144. [Google Scholar] [CrossRef]
- Baino, F.; Vitale-Brovarone, C. Mechanical properties and reliability of glass-ceramic foam scaffolds for bone repair. Mater. Lett. 2014, 118, 27–30. [Google Scholar] [CrossRef]
- Jordan, D.R.; Hwang, I.; Brownstein, S.; McEachren, T.; Gilberg, S.; Grahovac, S.; Mawn, L. The Molteno M-Sphere. Ophthal. Plast. Reconstr. Surg. 2000, 16, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, K. First-principles study of substitutional magnesium and zinc in hydroxyapatite and octacalcium phosphate. J. Chem. Phys. 2008, 128, 245101. [Google Scholar] [CrossRef] [PubMed]
- Baino, F. Porous glass-ceramic orbital implants: A feasibility study. Mater. Lett. 2018, 212, 12–15. [Google Scholar] [CrossRef]


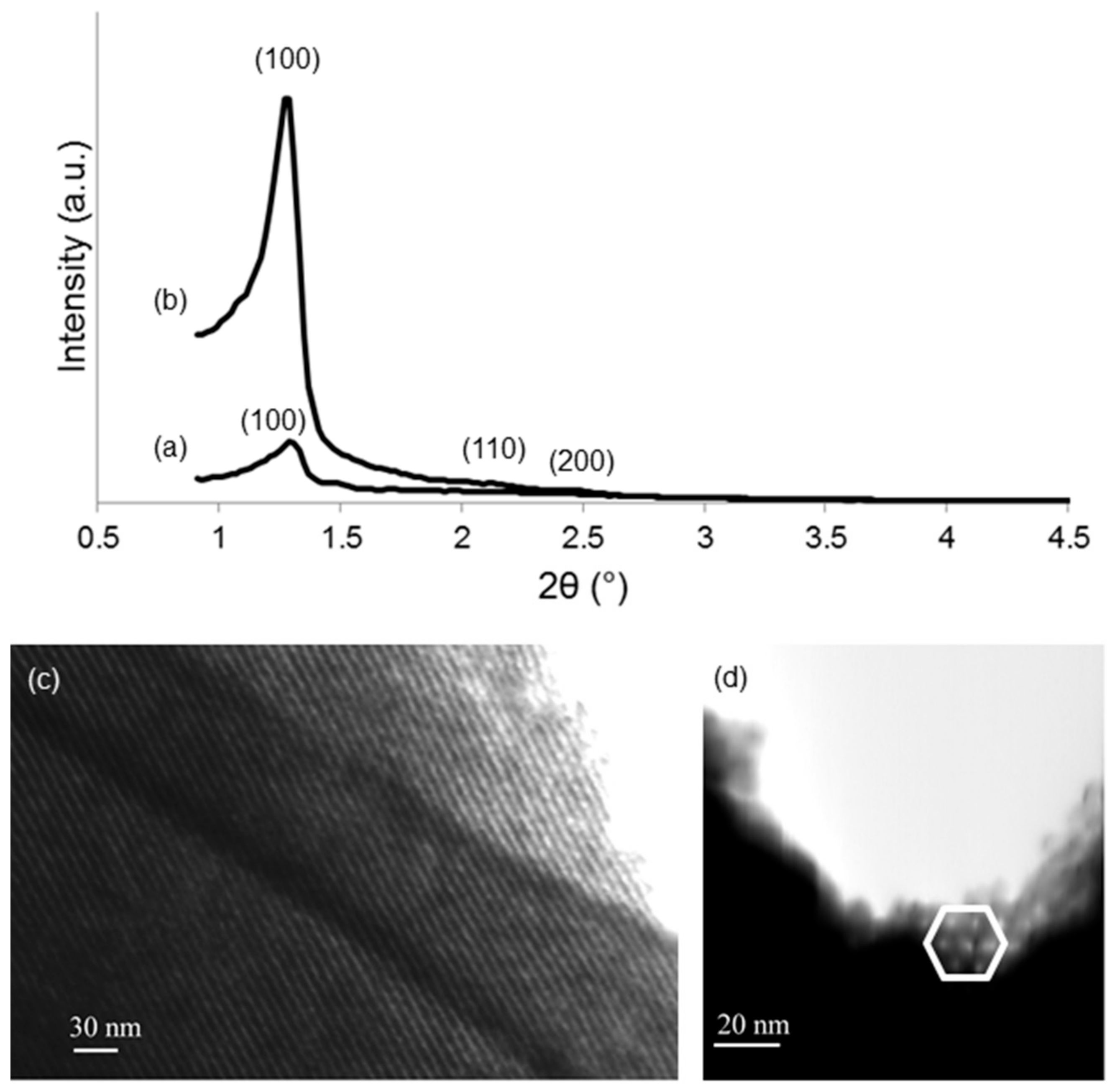
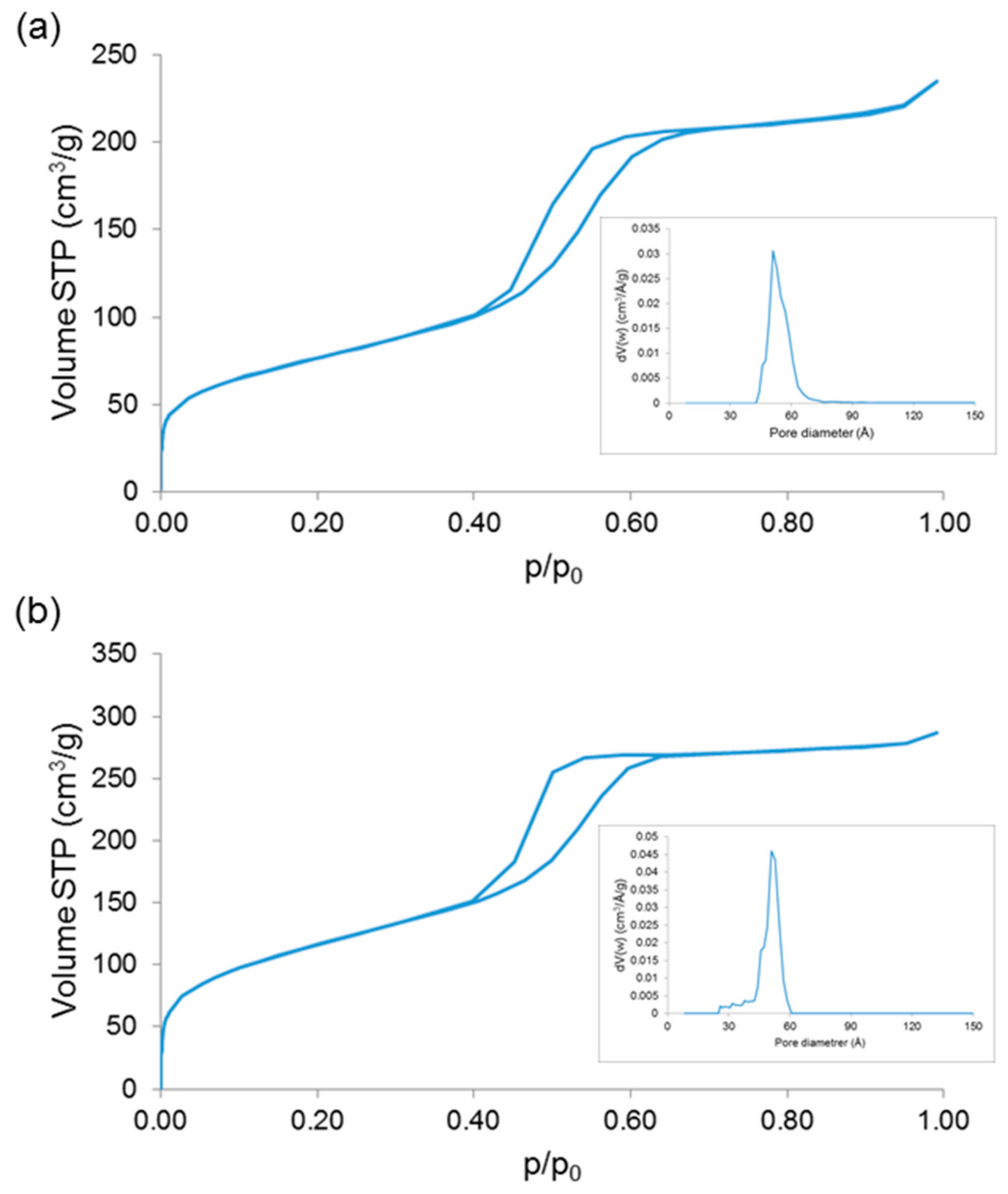

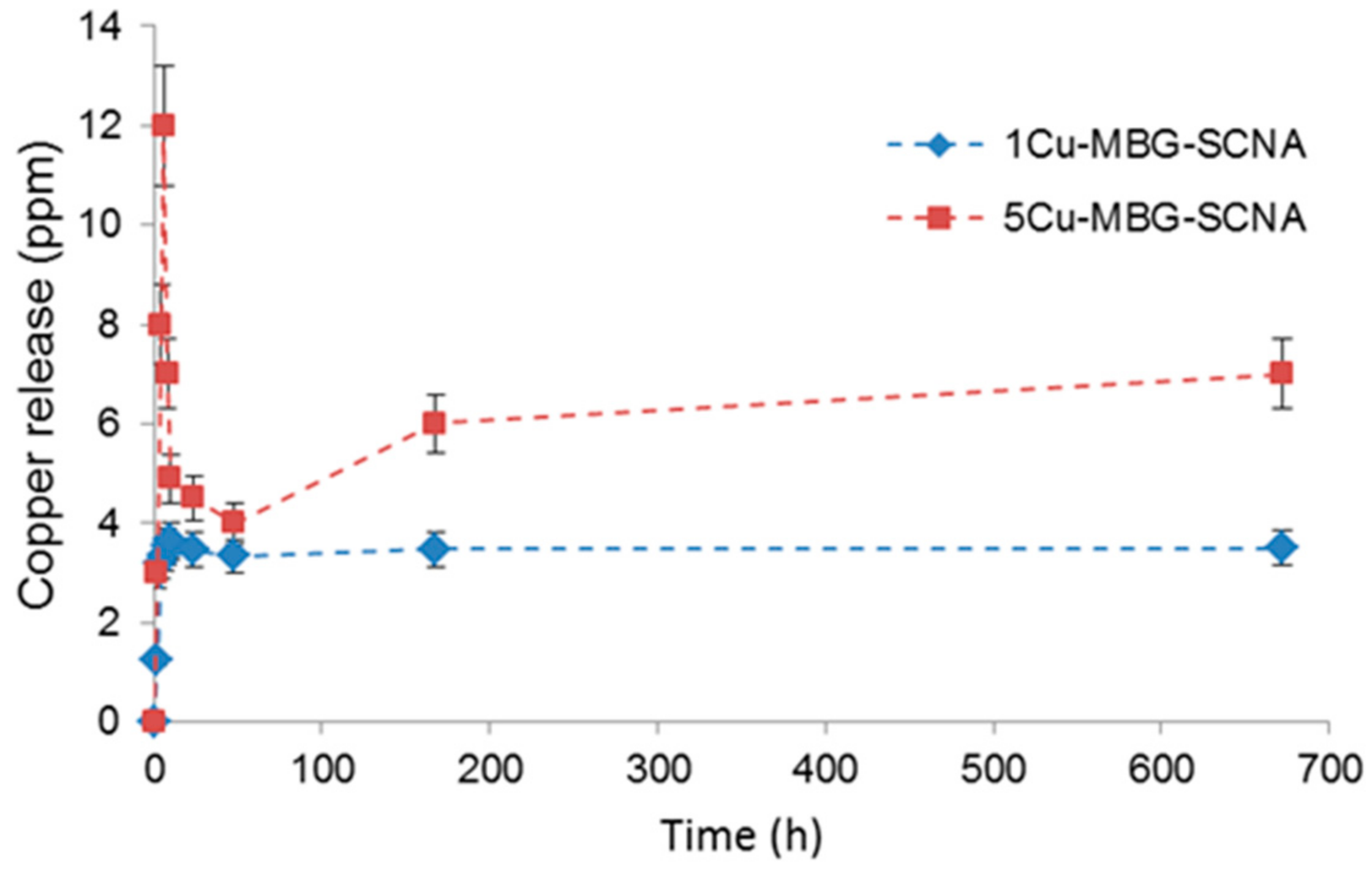

| Sample | SSA (m2/g) | Mean Pore Size (nm) |
|---|---|---|
| 1Cu-MBG | 275 | 5.1 |
| 5Cu-MBG | 432 | 5.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baino, F.; Potestio, I.; Vitale-Brovarone, C. Production and Physicochemical Characterization of Cu-Doped Silicate Bioceramic Scaffolds. Materials 2018, 11, 1524. https://doi.org/10.3390/ma11091524
Baino F, Potestio I, Vitale-Brovarone C. Production and Physicochemical Characterization of Cu-Doped Silicate Bioceramic Scaffolds. Materials. 2018; 11(9):1524. https://doi.org/10.3390/ma11091524
Chicago/Turabian StyleBaino, Francesco, Isabel Potestio, and Chiara Vitale-Brovarone. 2018. "Production and Physicochemical Characterization of Cu-Doped Silicate Bioceramic Scaffolds" Materials 11, no. 9: 1524. https://doi.org/10.3390/ma11091524
APA StyleBaino, F., Potestio, I., & Vitale-Brovarone, C. (2018). Production and Physicochemical Characterization of Cu-Doped Silicate Bioceramic Scaffolds. Materials, 11(9), 1524. https://doi.org/10.3390/ma11091524








