Injectable Hyaluronic Acid-co-Gelatin Cryogels for Tissue-Engineering Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of HAGM and Gelatin (MA-Gelatin)
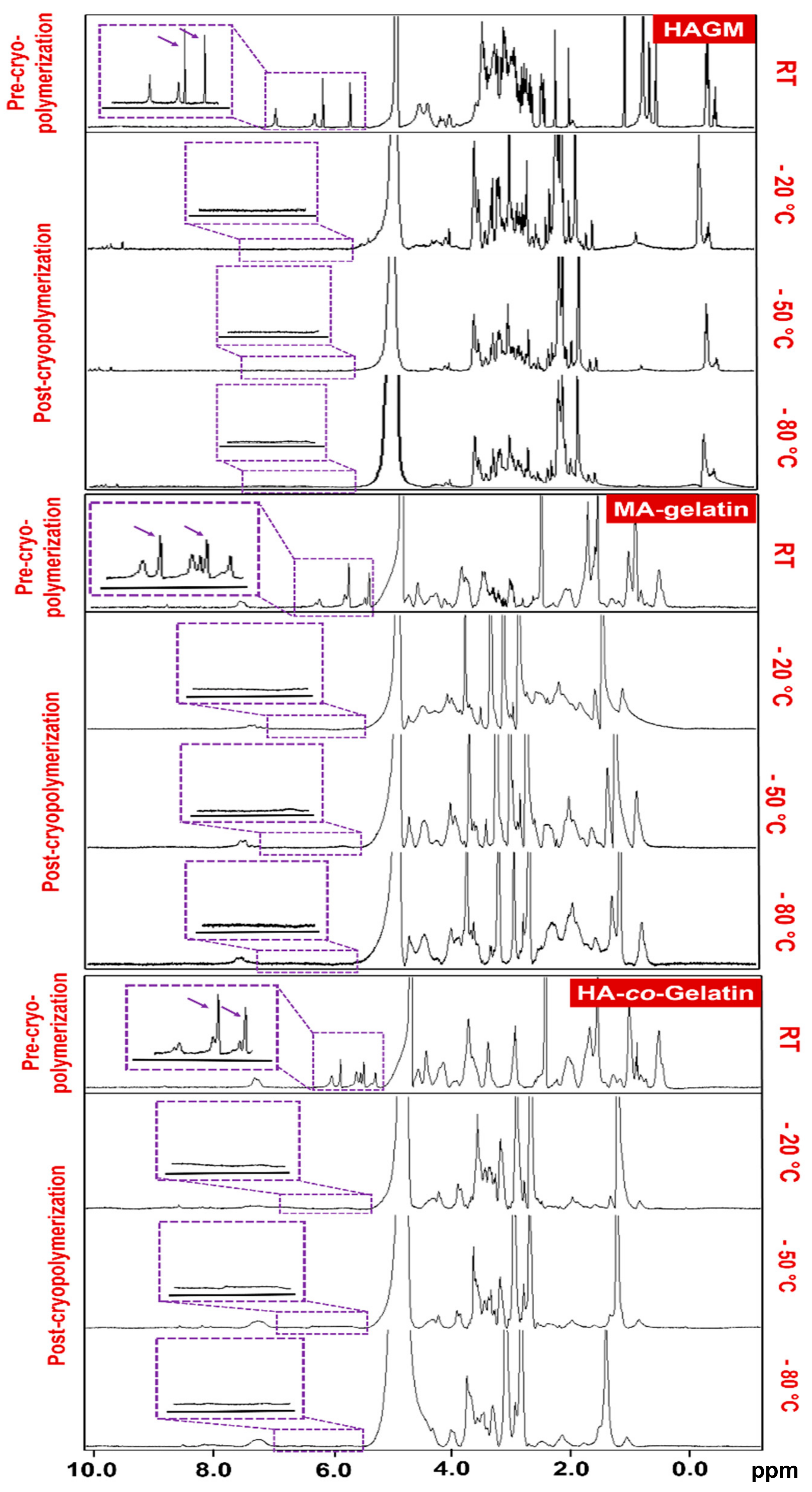
2.2. Chemical Characterization of Polymers and Cryogels by 1H NMR
2.3. Preparation of HAGM (4% w/v), MA-Gelatin (4% w/v), and HA-co-Gelatin (2% HAGM + 2% MA-Gelatin, wt/v) Cryogels
2.4. Physical Characterization of Cryogels
2.5. Injectability Test
2.6. Scanning Electron Microscopy (SEM)
2.7. Biological Properties
2.8. In Vitro Cell Viability and Cytoskeleton Staining
2.9. Generation of Bone Marrow-Derived Dendritic Cells (BMDC) and In Vitro Dendritic Cells (DCs) Activation Assays
2.10. Statistical Analysis
3. Results
3.1. Polymer Synthesis and Characterization
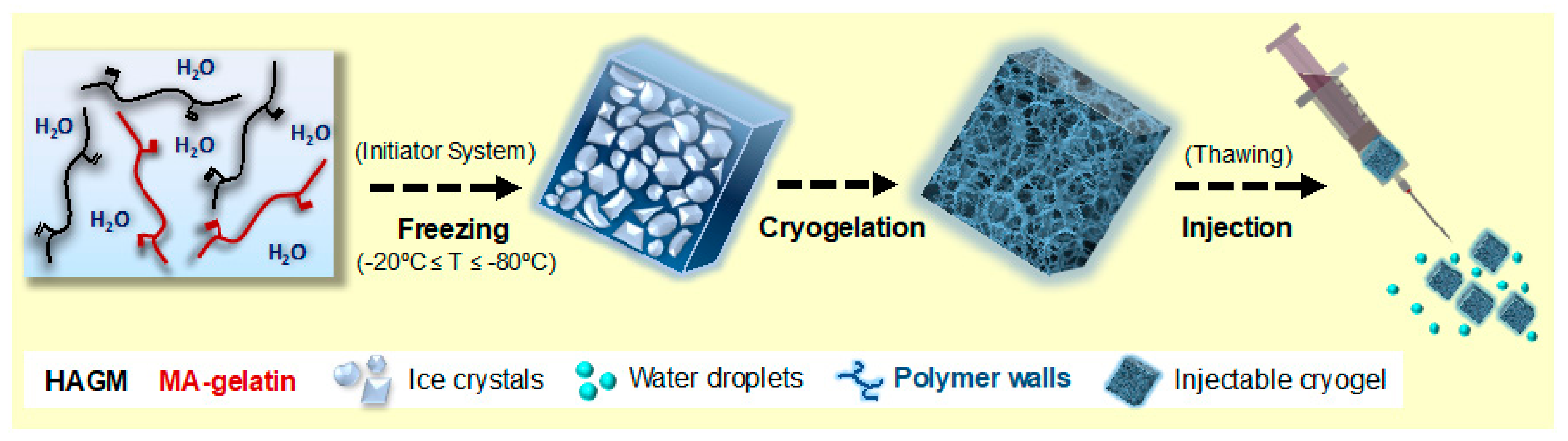
3.2. Fabrication of HAGM, MA-Gelatin, and HA-co-Gelatin Cryogels
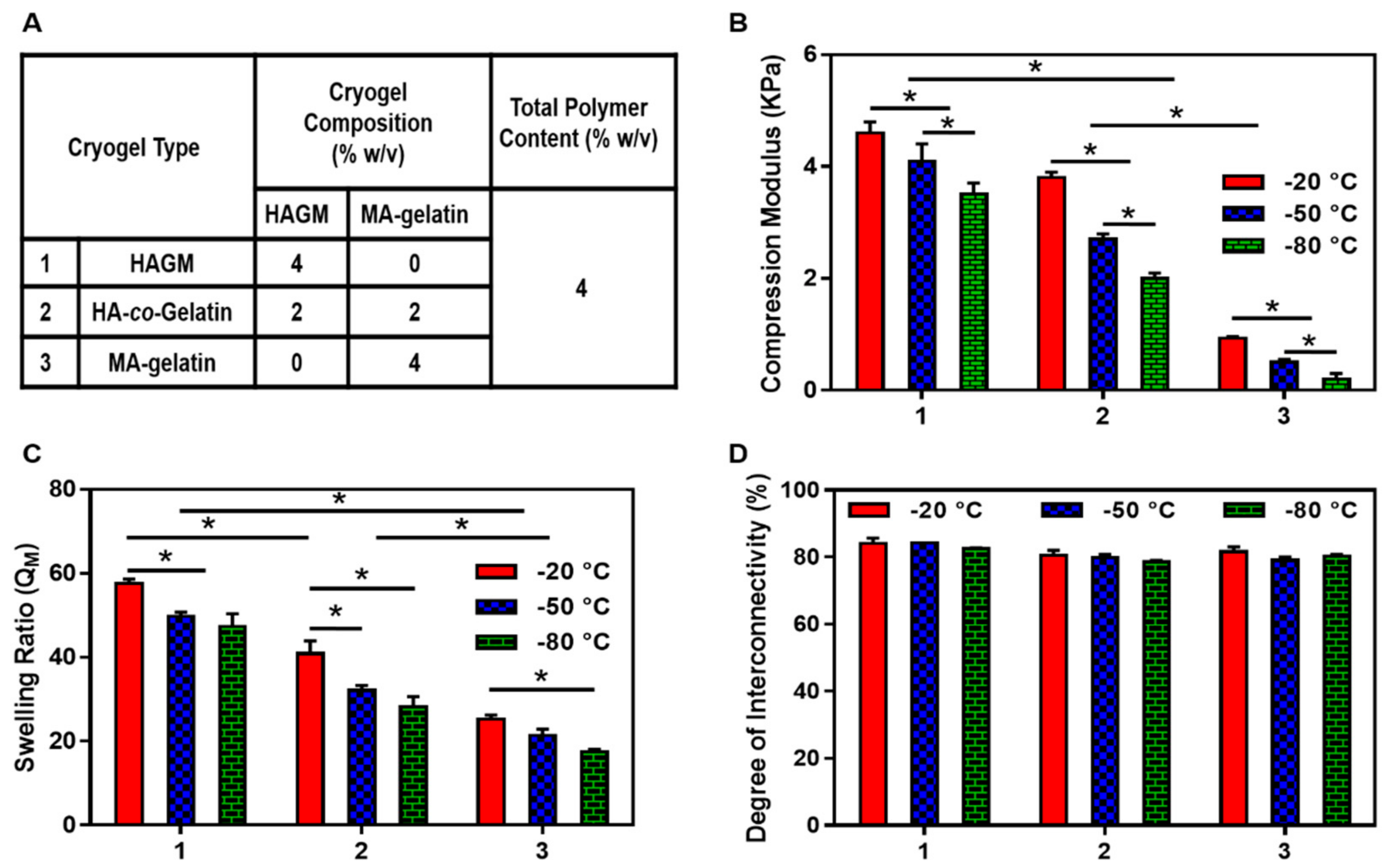
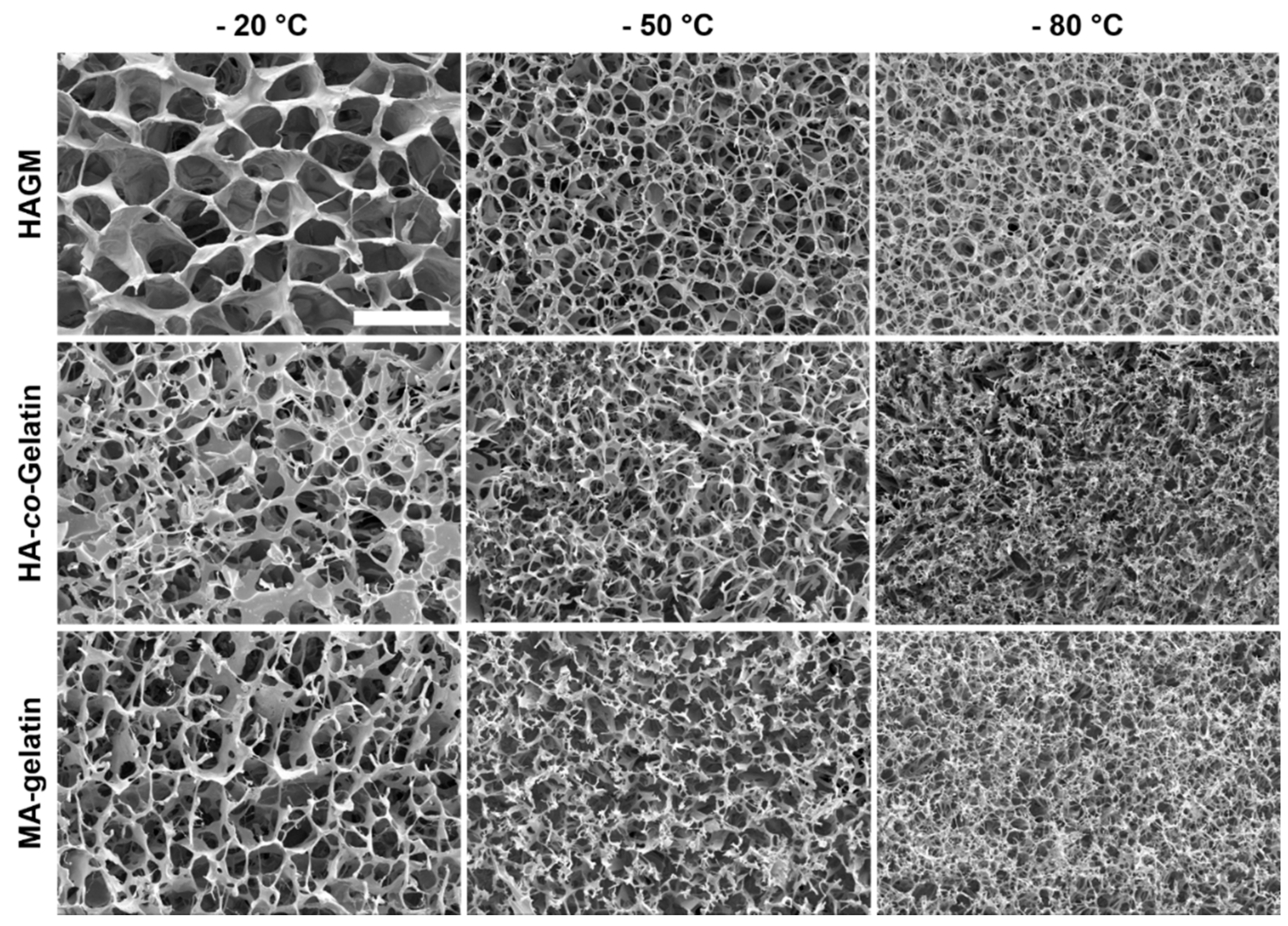
3.3. Physical Characterization
3.4. Injectability of Cryogels
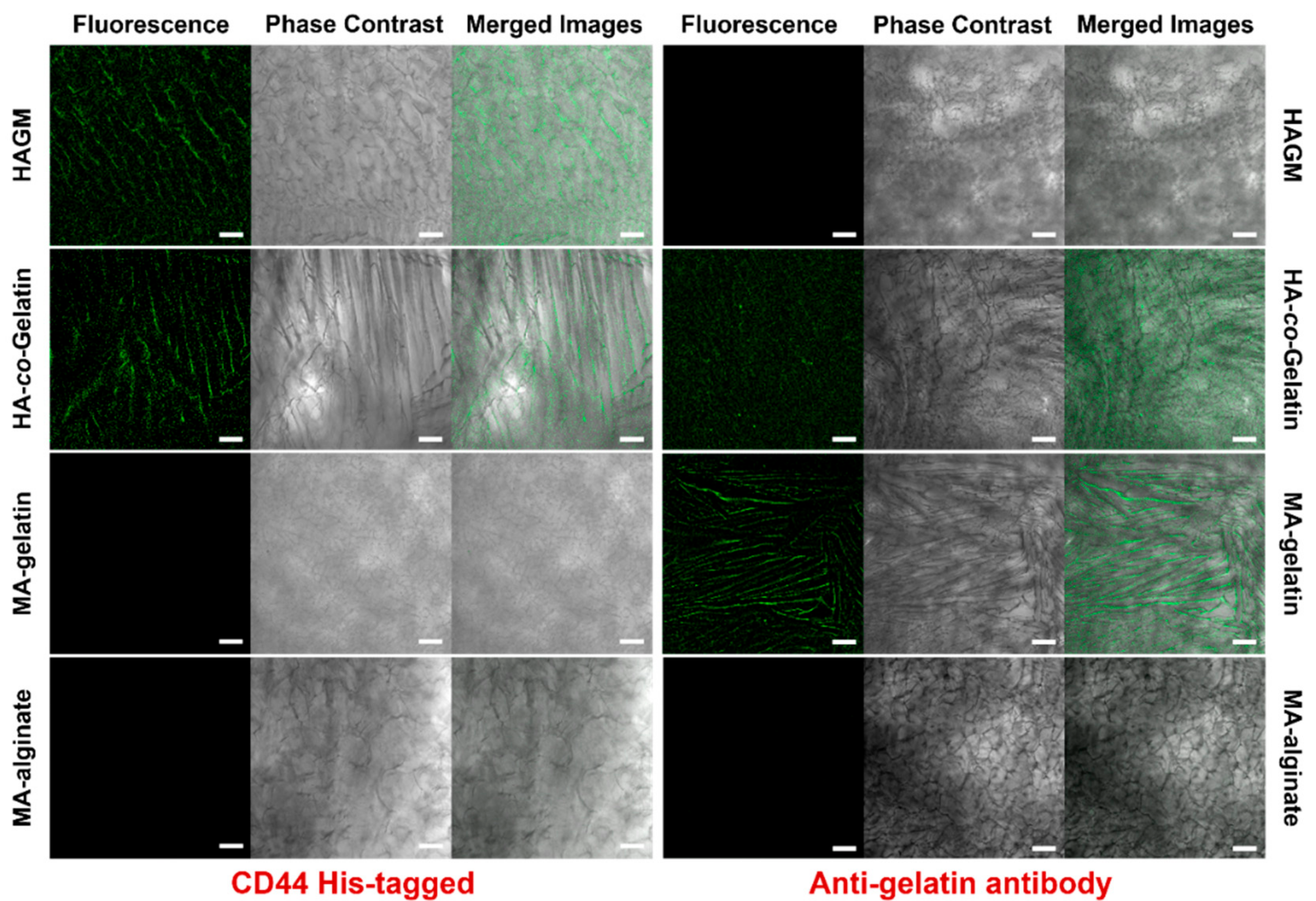
3.5. Biological Properties of Cryogels
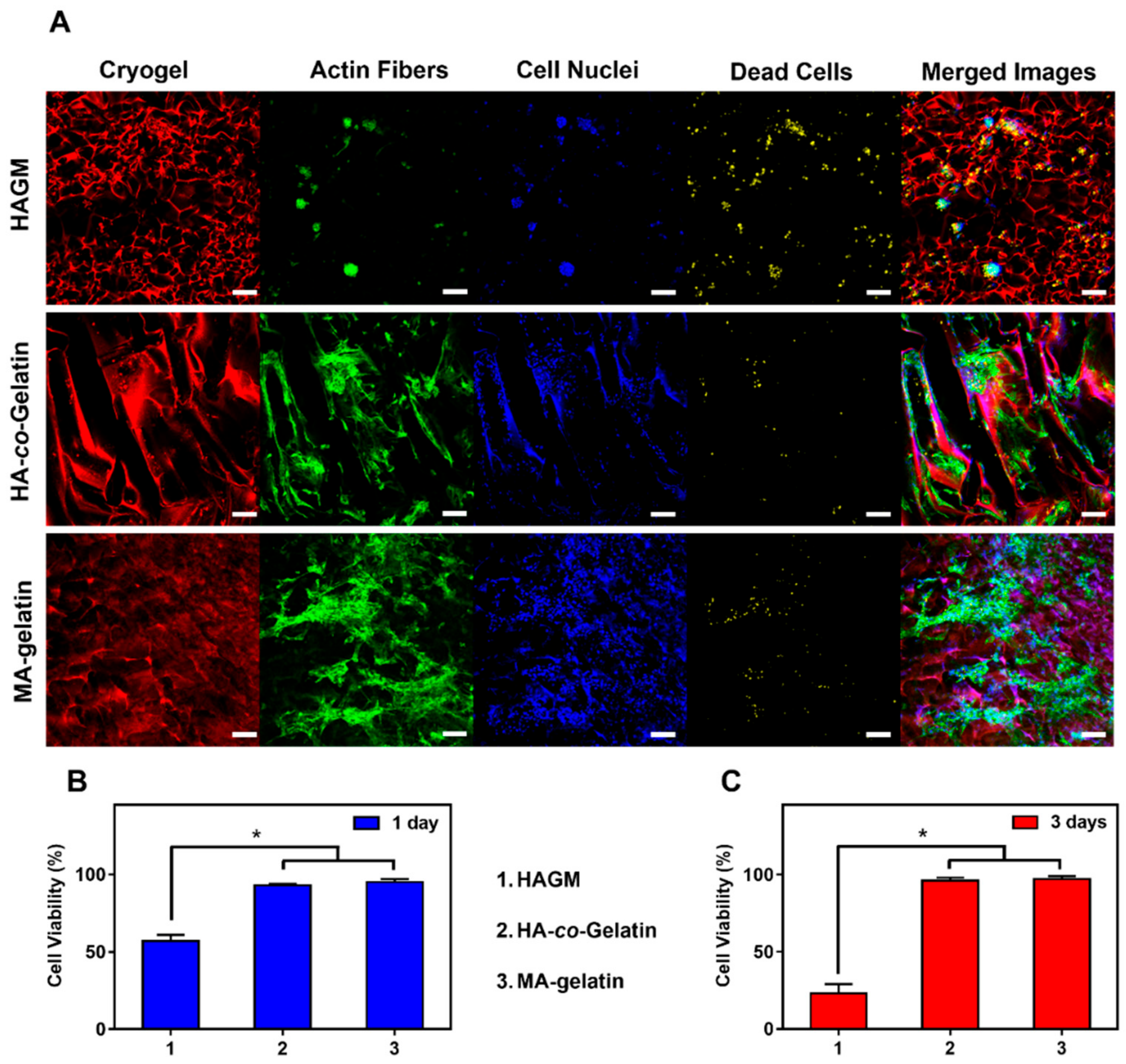
3.6. In Vitro Cell Attachment, Survival, and Spreading
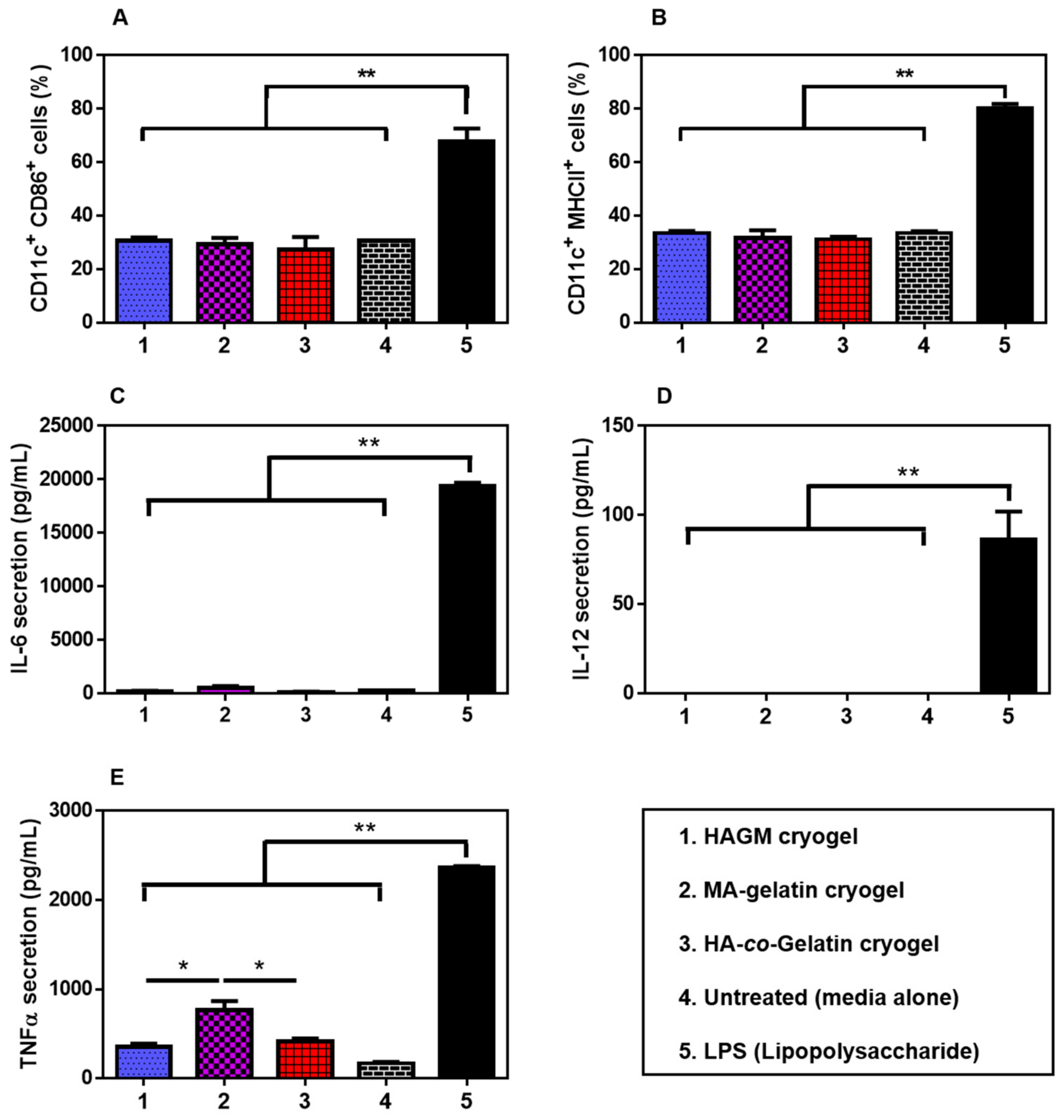
3.7. In Vitro Stimulation of BMDCs
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gurtner, G.C.; Werner, S.; Barrandon, Y.; Longaker, M.T. Wound Repair and Regeneration. Nature 2008, 453, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.W. Engineered Tissue Grafts: Opportunities and Challenges in Regenerative Medicine. Wiley Interdiscip. Rev. Syst. Biol. Med. 2012, 4, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Gurtner, G.C.; Callaghan, M.J.; Longaker, M.T. Progress and Potential for Regenerative Medicine. Annu. Rev. Med. 2007, 58, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Langer, R.; Vacanti, J.P. Tissue Engineering. Science 1993, 260, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Mikos, A.G.; Herring, S.W.; Ochareon, P.; Lu, H.H.; Kandel, R.; Schoen, F.J.; Toner, M.; Mooney, D.; Atala, A.; Van Dyke, M.E. Engineering Complex Tissues. Tissue Eng. 2006, 12, 1–55. [Google Scholar] [CrossRef] [PubMed]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Utkan, D. Engineering Hydrogels as Extracellular Matrix Mimics Hikmet. Nanomedicine 2011, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Brien, F.J.O. Biomaterials & Scaffolds Every Day Thousands of Surgical Procedures are Performed to Replace. Mater. Today 2011, 14, 88–95. [Google Scholar]
- Chan, B.P.; Leong, K.W. Scaffolding in Tissue Engineering: General Approaches and Tissue-Specific Considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef] [PubMed]
- Mallick, K.K.; Cox, S.C. Biomaterial Scaffolds for Tissue Engineering. Front. Biosci. Elit. 2013, 5, 341–360. [Google Scholar] [CrossRef]
- Xing, Q.; Yates, K.; Vogt, C.; Qian, Z.; Frost, M.C.; Zhao, F. Increasing Mechanical Strength of Gelatin Hydrogels by Divalent Metal Ion Removal. Sci. Rep. 2014, 4, 4706. [Google Scholar] [CrossRef] [PubMed]
- Vedadghavami, A.; Minooei, F.; Mohammadi, M.H.; Khetani, S.; Rezaei Kolahchi, A.; Mashayekhan, S.; Sanati-Nezhad, A. Manufacturing of Hydrogel Biomaterials with Controlled Mechanical Properties for Tissue Engineering Applications. Acta Biomater. 2017, 62, 42–63. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Deng, L.; Hsia, H.; Xu, K.; He, Y.; Huang, Q.; Peng, Y.; Zhou, Z.; Peng, C. Evaluation of Gelatin-Hyaluronic Acid Composite Hydrogels for Accelerating Wound Healing. J. Biomater. Appl. 2017, 31, 1380–1390. [Google Scholar] [CrossRef] [PubMed]
- Fallahi, A.; Khadivi, N.; Roohpour, N.; Middleton, A.M.; Kazemzadeh-Narbat, M.; Annabi, N.; Khademhosseini, A.; Tamayol, A. Characterization, Mechanistic Analysis and Improving the Properties of Denture Adhesives. Dent. Mater. 2018, 34, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.W.; Xie, R.; Ju, X.J.; Wang, W.; Chen, Q.; Chu, L.Y. Nano-Structured Smart Hydrogels with Rapid Response and High Elasticity. Nat. Commun. 2013, 4, 2226. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Braschler, T.M.; Renaud, P. Advances in the Design of Macroporous Polymer Scaffolds for Potential Applications in Dentistry. J. Period. Implant Sci. 2013, 43, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Gsib, O.; Duval, J.L.; Goczkowski, M.; Deneufchatel, M.; Fichet, O.; Larreta-Garde, V.; Bencherif, S.A.; Egles, C. Evaluation of Fibrin-Based Interpenetrating Polymer Networks as Potential Biomaterials for Tissue Engineering. Nanomaterials 2017, 7, 436. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, S.; Bencherif, S.; Norton, D.; Weinstock, L.; Mehta, M.; Mooney, D. Rapid and Extensive Collapse from Electrically Responsive Macroporous Hydrogels. Adv. Healthc. Mater. 2014, 3, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Béduer, A.; Braschler, T.; Peric, O.; Fantner, G.E.; Mosser, S.; Fraering, P.C.; Benchérif, S.; Mooney, D.J.; Renaud, P. A Compressible Scaffold for Minimally Invasive Delivery of Large Intact Neuronal Networks. Adv. Healthc. Mater. 2015, 4, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Béduer, A.; Braschler, T.; Peric, O.; Fantner, G.; Mosser, S.; Fraering, P.; Bencherif, S.; Mooney, D.J.; Renaud, P. Injectable Cryogels for Neural Tissue Engineering Applications. In Proceedings of the 18th International Conference on Miniaturized Systems for Chemistry and Life Sciences, San Antonio, TX, USA, 26–30 October 2014. [Google Scholar]
- Bencherif, S.A.; Sands, R.W.; Bhatta, D.; Arany, P.; Verbeke, C.S.; Edwards, D.A.; Mooney, D.J. Injectable Preformed Scaffolds with Shape-Memory Properties. Proc. Natl. Acad. Sci. USA 2012, 109, 19590–19595. [Google Scholar] [CrossRef] [PubMed]
- Offeddu, G.S.; Mela, I.; Jeggle, P.; Henderson, R.M.; Smoukov, S.K.; Oyen, M.L. Cartilage-like Electrostatic Stiffening of Responsive Cryogel Scaffolds. Sci. Rep. 2017, 7, 42948. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Zo, S.M.; Kumar, A.; Han, S.S. Engineering Three-Dimensional Macroporous Hydroxyethyl Methacrylate- Alginate-Gelatin Cryogel for Growth and Proliferation of Lung Epithelial Cells. J. Biomater. Sci. Polym. Ed. 2013, 24, 1343–1359. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Sheehan, J.A.; Hollinger, J.O.; Walker, L.M.; Matyjaszewski, K.; Washburn, N.R. Influence of Cross-Linker Chemistry on Release Kinetics of PEG-Co-PGA Hydrogels. J. Biomed. Mater. Res. 2009, 90, 142–153. [Google Scholar] [CrossRef] [PubMed]
- Han, M.E.; Kim, S.H.; Kim, H.D.; Yim, H.G.; Bencherif, S.A.; Kim, T.I.; Hwang, N.S. Extracellular Matrix-Based Cryogels for Cartilage Tissue Engineering. Int. J. Biol. Macromol. 2016, 93, 1410–1419. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Bhat, S.; Vishnoi, T.; Nayak, V.; Kumar, A. Three-Dimensional Supermacroporous Carrageenan-Gelatin Cryogel Matrix for Tissue Engineering Applications. Biomed. Res. Int. 2013, 2013, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.; Zu, Y.; Li, Z.; Li, W.; Ying, L.; Yang, J.; Wang, X.; He, S.; Liu, D.; Zhu, Z.; et al. Kctd10 Regulates Heart Morphogenesis by Repressing the Transcriptional Activity of Tbx5a in Zebrafish. Nat. Commun. 2014, 5, 3153. [Google Scholar] [CrossRef] [PubMed]
- Zucker, S. Cardiac Jelly and Its Roles in Heart Development. Sci. J. Lander Coll. Arts Sci. 2011, 4, 23–29. [Google Scholar]
- Camenisch, T.D.; Biesterfeldt, J.; Brehm-Gibson, T.; Bradley, J.; McDonald, J.A. Regulation of Cardiac Cushion Development by Hyaluronan. Exp. Clin. Cardiol. 2001, 6, 4–10. [Google Scholar] [PubMed]
- Hjortnaes, J.; Camci-Unal, G.; Hutcheson, J.D.; Jung, S.M.; Schoen, J.F.; Kluin, J.; Aikawa, E.; Khademhosseini, A. Directing Valvular Interstitial Cell Myofibroblast-like Differentiation in a Hybrid Hydrogel Platform. Adv. Heal. Mater 2016, 74, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kutlusoy, T.; Oktay, B.; Apohan, N.K.; Süleymanoğlu, M.; Kuruca, S.E. Chitosan-Co-Hyaluronic Acid Porous Cryogels and Their Application in Tissue Engineering. Int. J. Biol. Macromol. 2017, 103, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Ji, K.; Shih, T.Y.; Haddad, A.; Giatsidis, G.; Mooney, D.J.; Orgill, D.P.; Nabzdyk, C.S. Injectable Shape-Memorizing Three-Dimensional Hyaluronic Acid Cryogels for Skin Sculpting and Soft Tissue Reconstruction. Tissue Eng. Part A 2017, 23, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Siegwart, D.J.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Washburn, N.R.; Matyjaszewski, K. Nanostructured Hybrid Hydrogels Prepared by a Combination of Atom Transfer Radical Polymerization and Free Radical Polymerization. Biomaterials 2009, 30, 5270–5278. [Google Scholar] [CrossRef] [PubMed]
- Oelschlaeger, C.; Bossler, F.; Willenbacher, N. Synthesis, Structural and Micromechanical Properties of 3D Hyaluronic Acid-Based Cryogel Scaffolds. Biomacromolecules 2016, 17, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Kuo, C.Y.; Wang, Y.J.; Chen, J.P. Dual Function of Glucosamine in Gelatin/Hyaluronic Acid Cryogel to Modulate Scaffold Mechanical Properties and to Maintain Chondrogenic Phenotype for Cartilage Tissue Engineering. Int. J. Mol. Sci. 2016, 17, 1957. [Google Scholar] [CrossRef] [PubMed]
- Washburn, N.R.; Bencherif, S.A.; Srinivasan, A.; Jeffrey, O. Synthesis and Characteristic of Highly Crosslinke Hyaluronan Gels. Proc. Am. Chem. Soc. 2008, 181, 1194–1205. [Google Scholar]
- Kumari, J.; Kumar, A. Development of Polymer Based Cryogel Matrix for Transportation and Storage of Mammalian Cells. Sci. Rep. 2017, 7, 41551. [Google Scholar] [CrossRef] [PubMed]
- Cloyd, J.M.; Malhotra, N.R.; Weng, L.; Chen, W.; Mauck, R.L.; Elliott, D.M. Material Properties in Unconfined Compression of Human Nucleus Pulposus, Injectable Hyaluronic Acid-Based Hydrogels and Tissue Engineering Scaffolds. Eur. Spine J. 2007, 16, 1892–1898. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Walczak, P.; Bulte, J.W.M. The Survival of Engrafted Neural Stem Cells Within Hyaluronic Acid Hydrogels. Biomaterials 2014, 34, 5521–5529. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Kasyanov, V.; Xiao, Z.S.; Eisenberg, C.; Eisenberg, L.; Gonda, S.; Trusk, T.; Markwald, R.R.; Prestwich, G.D. Fabrication of Tubular Tissue Constructs by Centrifugal Casting of Cells Suspended in an in Situ Crosslinkable Hyaluronan-Gelatin Hydrogel. Biomaterials 2005, 26, 7628–7635. [Google Scholar] [CrossRef] [PubMed]
- Koshy, S.T.; Ferrante, T.C.; Lewin, S.A.; Mooney, D.J. Injectable, Porous, and Cell-Responsive Gelatin Cryogels. Biomaterials 2014, 35, 2477–2487. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, Y.; Ma, S.; Duan, S.; Yang, X.; Gao, P.; Zhang, X.; Cai, Q. Effective Bone Regeneration Using Thermosensitive Poly(N-Isopropylacrylamide) Grafted Gelatin as Injectable Carrier for Bone Mesenchymal Stem Cells. ACS Appl. Mater. Interfaces 2015, 7, 19006–19015. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Cheng, A.W.M.; Alexander, P.G.; Beck, A.M.; Tuan, R.S. Cartilage Tissue Engineering Application of Injectable Gelatin Hydrogel with In Situ Visible-Light-Activated Gelation Capability in Both Air and Aqueous Solution. Tissue Eng. Part A 2014, 20, 2402–2411. [Google Scholar] [CrossRef] [PubMed]
- Nikkhah, M.; Eshak, N.; Zorlutuna, P.; Annabi, N.; Castello, M.; Kim, K.; Dolatshahi-Pirouz, A.; Edalat, F.; Bae, H.; Yang, Y.; et al. Directed Endothelial Cell Morphogenesis in Micropatterned Gelatin Methacrylate Hydrogels. Biomaterials 2012, 33, 9009–9018. [Google Scholar] [CrossRef] [PubMed]
- Han, M.E.; Kang, B.J.; Kim, S.H.; Kim, H.D.; Hwang, N.S. Gelatin-Based Extracellular Matrix Cryogels for Cartilage Tissue Engineering. J. Ind. Eng. Chem. 2017, 45, 421–429. [Google Scholar] [CrossRef]
- Van Vlierberghe, S.; Dubruel, P.; Lippens, E.; Cornelissen, M.; Schacht, E. Correlation between Cryogenic Parameters and Physico-Chemical Properties of Porous Gelatin Cryogels. J. Biomater. Sci. Polym. Ed. 2009, 20, 1417–1438. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, V.; Becker, M.L. Degradable Adhesives for Surgery and Tissue Engineering. Biomacromolecules 2017, 18, 3009–3039. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.W.; Wu, H.C.; Huang, Y.C.; Sun, J.S.; Lin, F.H. Biomimetic Bilayered Gelatin-Chondroitin 6 Sulfate-Hyaluronic Acid Biopolymer as a Scaffold for Skin Equivalent Tissue Engineering. Artif. Organs 2006, 30, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Bencherif, S.A.; Li, W.A.; Mooney, D.J. Cell-Friendly Inverse Opal-like Hydrogels for a Spatially Separated Co-Culture System. Macromol. Rapid Commun. 2014, 35, 1578–1586. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Sands, R.W.; Ali, O.A.; Li, W.A.; Lewin, S.A.; Braschler, T.M.; Shih, T.Y.; Verbeke, C.S.; Bhatta, D.; Dranoff, G.; et al. Injectable Cryogel-Based Whole-Cell Cancer Vaccines. Nat. Commun. 2015, 6, 7556. [Google Scholar] [CrossRef] [PubMed]
- Dubbini, A.; Censi, R.; Butini, M.E.; Sabbieti, M.G.; Agas, D.; Vermonden, T.; Di Martino, P. Injectable Hyaluronic Acid/PEG-p(HPMAm-Lac)-Based Hydrogels Dually Cross-Linked by Thermal Gelling and Michael Addition. Eur. Polym. J. 2015, 72, 423–437. [Google Scholar] [CrossRef]
- Hahn, S.K.; Oh, E.J.; Miyamoto, H.; Shimobouji, T. Sustained Release Formulation of Erythropoietin Using Hyaluronic Acid Hydrogels Crosslinked by Michael Addition. Int. J. Pharm. 2006, 322, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.C.; Fisher, S.A.; Tam, R.Y.; Nimmo, C.M.; Shoichet, M.S. Hyaluronic Acid Click Hydrogels Emulate the Extracellular Matrix. Langmuir 2013, 29, 7393–7400. [Google Scholar] [CrossRef] [PubMed]
- Khunmanee, S.; Jeong, Y.; Park, H. Crosslinking Method of Hyaluronic-Based Hydrogel for Biomedical Applications. J. Tissue Eng. 2017. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Ren, J.; Chen, G.; Li, G.; Wu, X.; Wang, G.; Gu, G.; Li, J. Injectable in Situ Cross-Linking Chitosan-Hyaluronic Acid Based Hydrogels for Abdominal Tissue Regeneration. Sci. Rep. 2017, 7, 2699. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, T.; Kageyama, T.; Ohta, S.; Fukuda, J.; Ito, T. Injectable Hydrogel with Slow Degradability Composed of Gelatin and Hyaluronic Acid Cross-Linked by Schiff’s Base Formation. Biomacromolecules 2018, 19, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Annabi, N.; Zhang, Y.N.; Assmann, A.; Sani, E.S.; Cheng, G.; Lassaletta, A.D.; Vegh, A.; Dehghani, B.; Ruiz-Esparza, G.U.; Wang, X.; et al. Engineering a Highly Elastic Human Protein-Based Sealant for Surgical Applications. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Kazemzadeh-Narbat, M.; Rouwkema, J.; Annabi, N.; Cheng, H.; Ghaderi, M.; Cha, B.H.; Aparnathi, M.; Khalilpour, A.; Byambaa, B.; Jabbari, E.; et al. Engineering Photocrosslinkable Bicomponent Hydrogel Constructs for Creating 3D Vascularized Bone. Adv. Healthc. Mater. 2017, 6, 1601122. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.T.; Bencherif, S.A.; Gilbert, T.W.; Lotze, M.T.; Washburn, N.R. Design Principles for Cytokine-Neutralizing Gels: Cross-Linking Effects. Acta Biomater. 2010, 6, 4708–4715. [Google Scholar] [CrossRef] [PubMed]
- Gsib, O.; Deneufchatel, M.; Goczkowski, M.; Trouillas, M.; Resche-Guigon, M.; Bencherif, S.; Fichet, O.; Lataillade, J.J.; Larreta-Garde, V.; Egles, C. FibriDerm: Interpenetrated Fibrin Scaffolds for the Construction of Human Skin Equivalents for Full Thickness Burns. IRBM 2017, 1, 6–11. [Google Scholar] [CrossRef]
- Sepantafar, M.; Maheronnaghsh, R.; Mohammadi, H.; Rajabi-Zeleti, S.; Annabi, N.; Aghdami, N.; Baharvand, H. Stem Cells and Injectable Hydrogels: Synergistic Therapeutics in Myocardial Repair. Biotechnol. Adv. 2016, 34, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Shu, X.Z.; Prestwich, G.D. Osteochondral Defect Repair with Autologous Bone Marrow–Derived Mesenchymal Stem Cells in an Injectable, In Situ, Cross-Linked Synthetic Extracellular Matrix. Tissue Eng. 2006, 12, 3405–3416. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Balikov, D.A.; Lee, J.B.; Lee, S.H.; Lee, S.H.; Lee, J.H.; Park, K.D.; Sung, H.J. In Situ Forming Gelatin Hydrogels-Directed Angiogenic Differentiation and Activity of Patient-Derived Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2017, 18, 1705. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In Situ Forming Hydrogels Based on Chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Srinivasan, A.; Horkay, F.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. Influence of the Degree of Methacrylation on Hyaluronic Acid Hydrogels Properties. Biomaterials 2008, 29, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Lin-Gibson, S.; Bencherif, S.; Cooper, J.A.; Wetzel, S.J.; Antonucci, J.M.; Vogel, B.M.; Horkay, F.; Washburn, N.R. Synthesis and Characterization of PEG Dimethacrylates and Their Hydrogels. Biomacromolecules 2004, 5, 1280–1287. [Google Scholar] [CrossRef] [PubMed]
- Prata, J.E.; Barth, T.A.; Bencherif, S.A.; Washburn, N.R. Complex Fluids Based on Methacrylated Hyaluronic Acid. Biomacromolecules 2010, 11, 769–775. [Google Scholar] [CrossRef] [PubMed]
- Kellett-Clarke, H.; Stegmann, M.; Barclay, A.N.; Metcalfe, C. CD44 Binding to Hyaluronic Acid Is Redox Regulated by a Labile Disulfide Bond in the Hyaluronic Acid Binding Site. PLoS ONE 2015, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Venien, A.; Levieux, D. Differentiation of Bovine from Porcine Gelatines Using Polyclonal Anti-Peptide Antibodies in Indirect and Competitive Indirect ELISA. J. Pharm. Biomed. Anal. 2005, 39, 418–424. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.B.; Kukutsch, N.; Ogilvie, A.L.J.; Roßner, S.; Koch, F.; Romani, N.; Schuler, G. An Advanced Culture Method for Generating Large Quantities of Highly Pure Dendritic Cells from Mouse Bone Marrow. J. Immunol. Methods 1999, 223, 77–92. [Google Scholar] [CrossRef]
- Misra, S.; Ghatak, S. Interactions between Hyaluronan and Its Receptors (CD44, RHAMM) Regulate the Activities of inflammation and cancer. Front. Immunol. 2015. [Google Scholar] [CrossRef] [PubMed]
- Granucci, F.; Zanoni, I.; Ricciardi-Castagnoli, P. Central Role of Dendritic Cells in the Regulation and Deregulation of Immune Responses. Cell. Mol. Life Sci. 2008, 65, 1683–1697. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Polymeric Scaffolds in Tissue Engineering Application: A Review. Int. J. Polym. Sci. 2011, 2011, 290602. [Google Scholar] [CrossRef]
- Montgomery, M.; Ahadian, S.; Davenport Huyer, L.; Lo Rito, M.; Civitarese, R.A.; Vanderlaan, R.D.; Wu, J.; Reis, L.A.; Momen, A.; Akbari, S.; et al. Flexible Shape-Memory Scaffold for Minimally Invasive Delivery of Functional Tissues. Nat. Mater. 2017, 16, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Madan, M.; Bajaj, A.; Lewis, S.; Udupa, N.; Baig, J. In Situ Forming Polymeric Drug Delivery Systems. Indian J. Pharm. Sci. 2009, 71, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Bencherif, S.A.; Washburn, N.R.; Matyjaszewski, K. Synthesis by AGET ATRP of Degradable Nanogel Precursors for in Situ Formation of Nanostructured Hyaluronic Acid Hydrogel. Biomacromolecules 2009, 10, 2499–2507. [Google Scholar] [CrossRef] [PubMed]
- Siegwart, D.J.; Srinivasan, A.; Bencherif, S.A.; Karunanidhi, A.; Jung, K.O.; Vaidya, S.; Jin, R.; Hollinger, J.O.; Matyjaszewski, K. Cellular Uptake of Functional Nanogels Prepared by Inverse Miniemulsion ATRP with Encapsulated Proteins, Carbohydrates, and Gold Nanoparticles. Biomacromolecules 2009, 10, 2300–2309. [Google Scholar] [CrossRef] [PubMed]
- Kharaziha, M.; Memic, A.; Akbari, M.; Brafman, D.A.; Nikkhah, M. Nano-Enabled Approaches for Stem Cell-Based Cardiac Tissue Engineering. Adv. Healthc. Mater. 2016, 5, 1533–1553. [Google Scholar] [CrossRef] [PubMed]
- Memic, A.; Alhadrami, H.A.; Hussain, M.A.; Aldhahri, M.; Al Nowaiser, F.; Al-Hazmi, F.; Oklu, R.; Khademhosseini, A. Hydrogels 2.0: Improved Properties with Nanomaterial Composites for Biomedical Applications. Biomed. Mater. 2016, 11, 014104. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A. Supermacroporous Cryogels: Biomedical and Biotechnological Applications; CRC Press: Karaton, FL, USA, 2016. [Google Scholar]
- Zhu, J. Bioactive Modification of Poly(Ethylenglykol) Hydrogels for Tissue Engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Hern, D.L.; Hubbell, J.A. Incorporation of Adhesion Peptides into Nonadhesive Hydrogels Useful for Tissue Resurfacing. J. Biomed. Mater. Res. 1998, 39, 266–276. [Google Scholar] [CrossRef]
- Bencherif, S.A.; Srinivasan, A.; Sheehan, J.A.; Walker, L.M.; Gayathri, C.; Gil, R.; Hollinger, J.O.; Matyjaszewski, K.; Washburn, N.R. End-Group Effects on the Properties of PEG-Co-PGA Hydrogels. Acta Biomater. 2009, 5, 1872–1883. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-Laden Microengineered Gelatin Methacrylate Hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rezaeeyazdi, M.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable Hyaluronic Acid-co-Gelatin Cryogels for Tissue-Engineering Applications. Materials 2018, 11, 1374. https://doi.org/10.3390/ma11081374
Rezaeeyazdi M, Colombani T, Memic A, Bencherif SA. Injectable Hyaluronic Acid-co-Gelatin Cryogels for Tissue-Engineering Applications. Materials. 2018; 11(8):1374. https://doi.org/10.3390/ma11081374
Chicago/Turabian StyleRezaeeyazdi, Mahboobeh, Thibault Colombani, Adnan Memic, and Sidi A. Bencherif. 2018. "Injectable Hyaluronic Acid-co-Gelatin Cryogels for Tissue-Engineering Applications" Materials 11, no. 8: 1374. https://doi.org/10.3390/ma11081374
APA StyleRezaeeyazdi, M., Colombani, T., Memic, A., & Bencherif, S. A. (2018). Injectable Hyaluronic Acid-co-Gelatin Cryogels for Tissue-Engineering Applications. Materials, 11(8), 1374. https://doi.org/10.3390/ma11081374





