Micro/Mesoporous Fe3O4/Fe-Phthalocyanine Microspheres and Effects of Their Surface Morphology on the Crystallization and Properties of Poly(Arylene Ether Nitrile) Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
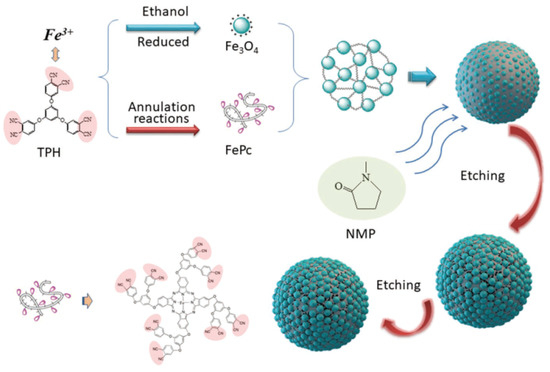
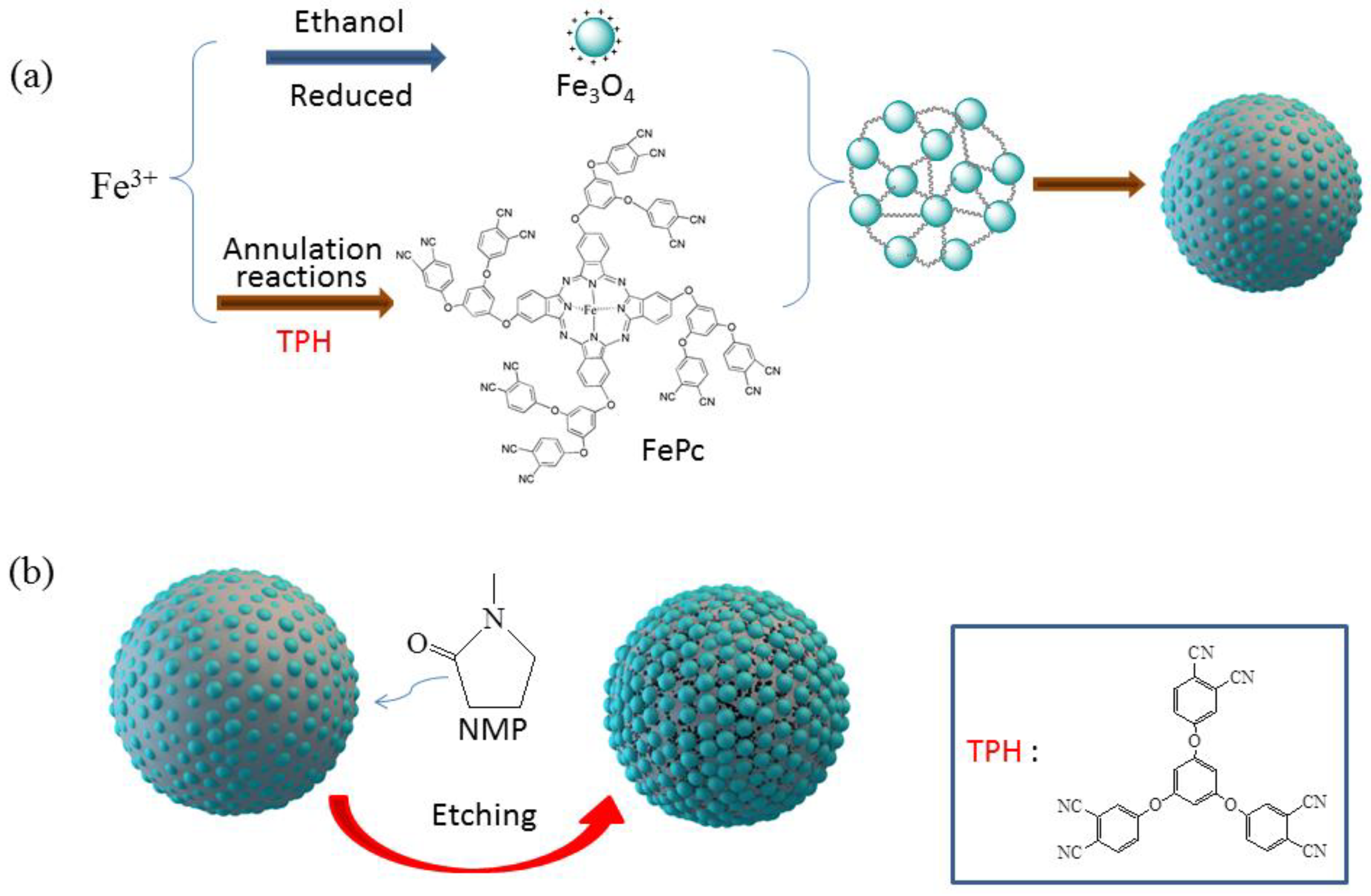
2.2. Preparation of Original Fe3O4/FePc Magnetic Hybrid Microspheres
2.3. Preparation of Etched Fe3O4/FePc Hybrid Microspheres
2.4. Preparation of Crystallizable PEN Coated Fe3O4/FePc Magnetic Microspheres
2.5. Preparation of Fe3O4/FePc Reinforced PEN Magnetic Nanocomposites
2.6. Characterizations
3. Results
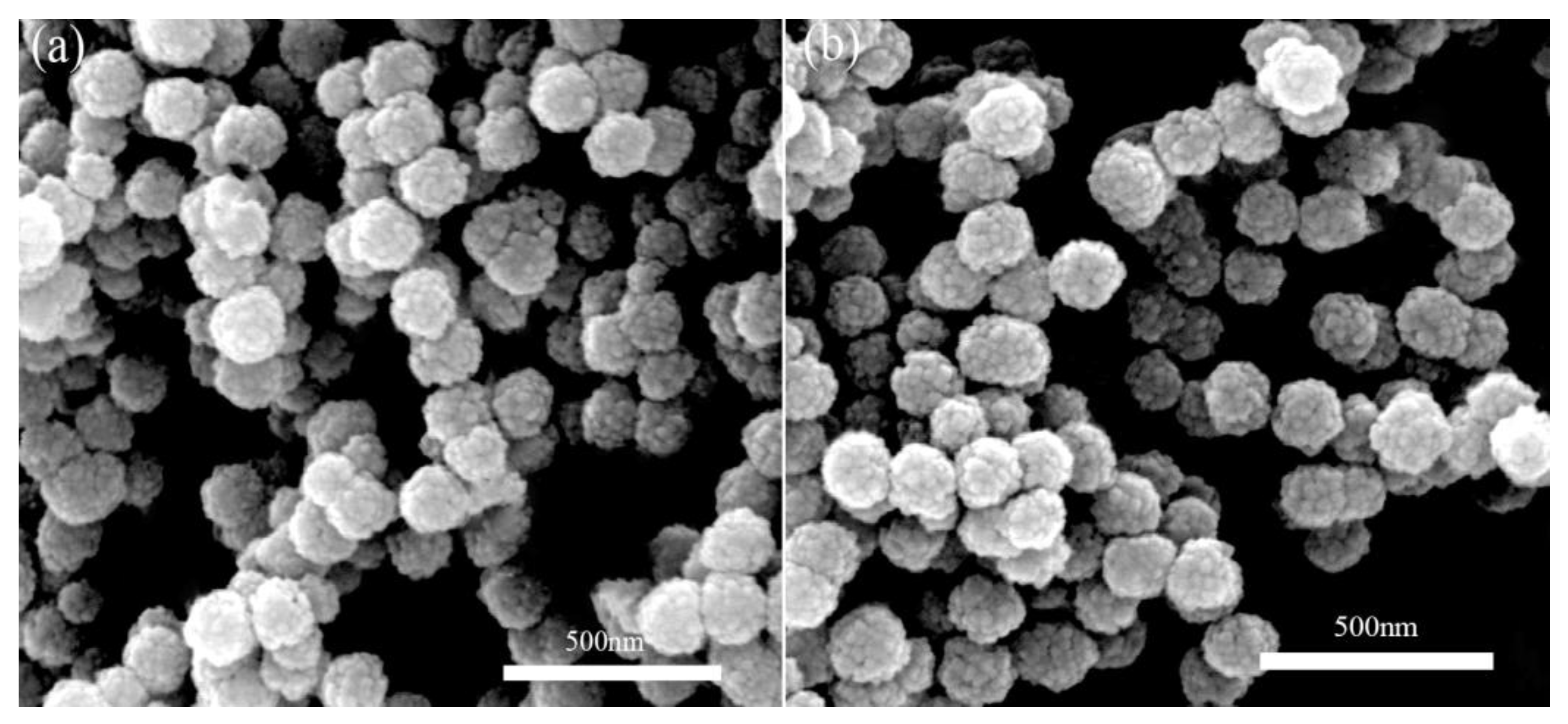
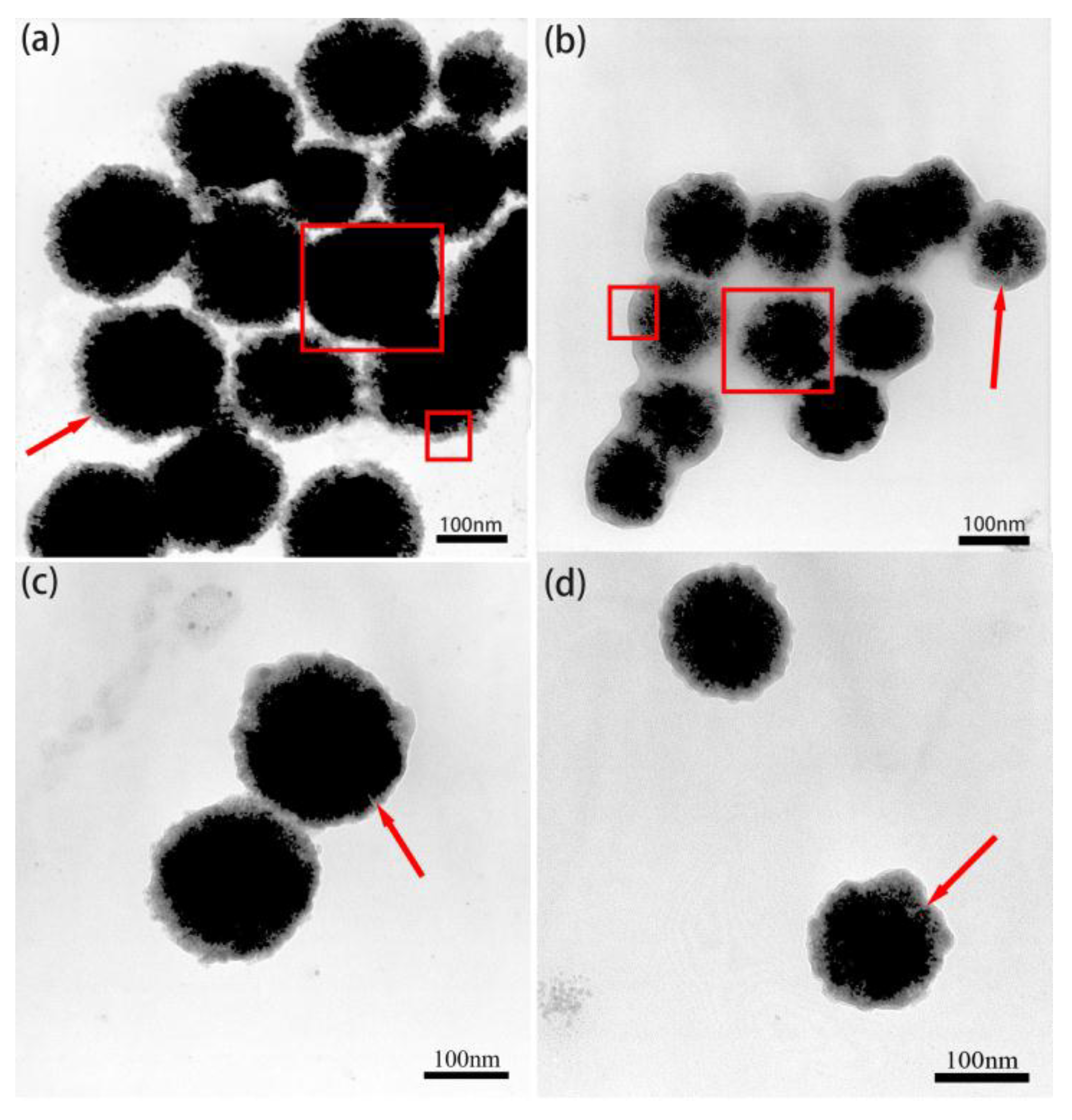
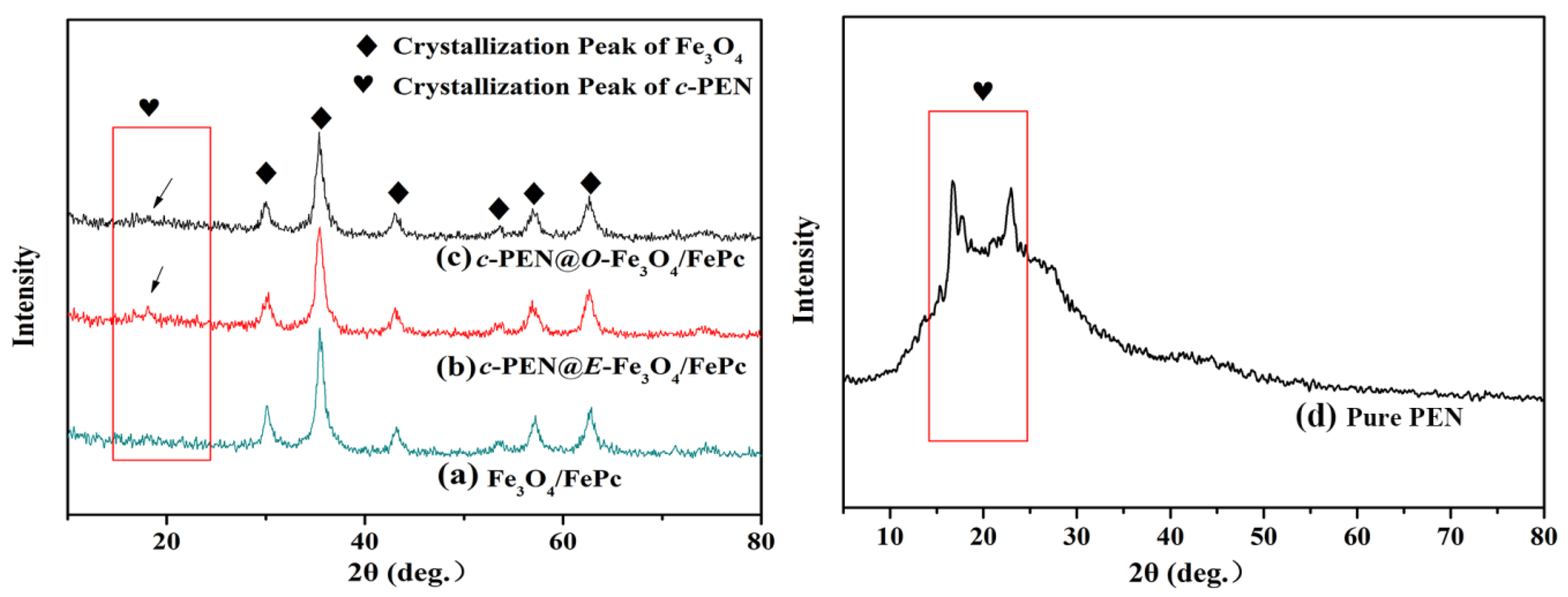
3.1. The Microcosmic Morphology of the Various Fe3O4/FePc
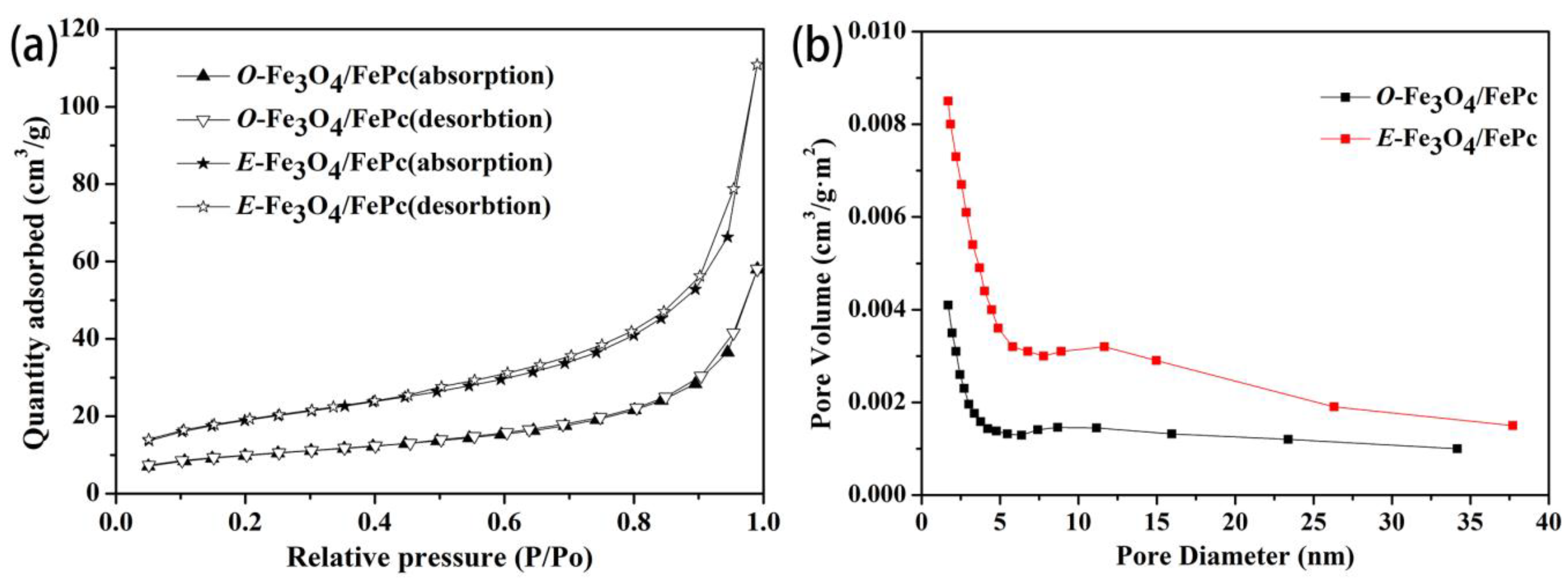
3.2. The Surface Characteristics of the Various Fe3O4/FePc
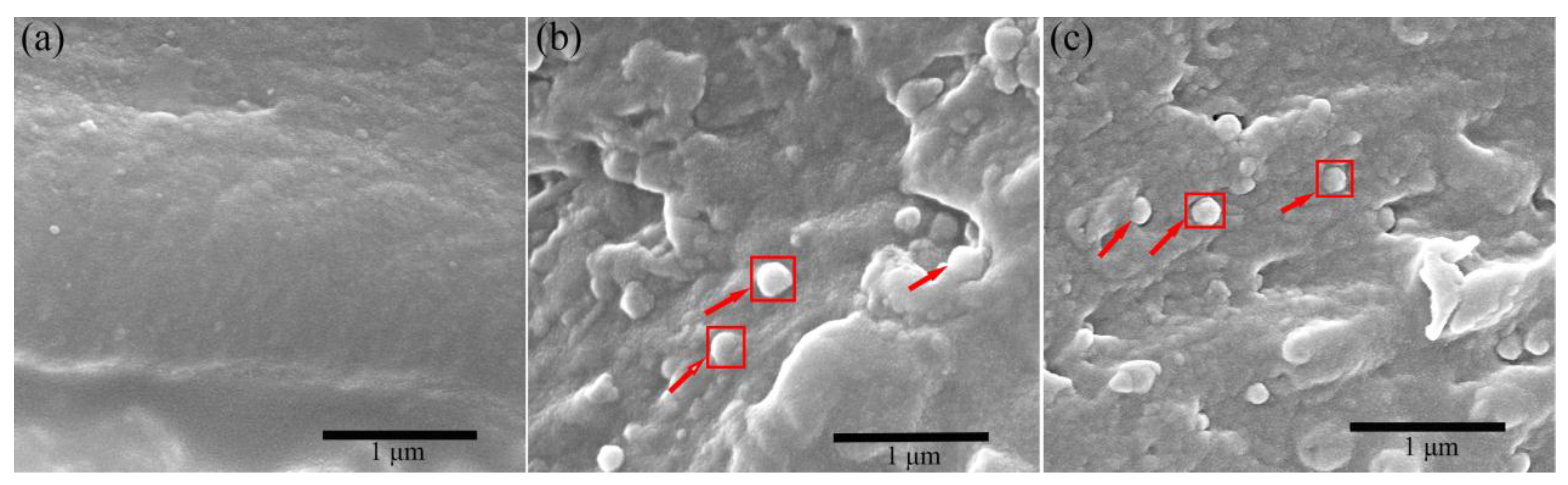
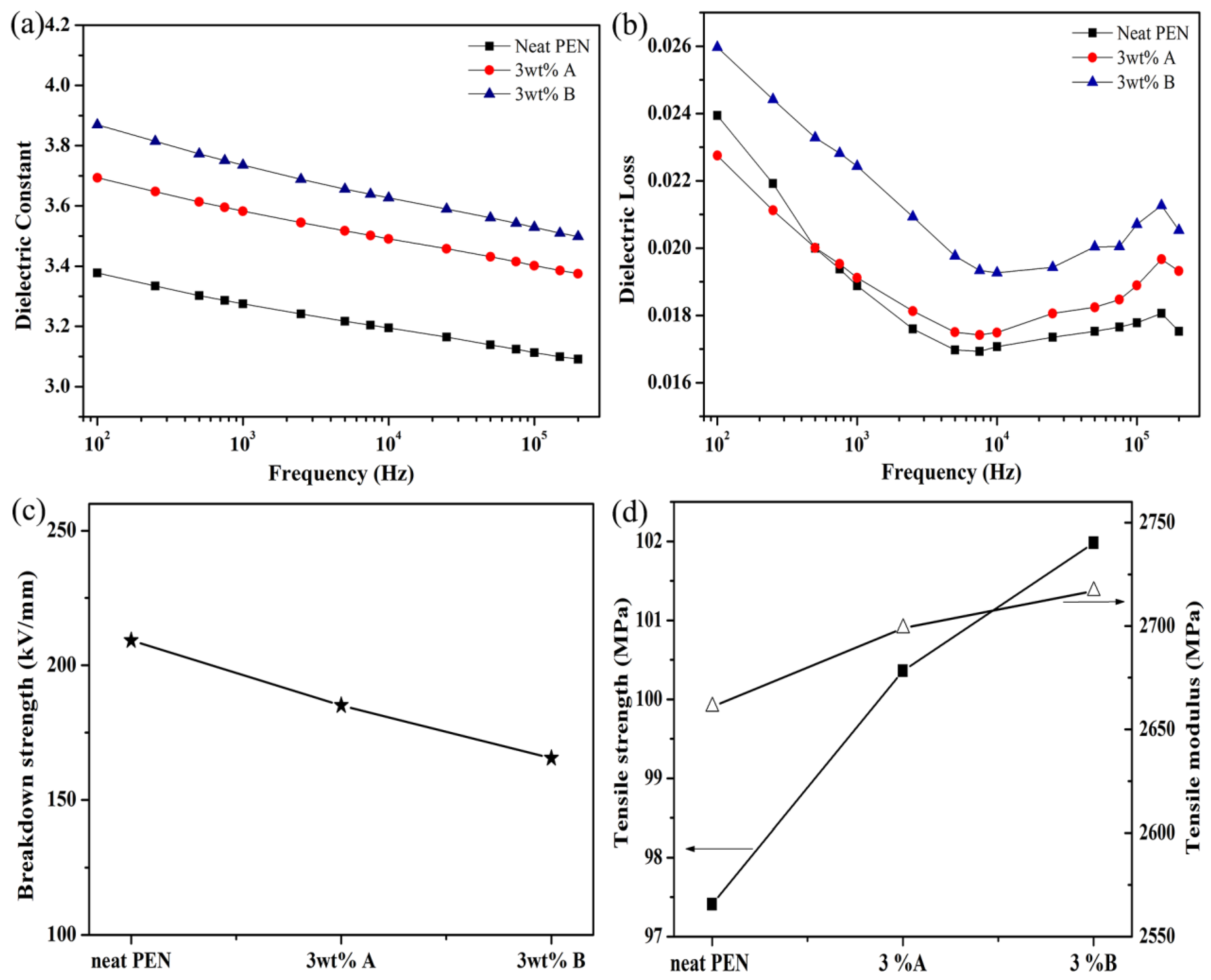
3.3. The Interfacial Characteristics and the Dielectric Properties of the Various Fe3O4/FePc/H-PEN Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wan, Y.J.; Tang, L.C.; Gong, L.X.; Yan, D.; Li, Y.B.; Wu, L.B.; Jiang, J.X.; Lai, G.Q. Grafting of epoxy chains onto graphene oxide for epoxy composites with improved mechanical and thermal properties. Carbon 2014, 69, 467–480. [Google Scholar] [CrossRef]
- Jin, F.; Feng, M.; Huang, X.; Long, C.; Jia, K.; Liu, X. Effect of SiO2 grafted MWCNTs on the mechanical and dielectric properties of PEN composite films. Appl. Surf. Sci. 2015, 357, 704–711. [Google Scholar] [CrossRef]
- Song, Y.; Shen, Y.; Liu, H.Y.; Lin, Y.H.; Li, M.; Nan, C.W. Improving the dielectric constants and breakdown strength of polymer composites: Effects of the shape of the BaTiO3 nanoinclusions, surface modification and polymer matrix. J. Mater. Chem. 2012, 22, 16491–16498. [Google Scholar] [CrossRef]
- Tang, H.; Wang, P.; Zheng, P.; Liu, X. Core-shell structured BaTiO3@polymer hybrid nanofiller for poly(arylene ether nitrile) nanocomposites with enhanced dielectric properties and high thermal stability. Compos. Sci. Technol. 2016, 123, 134–142. [Google Scholar] [CrossRef]
- Liu, S.H.; Zhai, J.W.; Wang, J.W.; Xue, S.X.; Zhang, W.Q. Enhanced Energy Storage Density in Poly(Vinylidene Fluoride) Nanocomposites by a Small Loading of Suface-Hydroxylated Ba0.6Sr0.4TiO3 Nanofibers. ACS Appl. Mater. Interfaces 2014, 6, 1533–1540. [Google Scholar]
- Zhang, X.H.; Ma, Y.H.; Zhao, C.W.; Yang, W.T. High dielectric constant and low dielectric loss hybrid nanocomposites fabricated with ferroelectric polymer matrix and BaTiO3 nanofibers modified with perfluoroalkylsilane. Appl. Surf. Sci. 2014, 305, 531–538. [Google Scholar] [CrossRef]
- Li, K.; Xu, M.; Tong, L.; Tang, X.; Liu, X. Promoted crystallization of Poly(arylene ether nitrile) reinforced with Fe3O4/FePc nano-hybrid microsphere. Mater. Today Commun. 2017, 13, 72–79. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, R.; Xu, M.; Zhan, Y.; Lei, Y.; Zhong, J.; Liu, X. Fe–phthalocyanine oligomer/Fe3O4 nano-hybrid particles and their effect on the properties of polyarylene ether nitriles magnetic nanocomposites. Colloids Surf. A Physicochem. Eng. Asp. 2011, 375, 245–251. [Google Scholar] [CrossRef]
- Bera, B.; Das, J.K.; Das, N. Mesoporous silica based composite membrane formation by in-situ cross-linking of phenol and formaldehyde at room temperature for enhanced CO2 separation. Microporous Mesoporous Mater. 2018, 256, 177–189. [Google Scholar] [CrossRef]
- Dou, B.; Li, J.; Hu, Q.; Ma, C.; He, C.; Li, P.; Hu, Q.; Hao, Z.; Qiao, S. Hydrophobic micro/mesoporous silica spheres assembled from zeolite precursors in acidic media for aromatics adsorption. Microporous Mesoporous Mater. 2010, 133, 115–123. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wen, X.; Li, L.; Wang, F.; Zhao, N.; Xiao, F.K.; Wei, W.; Sun, Y.H. Synthesis of amine-modified mesoporous materials for CO2 capture by a one-pot template-free method. J. Sol-Gel Sci. Technol. 2013, 66, 353–362. [Google Scholar] [CrossRef]
- Ma, X.L.; Wang, X.X.; Song, C.S. “Molecular Basket” Sorbents for Separation of CO2 and H2S from Various Gas Streams. J. Am. Chem. Soc. 2009, 131, 5777–5783. [Google Scholar] [CrossRef] [PubMed]
- Coriolano, A.C.F.; Silva, C.G.C.; Costa, M.J.F.; Pergher, S.B.C.; Caldeira, V.P.S.; Araujo, A.S. Development of HZSM-5/AlMCM-41 hybrid micro-mesoporous material and application for pyrolysis of vacuum gasoil. Microporous Mesoporous Mater. 2013, 172, 206–212. [Google Scholar] [CrossRef]
- Sue, Y.C.; Wu, J.W.; Chung, S.E.; Kang, C.H.; Tung, K.L.; Wu, K.C.W.; Shieh, F.K. Synthesis of Hierarchical Micro/Mesoporous Structures via Solid-Aqueous Interface Growth: Zeolitic Imidazolate Framework-8 on Siliceous Mesocellular Foams for Enhanced Pervaporation of Water/Ethanol Mixtures. ACS Appl. Mater. Interfaces 2014, 6, 5192–5198. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Liu, P.F.; Zhu, B.; Chen, S.F.; Yao, K.L.; Han, R. Microporous carbon derived from acacia gum with tuned porosity for high-performance electrochemical capacitors. Int. J. Hydrogen Energy 2015, 40, 6188–6196. [Google Scholar] [CrossRef]
- Jia, Z.; Wang, Q.; Ren, D.; Zhu, R. Fabrication of one-dimensional mesoporous α-Fe2O3 nanostructure via self-sacrificial template and its enhanced Cr(VI) adsorption capacity. Appl. Surf. Sci. 2013, 264, 255–260. [Google Scholar] [CrossRef]
- Fang, Y.M.; Hu, H.Q. An ordered mesoporous aluminosilicate with completely crystalline zeolite wall structure. J. Am. Chem. Soc. 2006, 128, 10636–10637. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.A.; Gao, J.S.; Xu, C.M.; Shen, B.J. Alkali-treatment of ZSM-5 zeolites with different SiO2/Al2O3 ratios and light olefin production by heavy oil cracking. Fuel Process. Technol. 2011, 92, 414–420. [Google Scholar] [CrossRef]
- Xu, M.; Meng, F.; Zhao, R.; Zhan, Y.; Lei, Y.; Liu, X. Iron phthalocyanine oligomer/Fe3O4 hybrid microspheres and their microwave absorption property. J. Magn. Magn. Mater. 2011, 323, 2174–2178. [Google Scholar] [CrossRef]
- Zou, Y.K.; Liu, X.B. Morphology, thermal and mechanical properties of glass fiber-reinforced crosslinkable poly(arylene ether nitrile). J. Appl. Polym. Sci. 2013, 129, 130–137. [Google Scholar] [CrossRef]
- Tong, L.; Jia, K.; Liu, X. Novel phthalonitrile-terminated polyarylene ether nitrile with high glass transition temperature and enhanced thermal stability. Mater. Lett. 2014, 128, 267–270. [Google Scholar] [CrossRef]
- Zhao, R.; Jia, K.; Wei, J.-J.; Pu, J.-X.; Liu, X.-B. Hierarchically nanostructured Fe3O4 microspheres and their novel microwave electromagnetic properties. Mater. Lett. 2010, 64, 457–459. [Google Scholar] [CrossRef]
- Dong, S.; Xu, M.; Wei, J.; Yang, X.; Liu, X. The preparation and wide frequency microwave absorbing properties of tri-substituted-bisphthalonitrile/Fe3O4 magnetic hybrid microspheres. J. Magn. Magn. Mater. 2014, 349, 15–20. [Google Scholar] [CrossRef]
- Meng, F.; Zhao, R.; Zhan, Y.; Lei, Y.; Zhong, J.; Liu, X. One-step synthesis of Fe-phthalocyanine/Fe3O4 hybrid microspheres. Mater. Lett. 2011, 65, 264–267. [Google Scholar] [CrossRef]
- Goertzen, W.K.; Kessler, M.R. Dynamic mechanical analysis of fumed silica/cyanate ester nanocomposites. Compos. Part A Appl. Sci. Manuf. 2008, 39, 761–768. [Google Scholar] [CrossRef]
- Kim, P.; Doss, N.M.; Tillotson, J.P.; Hotchkiss, P.J.; Pan, M.J.; Marder, S.R.; Li, J.Y.; Calame, J.P.; Perry, J.W. High Energy Density Nanocomposites Based on Surface-Modified BaTiO3 and a Ferroelectric Polymer. ACS Nano 2009, 3, 2581–2592. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Pu, Z.; Chen, Z.; Huang, X.; Liu, X. Effect of nanosilica on the thermal, mechanical, and dielectric properties of polyarylene ether nitriles terminated with phthalonitrile. Polym. Compos. 2014, 35, 344–350. [Google Scholar] [CrossRef]

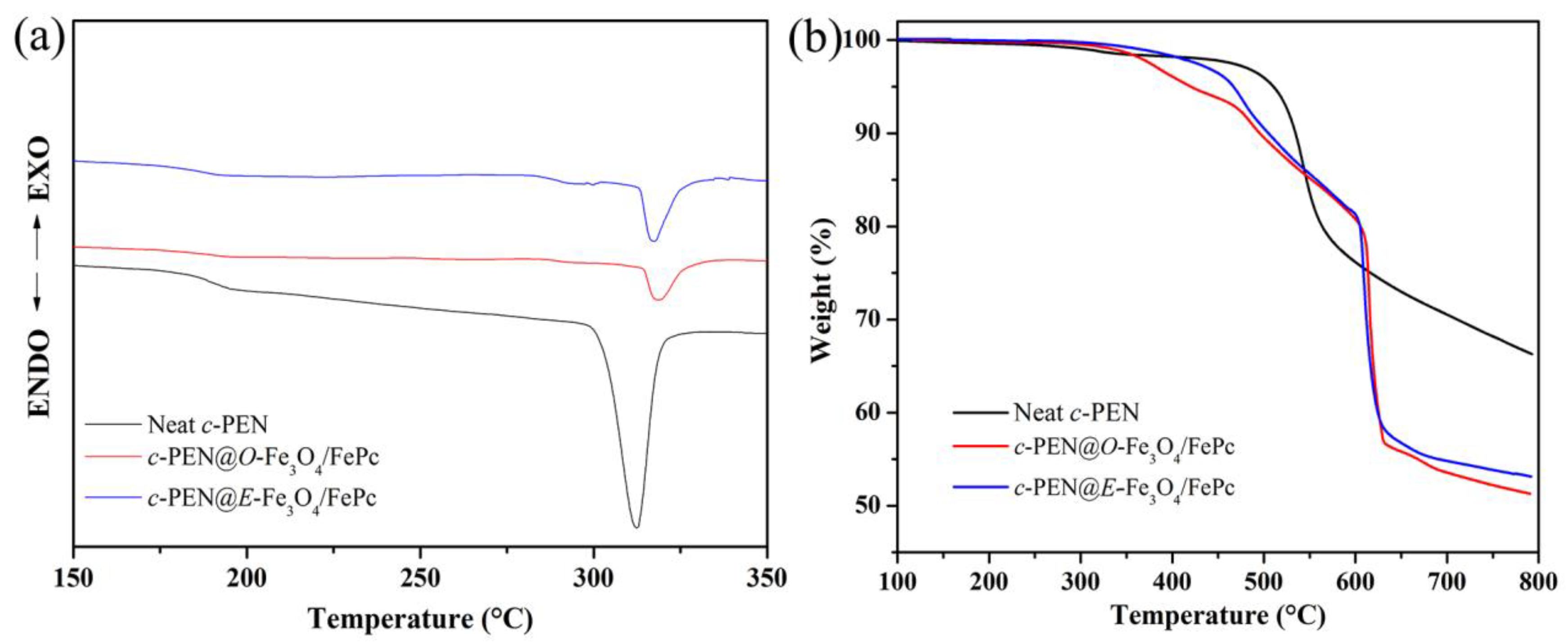
| Samples | Neat c-PEN | c-PEN@O-Fe3O4/FePc | c-PEN@E-Fe3O4/FePc |
|---|---|---|---|
| Tg (°C) | 192.2 | 189.7 | 188.6 |
| ΔH (J/g) | 22.16 | 6.87 | 8.90 |
| T5% (°C) | 507.7 | 421.2 | 463.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, K.; Ren, D.; Tang, X.; Xu, M.; Liu, X. Micro/Mesoporous Fe3O4/Fe-Phthalocyanine Microspheres and Effects of Their Surface Morphology on the Crystallization and Properties of Poly(Arylene Ether Nitrile) Composites. Materials 2018, 11, 1356. https://doi.org/10.3390/ma11081356
Li K, Ren D, Tang X, Xu M, Liu X. Micro/Mesoporous Fe3O4/Fe-Phthalocyanine Microspheres and Effects of Their Surface Morphology on the Crystallization and Properties of Poly(Arylene Ether Nitrile) Composites. Materials. 2018; 11(8):1356. https://doi.org/10.3390/ma11081356
Chicago/Turabian StyleLi, Kui, Dengxun Ren, Xianzhong Tang, Mingzhen Xu, and Xiaobo Liu. 2018. "Micro/Mesoporous Fe3O4/Fe-Phthalocyanine Microspheres and Effects of Their Surface Morphology on the Crystallization and Properties of Poly(Arylene Ether Nitrile) Composites" Materials 11, no. 8: 1356. https://doi.org/10.3390/ma11081356
APA StyleLi, K., Ren, D., Tang, X., Xu, M., & Liu, X. (2018). Micro/Mesoporous Fe3O4/Fe-Phthalocyanine Microspheres and Effects of Their Surface Morphology on the Crystallization and Properties of Poly(Arylene Ether Nitrile) Composites. Materials, 11(8), 1356. https://doi.org/10.3390/ma11081356