Open Source 3D Printed Lung Tumor Movement Simulator for Radiotherapy Quality Assurance
Abstract
1. Introduction
2. Materials and Methods
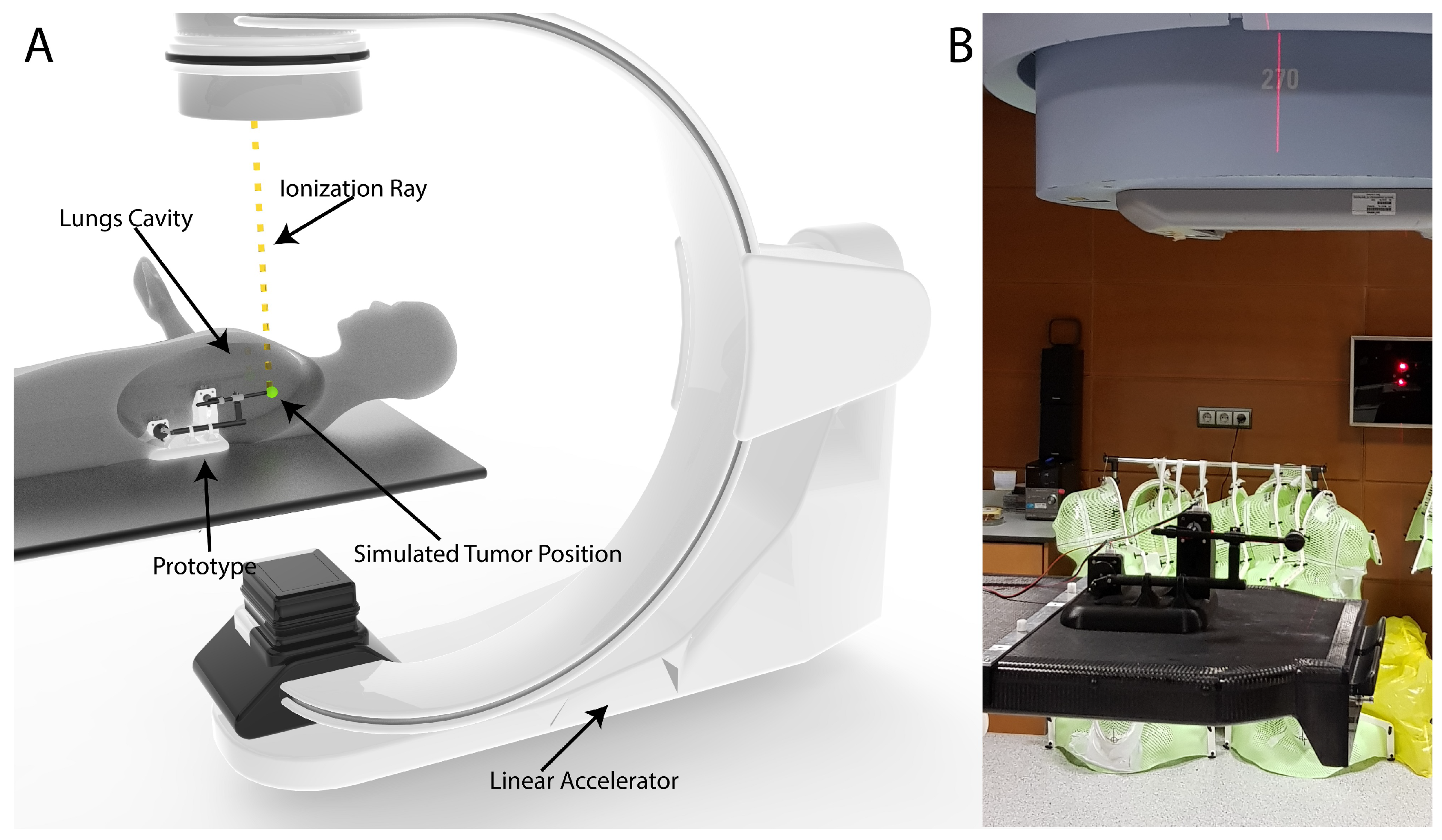
2.1. Linear Accelerator
2.2. Electromechanical Components
2.3. Microcontroller
2.4. Manufacture and Material
3. Results
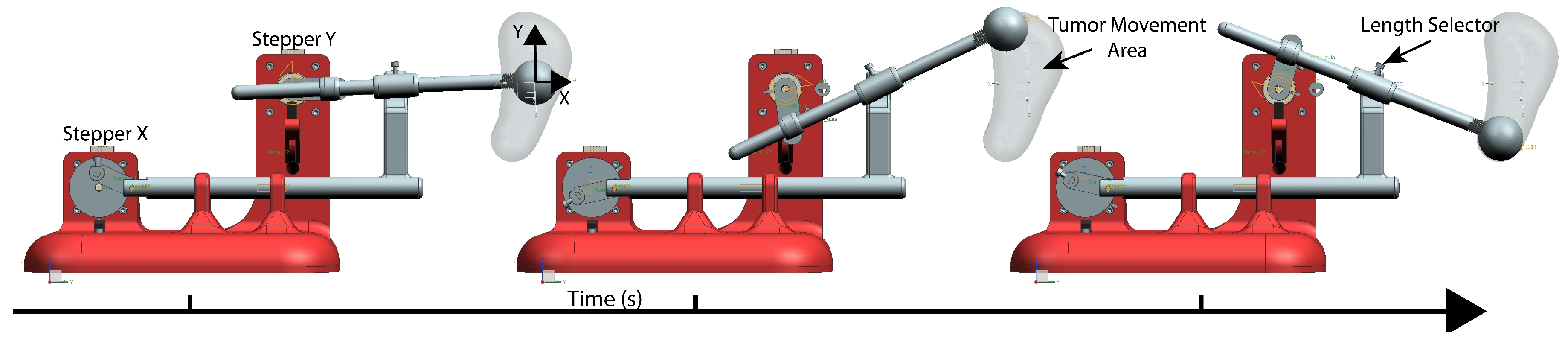
3.1. Prototype Design
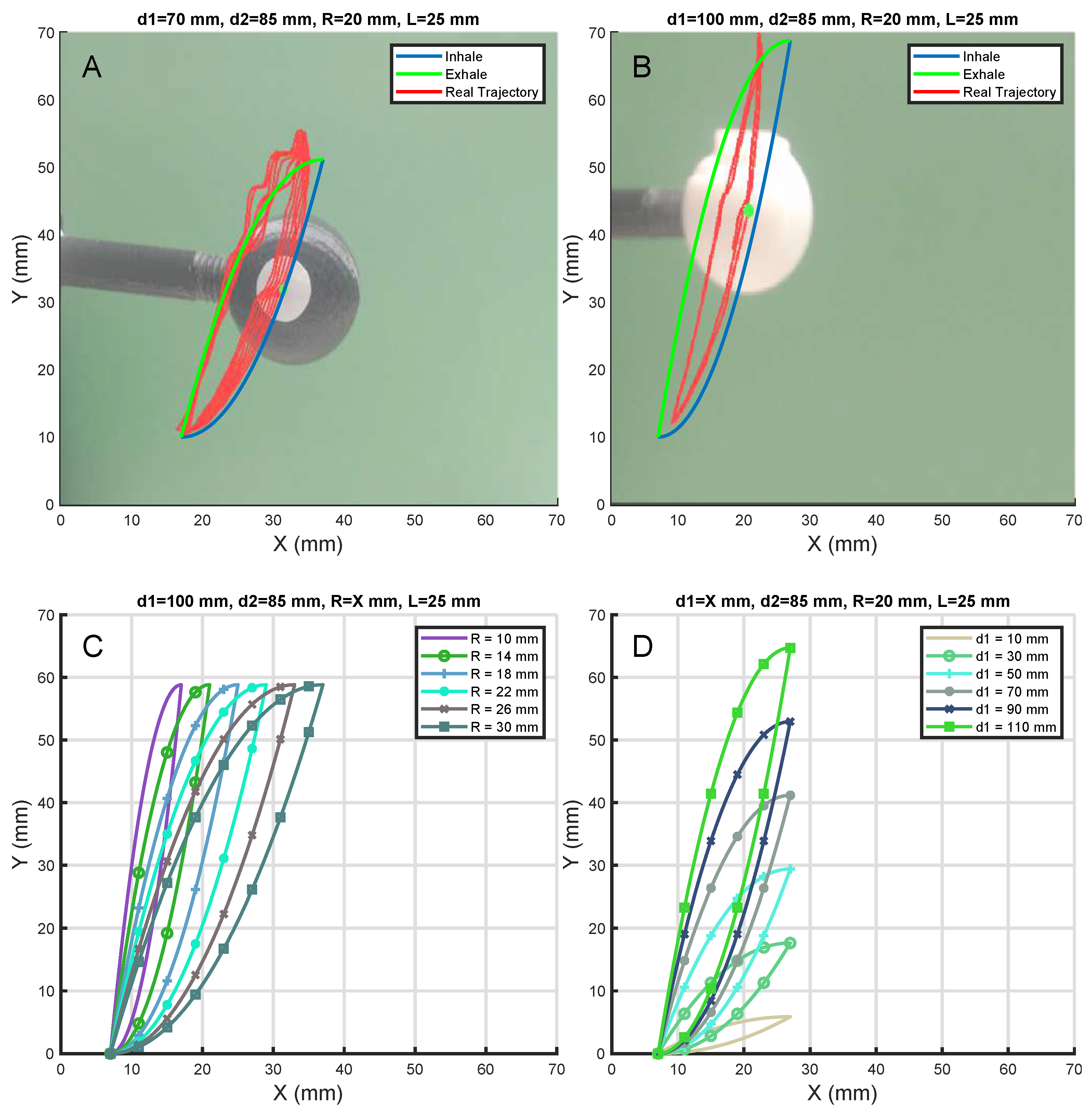
3.2. Movement and Path Simulation
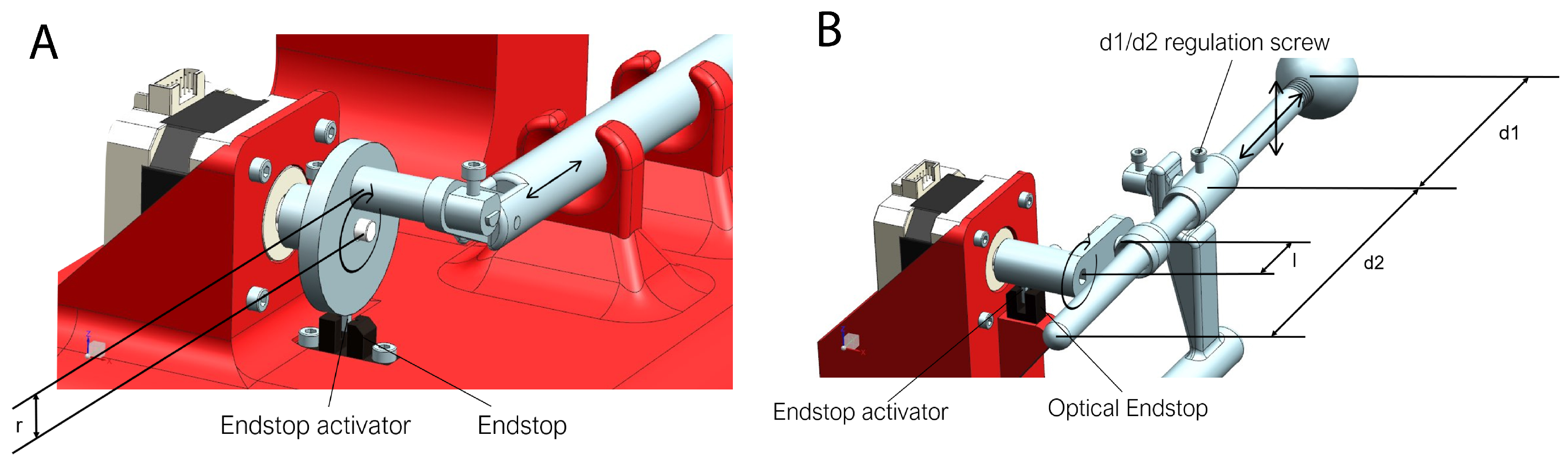
3.3. Synchronizing the Movements
- In width: The width can be modified by changing the distance “r”.
- In timing: In the serial console, the breathing time can be set to move at the same frequency as the patient does.
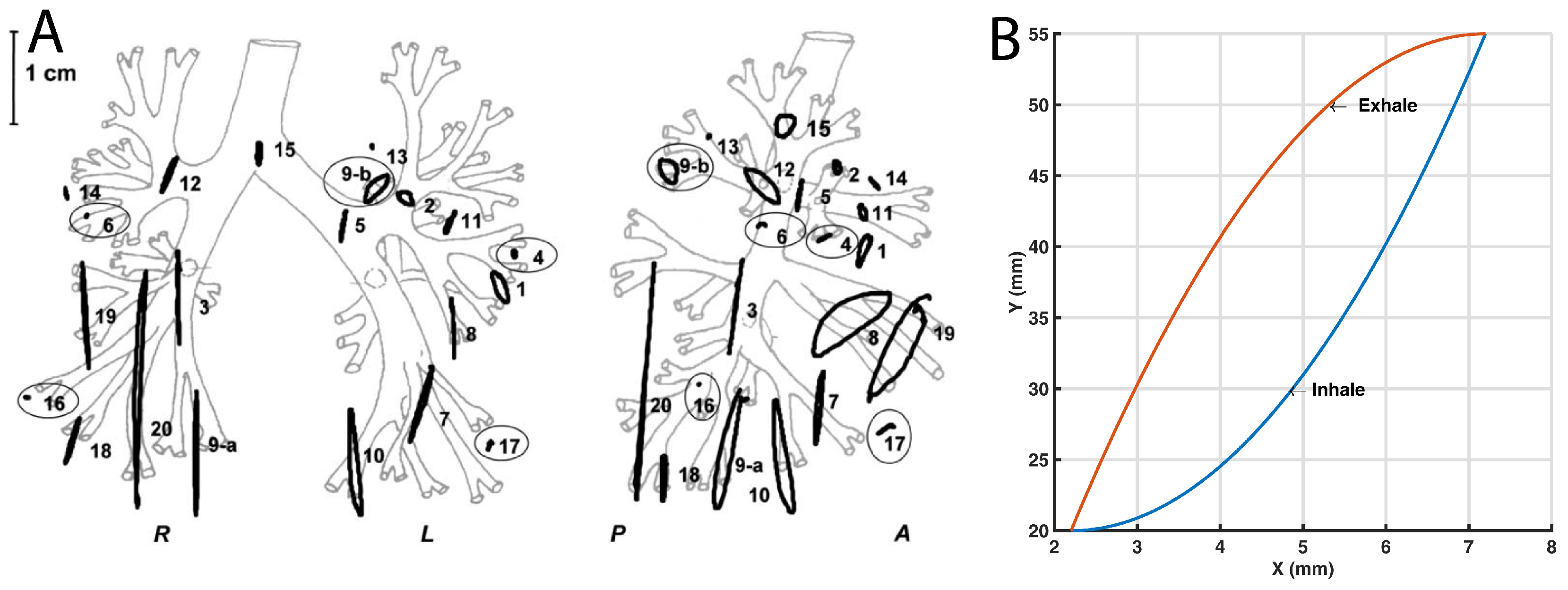
3.4. Path Verification
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Fitzmaurice, C.; Dicker, D.; Pain, A. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Mountain, C.F. Revisions in the international system for staging lung cancer. Chest 1997, 111, 1710–1717. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Siegel, R.L.; Lin, C.C.; Mariotto, A.B.; Kramer, J.L.; Rowland, J.H.; Stein, K.D.; Alteri, R.; Jemal, A. Cancer treatment and survivorship statistics, 2016. CA Cancer J. Clin. 2016, 66, 271–289. [Google Scholar] [CrossRef] [PubMed]
- Mayles, P.; Nahum, A. Handbook of Radiotherapy Physics; Taylor & Francis: London, UK, 2007. [Google Scholar] [CrossRef]
- Mageras, G.S.; Pevsner, A.; Yorke, E.D.; Rosenzweig, K.E.; Ford, E.C.; Hertanto, A.; Larson, S.M.; Lovelock, D.M.; Erdi, Y.E.; Nehmeh, S.A.; et al. Measurement of lung tumor motion using respiration-correlated CT. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Borm, K.J.; Oechsner, M.; Wilkens, J.J.; Berndt, J.; Molls, M.; Geinitz, H.; Duma, M.N. The impact of CT window settings on the contouring of a moving target: A phantom study. Clin. Radiol. 2014, 69, e331–e336. [Google Scholar] [CrossRef] [PubMed]
- Guyton, A.C.; Hall, J.E.; John, E.; Fernández Bernaldo de Quirós, I.; Agud Aparicio, J.L.; Alvarez Baleriola, I. Guyton & Hall: Tratado de Fisiología Medica, 12th ed.; Elsevier España S.A.: Madrid, Spain, 2011; p. 1112. [Google Scholar]
- Guidi, G.; Maffei, N.; Ciarmatori, A.; Mistretta, M.G.; Gottardi, G.; Costi, T.; Vecchi, C.; Baldazzi, G. Real-time lung tumour motion modeling for adaptive radiation therapy using lego mindstorms. J. Mech. Med. Biol. 2015, 15, 1540019. [Google Scholar] [CrossRef]
- Seppenwoolde, Y.; Shirato, H.; Kitamura, K.; Shimizu, S.; van Herk, M.; Lebesque, J.V.; Miyasaka, K. Precise and real-time measurement of 3D tumor motion in lung due to breathing and heartbeat, measured during radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 822–834. [Google Scholar] [CrossRef]
- Harris, R.S. Pressure-volume curves of the respiratory system. Respir. Care 2005, 50, 78–99. [Google Scholar] [PubMed]
- Aznar, M.C.; Persson, G.F.; Kofoed, I.M.; Nygaard, D.E.; Korreman, S.S. Irregular breathing during 4DCT scanning of lung cancer patients: Is the midventilation approach robust? Phys. Med. 2014, 30, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sarudis, S.; Karlsson Hauer, A.; Nyman, J.; Bäck, A. Systematic evaluation of lung tumor motion using four-dimensional computed tomography. Acta Oncol. 2017, 56, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Bao, Y.; Zhang, L.; Fan, W.; He, H.; Sun, Z.W.; Hu, X.; Huang, S.M.; Chen, M.; Deng, X.W. Assessment of respiration-induced motion and its impact on treatment outcome for lung cancer. BioMed Res. Int. 2013, 2013, 872739. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Shirato, H.; Ogura, S.; Akita-Dosaka, H.; Kitamura, K.; Nishioka, T.; Kagei, K.; Nishimura, M.; Miyasaka, K. Detection of lung tumor movement in real-time tumor-tracking radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2001, 51, 304–310. [Google Scholar] [CrossRef]
- Redmond, K.J.; Song, D.Y.; Fox, J.L.; Zhou, J.; Rosenzweig, C.N.; Ford, E. Respiratory Motion Changes of Lung Tumors Over the Course of Radiation Therapy Based on Respiration-Correlated Four-Dimensional Computed Tomography Scans. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Katoh, N.; Suzuki, R.; Ito, Y.M.; Shimizu, S.; Onimaru, R.; Inoue, T.; Miyamoto, N.; Shirato, H. Evaluation of the motion of lung tumors during stereotactic body radiation therapy (SBRT) with four-dimensional computed tomography (4DCT) using real-time tumor-tracking radiotherapy system (RTRT). Phys. Med. 2016, 32, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Knybel, L.; Cvek, J.; Molenda, L.; Stieberova, N.; Feltl, D. Analysis of Lung Tumor Motion in a Large Sample: Patterns and Factors Influencing Precise Delineation of Internal Target Volume. Int. J. Radiat. Oncol. Biol. Phys. 2016, 96, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Fassi, A.; Schaerer, J.; Fernandes, M.; Riboldi, M.; Sarrut, D.; Baroni, G. Tumor Tracking Method Based on a Deformable 4D CT Breathing Motion Model Driven by an External Surface Surrogate. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Ruben, J.; Seeley, A.; Panettieri, V.; Ackerly, T. Variation in Lung Tumour Breathing Motion between Planning Four-dimensional Computed Tomography and Stereotactic Ablative Radiotherapy Delivery and its Dosimetric Implications: Any Role for Four-dimensional Set-up Verification? Clin. Oncol. 2016, 28, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Wangler, T.P. RF Linear Accelerators; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; p. 450. [Google Scholar]
- D’Ausilio, A. Arduino: A low-cost multipurpose lab equipment. Behav. Res. Methods 2012, 44, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Teikari, P.; Najjar, R.P.; Malkki, H.; Knoblauch, K.; Dumortier, D.; Gronfier, C.; Cooper, H.M. An inexpensive Arduino-based LED stimulator system for vision research. J. Neurosci. Methods 2012, 211, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Besson, T.; Debayle, D.; Diochot, S.; Salinas, M.; Lingueglia, E. Low cost venom extractor based on Arduino board for electrical venom extraction from arthropods and other small animals. Toxicon 2016, 118, 156–161. [Google Scholar] [CrossRef] [PubMed]
- Sheinin, A.; Lavi, A.; Michaelevski, I. StimDuino: An Arduino-based electrophysiological stimulus isolator. J. Neurosci. Methods 2015, 243, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.S.; Zanzinger, Z.; Debose, D.; Stephens, B. Open Source Building Science Sensors (OSBSS): A low-cost Arduino-based platform for long-term indoor environmental data collection. Build. Environ. 2016, 100, 114–126. [Google Scholar] [CrossRef]
- Mathew, A.P.; Oksman, K.; Sain, M. Mechanical properties of biodegradable composites from poly lactic acid (PLA) and microcrystalline cellulose (MCC). J. Appl. Polym. Sci. 2005, 97, 2014–2025. [Google Scholar] [CrossRef]
- Lopes, G.; Bonacchi, N.; Frazão, J.; Neto, J.P.; Atallah, B.V.; Soares, S.; Moreira, L.; Matias, S.; Itskov, P.M.; Correia, P.A.; et al. Bonsai: An event-based framework for processing and controlling data streams. Front. Neuroinform. 2015, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Dunn, L.; Kron, T.; Johnston, P.N.; McDermott, L.N.; Taylor, M.L.; Callahan, J.; Franich, R.D. A programmable motion phantom for quality assurance of motion management in radiotherapy. Australas. Phys. Eng. Sci. Med. 2012, 35, 93–100. [Google Scholar] [CrossRef] [PubMed]
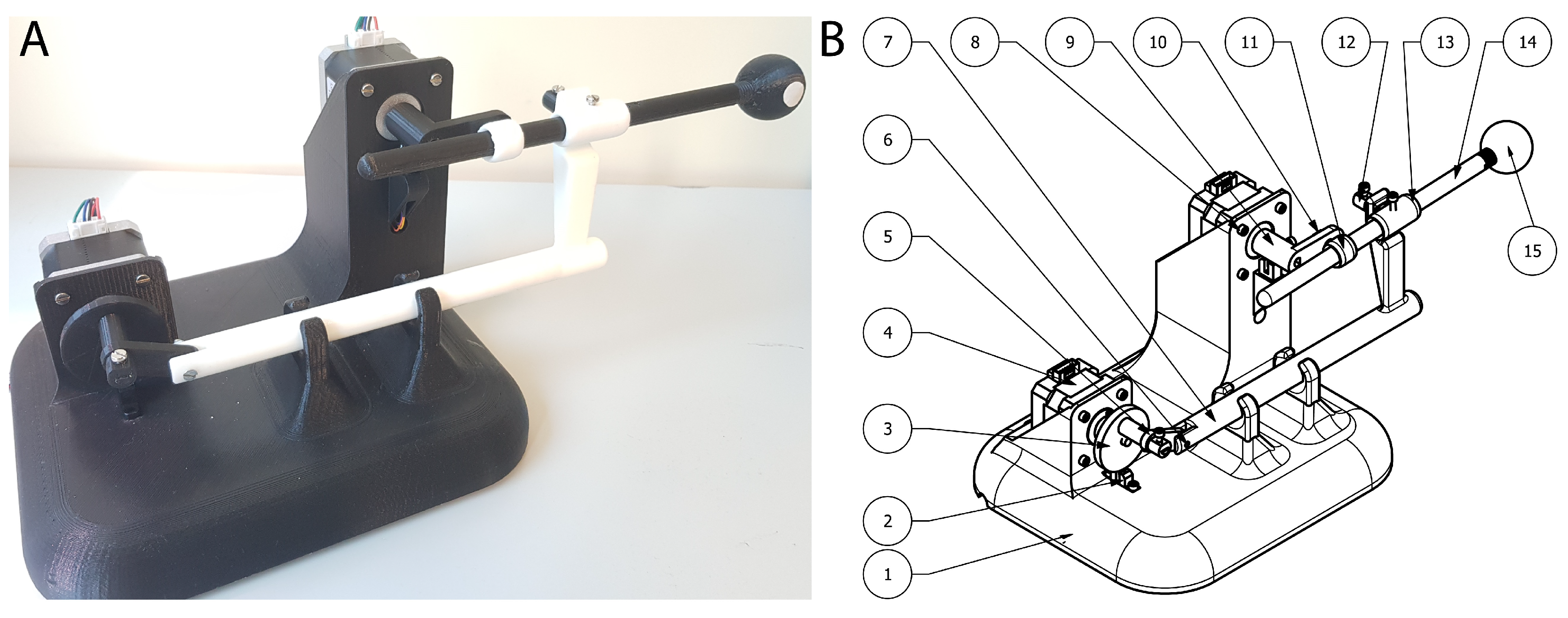

| PART (Figure 2) | Description | Quantity | X (mm) | Y (mm) | Z (mm) | Weight (g) | Material |
|---|---|---|---|---|---|---|---|
| 1 | Base | 1 | 209.50 | 180.00 | 145.00 | 550.00 | PLA |
| 2 | Endstop | 2 | - | - | - | - | - |
| 3 | Horizontal wheel | 1 | 45.10 | 40.00 | 51.00 | 9.00 | PLA |
| 4 | Stepper NEMA 17 | 2 | 42.00 | 42.00 | 38.00 | 285.00 | PLA |
| 5 | Union bar | 1 | 15.70 | 36.60 | 4.50 | 3.00 | PLA |
| 6 | Cylinder pin | 1 | 2.00 | 3.00 | 30.00 | 2.00 | Stainless Steel |
| 7 | Horizontal bar | 1 | 200.00 | 26.00 | 85.50 | 28.00 | PLA |
| 8 | Screw M3 x 6 | 15 | - | - | - | 5.00 | Stainless Steel |
| 9 | Vertical bar | 1 | 22.00 | 44.90 | 30.00 | 6.00 | PLA |
| 10 | Short retainer | 1 | 4.00 | 10.90 | 9.10 | 1.00 | PLA |
| 11 | Stem guide | 1 | 33.00 | 13.40 | 16.20 | 3.00 | PLA |
| 12 | Long retainer | 2 | 9.00 | 10.90 | 9.10 | 2.00 | PLA |
| 13 | Stem support | 1 | 22.00 | 44.90 | 30.00 | 6.00 | PLA |
| 14 | Stem | 1 | 200.10 | 10.00 | 14.90 | 12.00 | PLA |
| 15 | Tumor sphere (Tip) | 1 | 29.20 | 30.00 | 30.00 | 8.00 | PLA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Quiñones, D.R.; Soler-Egea, D.; González-Pérez, V.; Reibke, J.; Simarro-Mondejar, E.; Pérez-Feito, R.; García-Manrique, J.A.; Crispín, V.; Moratal, D. Open Source 3D Printed Lung Tumor Movement Simulator for Radiotherapy Quality Assurance. Materials 2018, 11, 1317. https://doi.org/10.3390/ma11081317
Quiñones DR, Soler-Egea D, González-Pérez V, Reibke J, Simarro-Mondejar E, Pérez-Feito R, García-Manrique JA, Crispín V, Moratal D. Open Source 3D Printed Lung Tumor Movement Simulator for Radiotherapy Quality Assurance. Materials. 2018; 11(8):1317. https://doi.org/10.3390/ma11081317
Chicago/Turabian StyleQuiñones, Darío R., David Soler-Egea, Víctor González-Pérez, Johanna Reibke, Elena Simarro-Mondejar, Ricardo Pérez-Feito, Juan A. García-Manrique, Vicente Crispín, and David Moratal. 2018. "Open Source 3D Printed Lung Tumor Movement Simulator for Radiotherapy Quality Assurance" Materials 11, no. 8: 1317. https://doi.org/10.3390/ma11081317
APA StyleQuiñones, D. R., Soler-Egea, D., González-Pérez, V., Reibke, J., Simarro-Mondejar, E., Pérez-Feito, R., García-Manrique, J. A., Crispín, V., & Moratal, D. (2018). Open Source 3D Printed Lung Tumor Movement Simulator for Radiotherapy Quality Assurance. Materials, 11(8), 1317. https://doi.org/10.3390/ma11081317






