Tensile Properties and Corrosion Behavior of Extruded Low-Alloyed Mg–1Sn–1Al–1Zn Alloy: The Influence of Microstructural Characteristics
Abstract
1. Introduction
2. Experimental Procedure
2.1. Specimen Preparation
2.2. Microstructural Characterization
2.3. Tensile Properties
2.4. Electrochemical Test
3. Results and Discussion
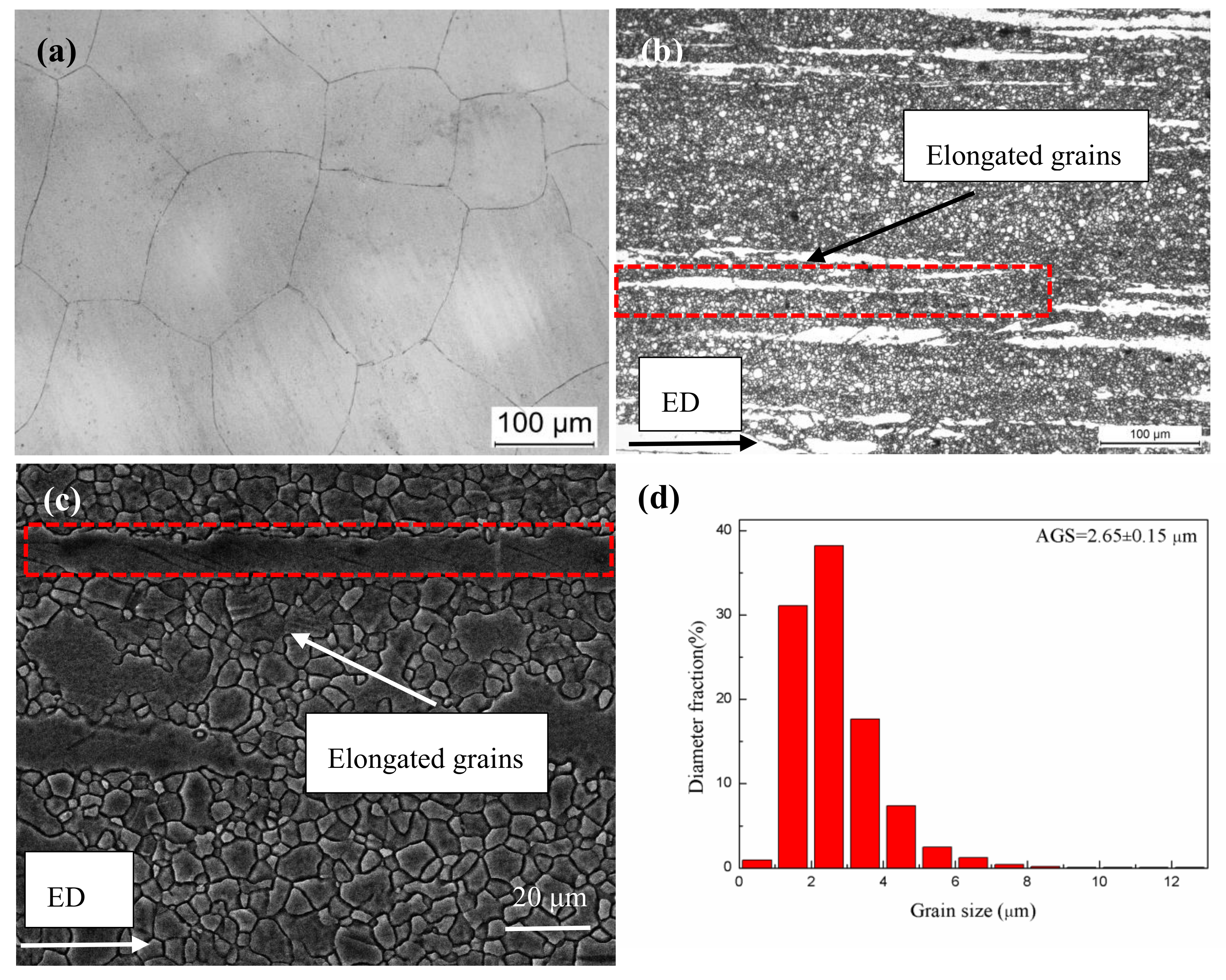
3.1. Microstructural Characteristics Prior to Corrosion
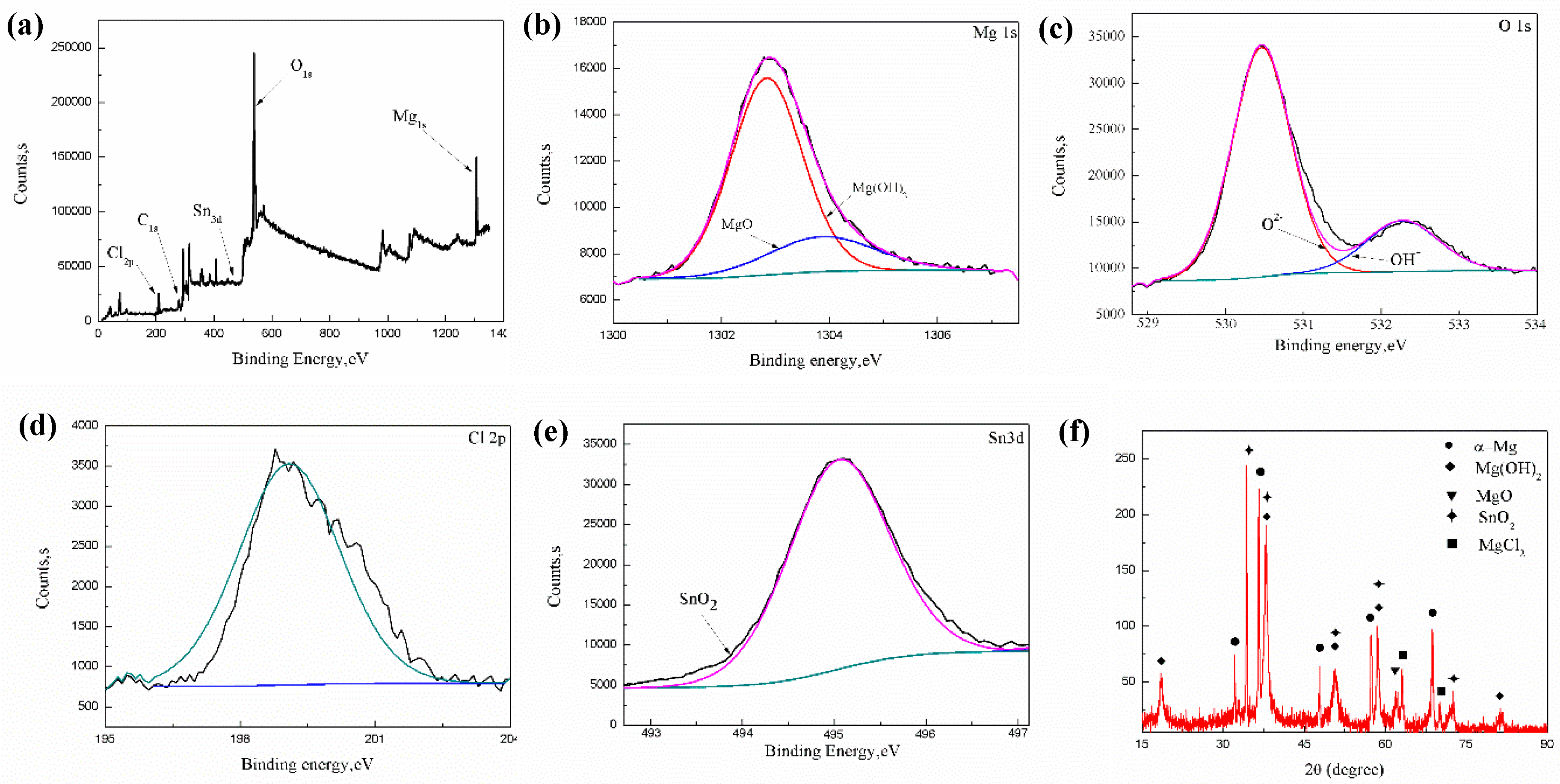
3.2. Phase Composition
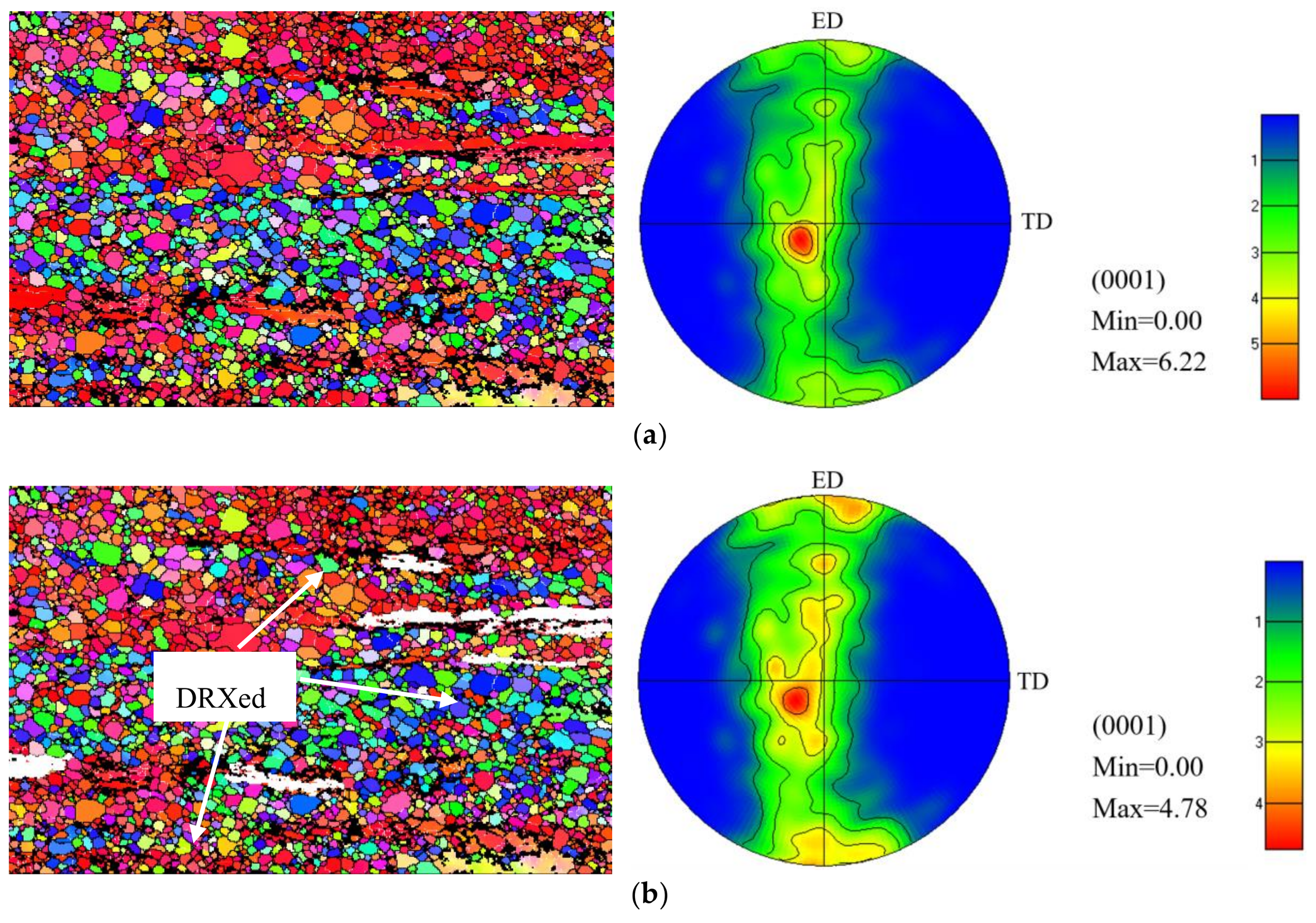
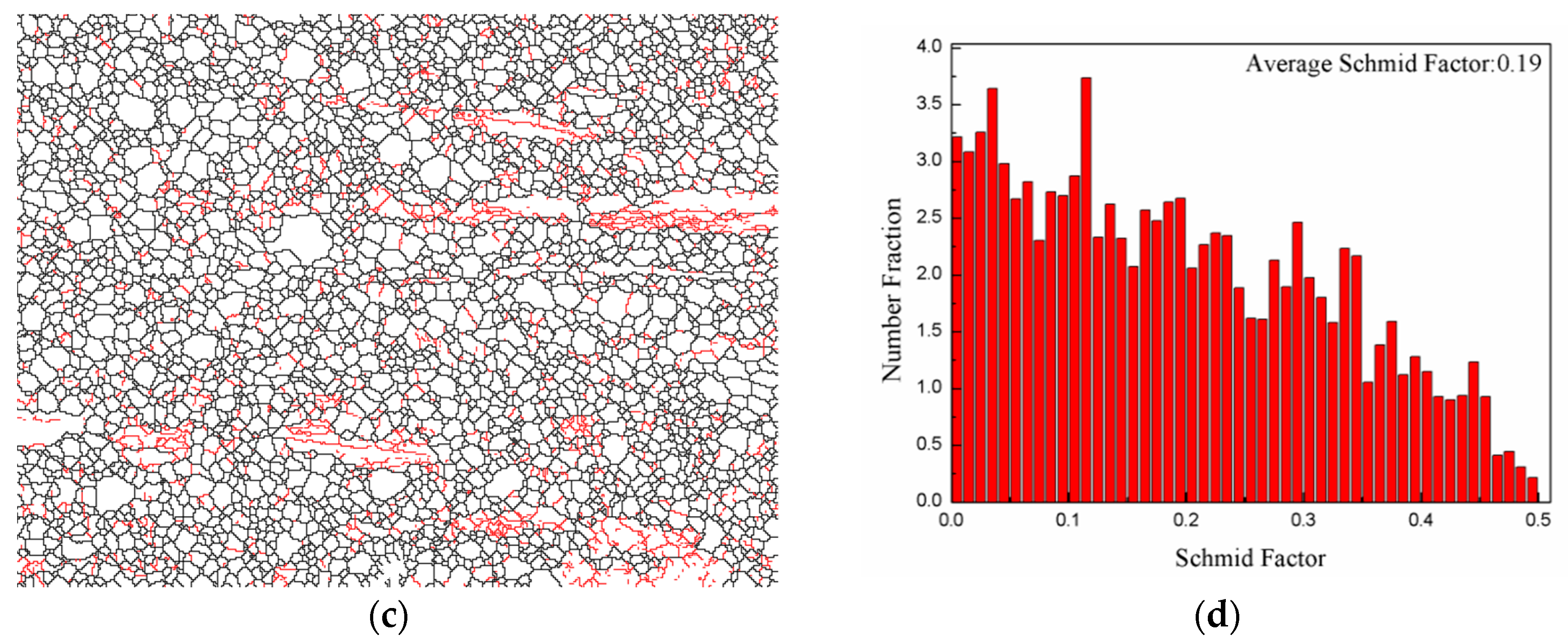
3.3. Texture
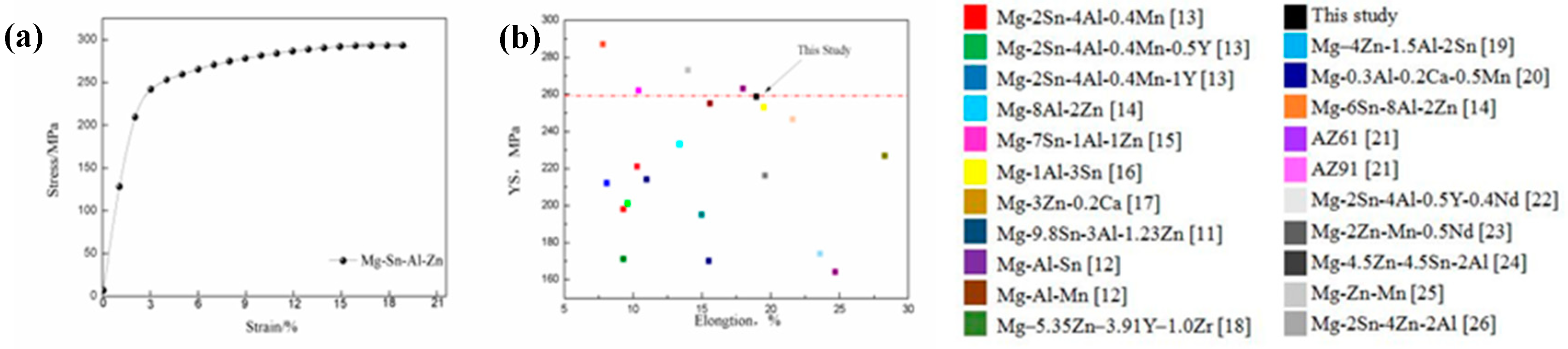
3.4. Tensile Properties
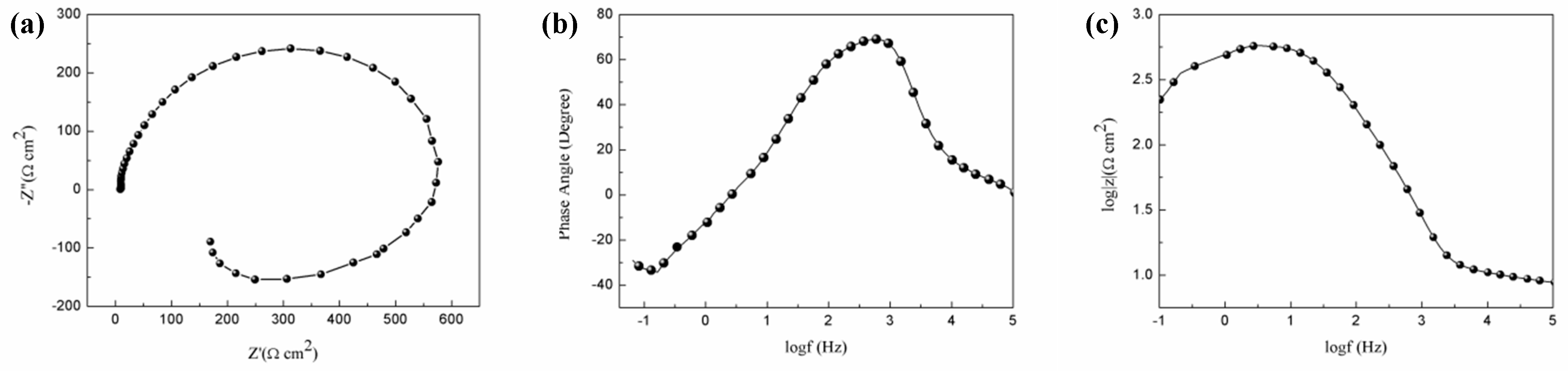
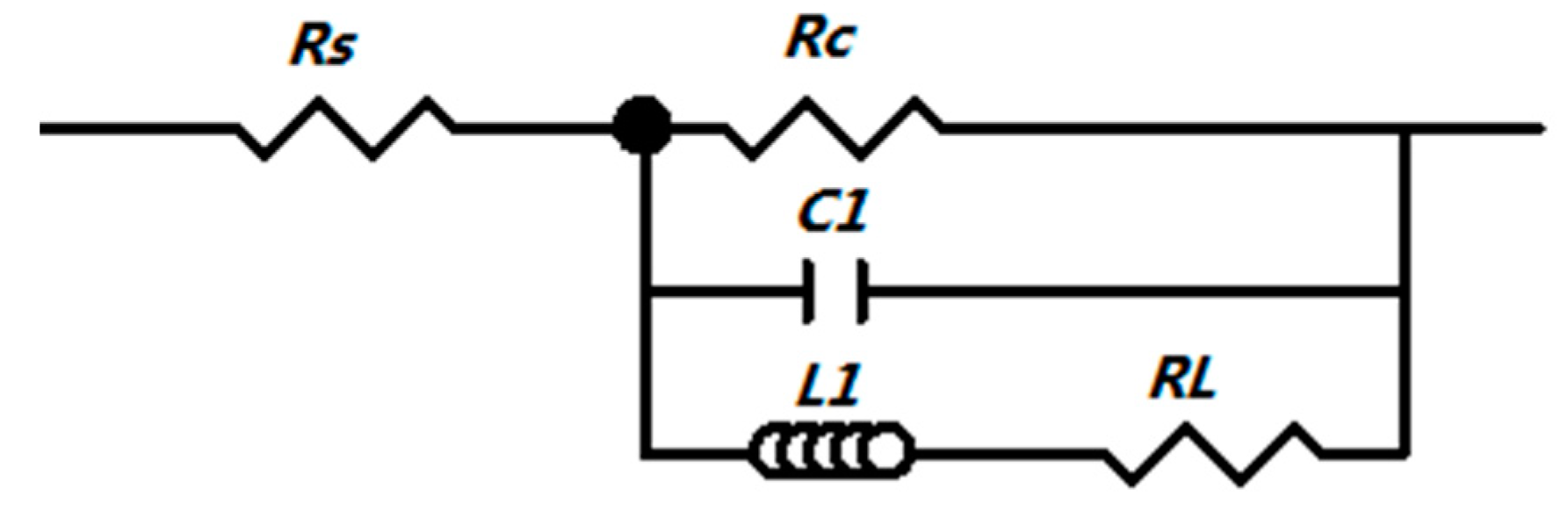
3.5. Corrosion Tests in the Simulated Body Fluid (SBF) Solution
4. Conclusions
- (1)
- The average grain size of homogenized alloy was 121.07 ± 32.15 μm. After extrusion, the alloy exhibited a microstructure consisting of fine dynamically recrystallized (DRXed) grains of ~2.65 μm and coarse unDRXed grains with strong texture.
- (2)
- The extruded alloy showed a high YS of 259 MPa, UTS of 297 MPa, and EL of 19.0%, which was ascribed to the grain boundary, solid solution and texture strengthening as well as a moderate SF value.
- (3)
- The presence of non-basal grains in the extruded alloy accelerate the corrosion process of the present extruded low-alloyed Mg–Sn–Al–Zn alloy. In addition, the corrosion product film mainly consisted of MgO, Mg(OH)2 and MgCl2, which cannot protect the matrix effectively.
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Zhao, C.; Chen, X.; Pan, F.; Gao, S.; Zhao, D.; Liu, X. Effect of Sn content on strain hardening behavior of as-extruded Mg-Sn alloys. Mater. Sci. Eng. A 2018, 713, 244–252. [Google Scholar] [CrossRef]
- Tong, L.B.; Zhang, Q.X.; Jiang, Z.H.; Zhang, J.B.; Meng, J.; Cheng, L.R.; Zhang, H.J. Microstructures, mechanical properties and corrosion resistances of extruded Mg–Zn–Ca–xCe/La alloys. J. Mech. Behav. Biomed. Mater. 2016, 62, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.Y.; Kang, J.Y.; Yang, J.; Yim, C.D.; You, B.S. Role of Sn in corrosion and passive behavior of extruded Mg-5wt%Sn alloy. Corros. Sci. 2016, 102, 355–362. [Google Scholar] [CrossRef]
- Feng, H.; Liu, S.H.; Du, Y.; Lei, T.; Zeng, R.C.; Yuan, T.C. Effect of the second phases on the corrosion behavior of the Mg–Al–Zn alloys. J. Alloys Compd. 2017, 695, 2330–2338. [Google Scholar] [CrossRef]
- Zeng, R.C.; Qi, W.C.; Cui, H.Z.; Zhang, F.; Li, S.Q.; Han, E.H. In vitro corrosion of as-extruded Mg-Ca alloys—The influence of Ca concentration. Corros. Sci. 2015, 96, 23–31. [Google Scholar] [CrossRef]
- Nakata, T.; Xu, C.; Matsumoto, Y.; Shimizu, K.; Sasaki, T.T.; Hono, K.; Kamado, S. Optimization of Mn content for high strengths in high-speed extruded Mg–0.3Al–0.3Ca (wt %) dilute alloy. Mater. Sci. Eng. A 2016, 673, 443–449. [Google Scholar] [CrossRef]
- Jiang, M.G.; Xu, C.; Nakata, T.; Yan, H.; Chen, R.S.; Kamado, S. Development of dilute Mg–Zn–Ca–Mn alloy with high performance via extrusion. J. Alloys Compd. 2016, 668, 13–21. [Google Scholar] [CrossRef]
- Zeng, Z.R.; Bian, M.Z.; Xu, S.W.; Davies, C.H.J.; Birbilis, N.; Nie, J.F. Texture evolution during cold rolling of dilute Mg alloys. J. Alloys Compd. 2017, 695, 1166–1174. [Google Scholar] [CrossRef]
- Cheng, W.L.; Ma, S.C.; Bai, Y.; Cui, Z.Q.; Wang, H.X. Corrosion behavior of Mg–6Bi–2Sn alloy in the simulated body fluid solution: The influence of microstructural characteristics. J. Alloys Compd. 2018, 731, 945–954. [Google Scholar] [CrossRef]
- Yang, B.; Cheng, W.L.; Ma, S.C.; Zhang, J.; Guo, C.; Zhang, Y. Influence of initial microstructure on the strengthening effect of extruded Mg–8Sn–4Zn–2Al alloys. Acta Metall. Sin. 2018, 31, 487–495. [Google Scholar]
- Elsayed, F.R.; Sasaki, T.T.; Ohkubo, T.; Takahashi, H.; Xu, S.W.; Kamado, S.; Hono, K. Effect of extrusion conditions on microstructure and mechanical properties of micro alloyed Mg–Sn–Al–Zn alloys. J. Mater. Sci. Eng. A 2013, 588, 318–328. [Google Scholar] [CrossRef]
- She, J.; Pan, F.; Zhang, J.; Tang, A.; Luo, S.; Yu, Z.; Song, K.; Rashad, M. Microstructure and mechanical properties of Mg–Al–Sn extruded alloys. J. Alloys Compd. 2016, 657, 893–905. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, C.; Meng, W.; Pan, F.; Zhou, J. Microstructure, mechanical and corrosion properties of Mg–4Al–2Sn–xY–0.4Mn alloys. J. Alloys Compd. 2017, 727, 491–500. [Google Scholar] [CrossRef]
- Park, S.H.; Jung, J.G.; Yoon, J.; You, B.S. Influence of Sn addition on the microstructure and mechanical properties of extruded Mg–8Al–2Zn alloy. Mater. Sci. Eng. A 2015, 626, 128–135. [Google Scholar] [CrossRef]
- Park, S.H.; You, B.S. Effect of homogenization temperature on the microstructure and mechanical properties of extruded Mg–7Sn–1Al–1Zn alloy. J. Alloys Compd. 2015, 637, 332–338. [Google Scholar] [CrossRef]
- Chen, Y.; Jin, L.; Song, Y.; Liu, H.; Ye, R. Effect of Zn on microstructure and mechanical property of Mg–3Sn–1Al alloys. Mater. Sci. Eng. A 2014, 612, 96–101. [Google Scholar] [CrossRef]
- Li, C.J.; Sun, H.F.; Li, X.W.; Zhang, J.L.; Fang, W.B.; Tan, Z.Y. Microstructure, texture and mechanical properties of Mg–3.0Zn–0.2Ca alloys fabricated by extrusion at various temperatures. J. Alloys Compd. 2015, 652, 122–131. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, X.; Wang, Z.; Le, Q.; Hu, W.; Bao, L.; Cui, J. Effects of phase composition and content on the microstructures and mechanical properties of high strength Mg–Y–Zn–Zr alloys. Mater. Des. 2015, 88, 915–923. [Google Scholar] [CrossRef]
- Wang, B.; Pan, F.; Chen, X.; Guo, W.; Mao, J. Microstructure and mechanical properties of as-extruded and as-aged Mg–Zn–Al–Sn alloys. Mater. Sci. Eng. A 2016, 656, 165–173. [Google Scholar] [CrossRef]
- Nakata, T.; Mezaki, T.; Ajima, R.; Xu, C.; Oh-ishi, K.; Shimizu, K.; Hanaki, S.; Sasaki, T.T.; Hono, K.; Kamado, S. High-speed extrusion of heat-treatable Mg–Al–Ca–Mn dilute alloy. Scr. Mater. 2015, 101, 28–31. [Google Scholar] [CrossRef]
- Kim, S.H.; You, B.S.; Park, S.H. Effect of billet diameter on hot extrusion behavior of Mg–Al–Zn alloys and its influence on microstructure and mechanical properties. J. Alloys Compd. 2017, 690, 417–423. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, Y.; Zhang, X.; Wang, Z. Studies on Extrusion Properties and Its Microstructure and Mechanical Properties of New Mg–Al–Sn–RE Magnesium Alloy. J. Alloys Compd. 2008, 466, 535–542. [Google Scholar]
- Peng, J.; Peng, Y.; Han, W. Effect of extruded temperature on microstructure and mechanical properties of Mg–2Zn–Mn–0.5Nd alloy. J. Mater. Eng. 2015, 43, 23–27. [Google Scholar]
- Chen, J.H.; Tai, H.H.; Yan, H.G.; Tian, X.Y.; Yin, H.; Zhou, X.P.; Ma, Z.J. Effect of extrusion on mechanical properties and corrosion behavior of Mg–Zn–Sn–Al Alloy. J. Hunan Univ. 2015, 42, 42–46. [Google Scholar]
- Yin, D.S.; Zhang, E.L.; Zeng, S.Y. Effcet of extrusion on mechanical properties and corrosion behavior of Mg–Mn–Zn alloy. Trans. Mater. Heat Treat. 2009, 30, 114–118. [Google Scholar]
- Zhao, D.; Zhou, J.; Liu, Y.; Dong, X.; Wang, J.; Yang, Y. Microstructure and mechanical properties of Mg–4Zn–2Al–2Sn alloys extruded at low temperatures. Acta Metall. Sin. 2014, 50, 41–48. [Google Scholar]
- Petch, N.J. The cleavage strength of polycrystals. J. Iron Steel Inst. 1953, 174, 25–28. [Google Scholar]
- Gypen, L.A.; Deruyttere, A. Multi-component intrinsic solid solution softening and hardening. J. Less-Common Met. 1977, 56, 91–101. [Google Scholar] [CrossRef]
- Cheng, W.L.; Tian, Q.W.; Yu, H.; Zhang, H.; You, B.S. Strengthening mechanisms of indirect-extruded Mg–Sn based alloys at room temperature. J. Magnes. Alloys 2014, 2, 299–304. [Google Scholar] [CrossRef]
- Vinogradov, A.; Orlov, D.; Danyuk, A.; Estrin, A. Effect of grain size on the mechanisms of plastic deformation in wrought Mg–Zn–Zr alloy revealed by acoustic emission measurements. Acta Mater. 2013, 61, 2044–2056. [Google Scholar] [CrossRef]
- Bakhsheshi-Rad, H.R.; Abdul-Kadir, M.R.; Idris, M.H.; Farahany, S. Relationship between the corrosion behavior and the thermal characteristics and microstructure of Mg–0.5Ca–xZn alloys. Corros. Sci. 2012, 64, 184–197. [Google Scholar] [CrossRef]
- Song, Y.; Han, E.H.; Dong, K.; Shan, D.Y.; Chang, D.Y.; Bong, S.Y. Microstructure and protection characteristics of the naturally formed oxide films on Mg–xZn alloys. Corros. Sci. 2013, 72, 133–143. [Google Scholar] [CrossRef]
- Wang, X.M.; Zeng, X.Q.; Zhou, Y.; Wu, G.S.; Yao, S.S.; Lai, Y.J. Early oxidation behaviors of Mg–Y alloys at high temperatures. J. Alloys Compd. 2008, 460, 368–374. [Google Scholar] [CrossRef]
- Liu, X.; Shan, D.; Song, Y.; Chen, R.; Han, E. Influences of the quantity of Mg2Sn phase on the corrosion behavior of Mg–7Sn magnesium alloy. Electrochim. Acta 2011, 56, 2582–2590. [Google Scholar] [CrossRef]
- Song, G.L.; Xu, Z.Q. The surface, microstructure and corrosion of magnesium alloy AZ31 sheet. Electrochim. Acta 2010, 55, 4148–4161. [Google Scholar] [CrossRef]
- Hagihara, K.; Okubo, M.; Yamasaki, M.; Nakano, T. Crystal-orientation-dependent corrosion behaviour of single crystals of a pure Mg and Mg–Al and Mg–Cu solid solutions. Corros. Sci. 2016, 109, 68–85. [Google Scholar] [CrossRef]
- Pawar, S.; Slater, T.J.A.; Burnett, T.L.; Zhou, X.; Scamans, G.M.; Fan, Z.; Thompson, G.E.; Withers, P.J. Crystallographic effects on the corrosion of twin roll cast AZ31 Mg alloy sheet. Acta Mater. 2017, 133, 90–99. [Google Scholar] [CrossRef]
- Fu, B.Q.; Liu, W.; Li, Z.L. Calculation of the surface energy of bcc-metals with the empirical electron theory. Appl. Surf. Sci. 2009, 255, 9348–9357. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An investigation of the corrosion mechanisms of WE43 Mg alloy in a modified simulated body fluid solution: The influence of immersion time. Corros. Sci. 2014, 87, 489–503. [Google Scholar] [CrossRef]




| Specimen | Ecorr (Vsce) | icorr (mA/cm2) | βa (mV) | βc (mV) | Pi (mm/a) |
|---|---|---|---|---|---|
| As-extruded | −1.48 | 4.93 × 10−2 | 54.71 | 382.29 | 11.2 |
| As-cast | −1.53 | 5.42 × 10−2 | 167.3 | 228.63 | 12.4 |
| Specimen | RS (Ω cm2) | C1 (F/cm2) | RC (Ω cm2) | RL (Ω cm2) | L1 (H/cm2) | RP (Ω cm2) | |
|---|---|---|---|---|---|---|---|
| C1 | n | ||||||
| As-extruded | 9.741 | 6.818 × 10−6 | 0.995 | 526.5 | 187.7 | 417.1 | 723.94 |
| As-cast | 1.663 | 5.655 × 10−5 | 0.852 | 110.3 | 75.46 | 6.77 | 187.33 |
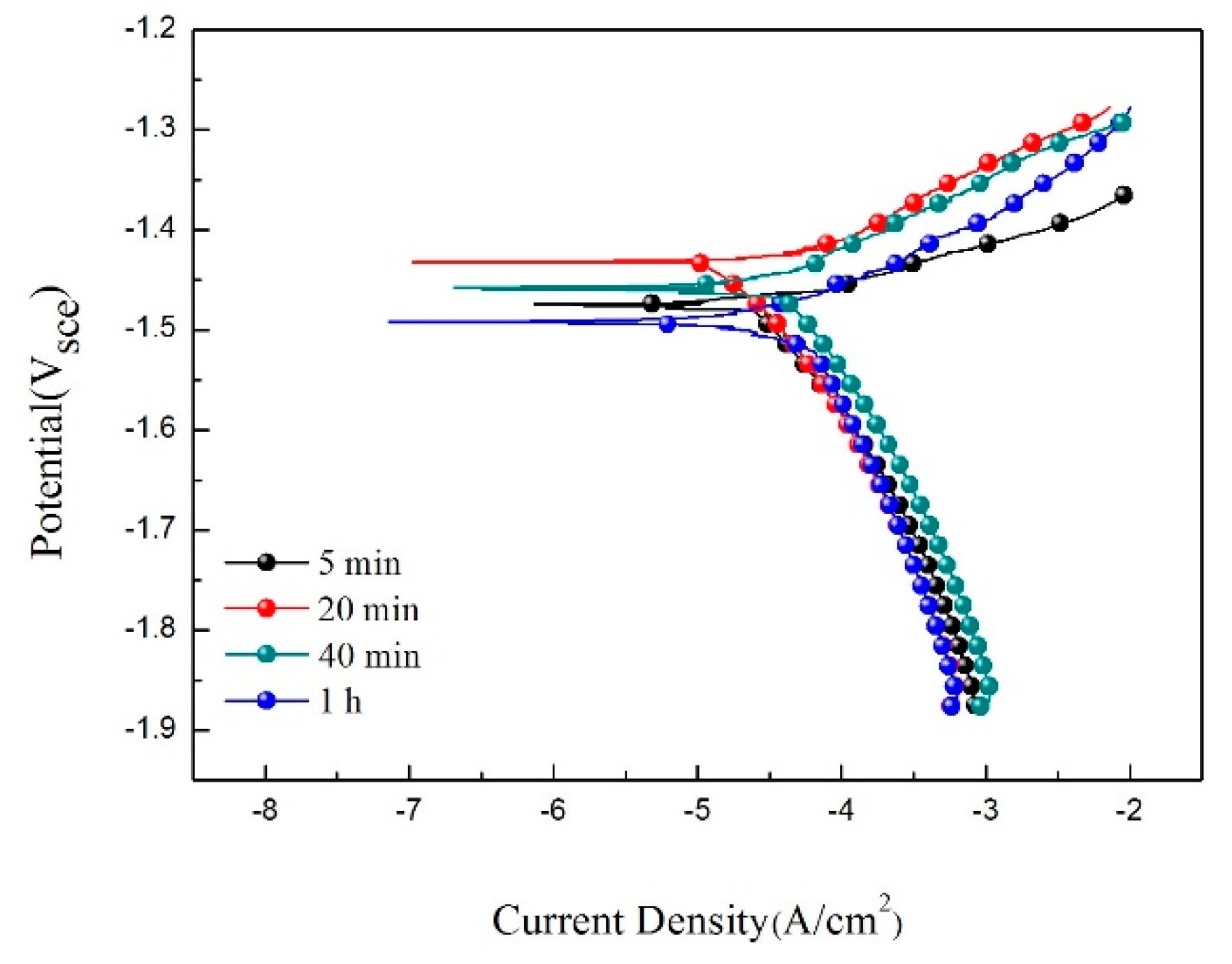
| Specimen | Ecorr (Vsce) | icorr (mA/cm2) | βa (mV) | βc (mV) | Pi (mm/a) |
|---|---|---|---|---|---|
| 5 min | −1.49 | 4.83 × 10−2 | 66.74 | 270.19 | 11.0 |
| 20 min | −1.43 | 2.93 × 10−2 | 62.40 | 348.26 | 6.6 |
| 40 min | −1.46 | 4.12 × 10−2 | 77.62 | 211.71 | 9.4 |
| 1 h | −1.49 | 4.80 × 10−2 | 80.06 | 252.76 | 10.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, W.; Zhang, Y.; Ma, S.; Arthanari, S.; Cui, Z.; Wang, H.-x.; Wang, L. Tensile Properties and Corrosion Behavior of Extruded Low-Alloyed Mg–1Sn–1Al–1Zn Alloy: The Influence of Microstructural Characteristics. Materials 2018, 11, 1157. https://doi.org/10.3390/ma11071157
Cheng W, Zhang Y, Ma S, Arthanari S, Cui Z, Wang H-x, Wang L. Tensile Properties and Corrosion Behavior of Extruded Low-Alloyed Mg–1Sn–1Al–1Zn Alloy: The Influence of Microstructural Characteristics. Materials. 2018; 11(7):1157. https://doi.org/10.3390/ma11071157
Chicago/Turabian StyleCheng, Weili, Yao Zhang, Shichao Ma, Srinivasan Arthanari, Zeqin Cui, Hong-xia Wang, and Lifei Wang. 2018. "Tensile Properties and Corrosion Behavior of Extruded Low-Alloyed Mg–1Sn–1Al–1Zn Alloy: The Influence of Microstructural Characteristics" Materials 11, no. 7: 1157. https://doi.org/10.3390/ma11071157
APA StyleCheng, W., Zhang, Y., Ma, S., Arthanari, S., Cui, Z., Wang, H.-x., & Wang, L. (2018). Tensile Properties and Corrosion Behavior of Extruded Low-Alloyed Mg–1Sn–1Al–1Zn Alloy: The Influence of Microstructural Characteristics. Materials, 11(7), 1157. https://doi.org/10.3390/ma11071157