Osteoblast Cell Response to Naturally Derived Calcium Phosphate-Based Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Ceramic Synthesis
2.2. Characterization of the Synthesized Products
- −
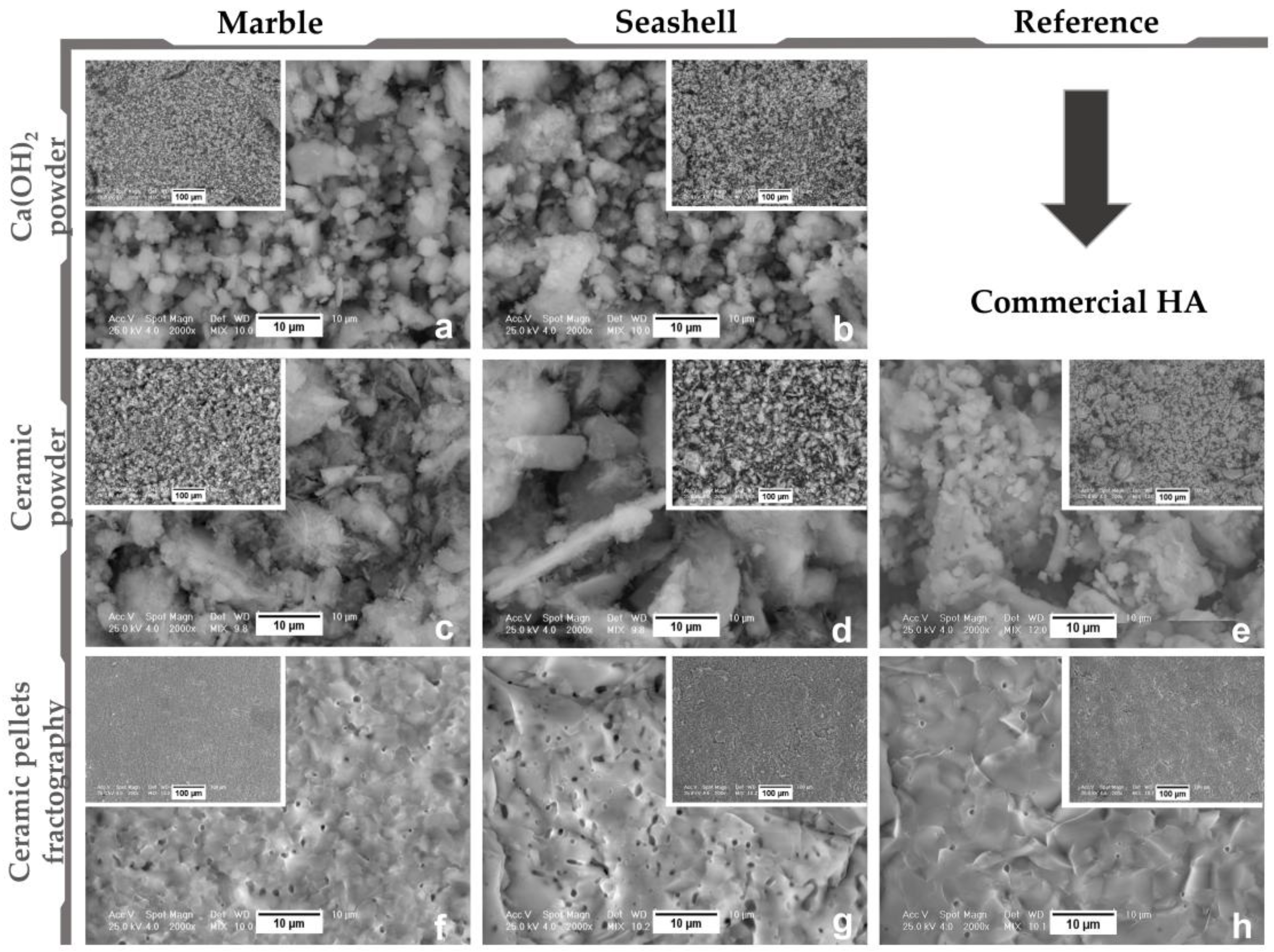
- Morpho-compositional analysis: by Scanning Electron Microscopy—Philips Xl 30 ESEM TMP (FEI/Phillips, Hillsboro, OR, USA) coupled with Energy Dispersive Spectroscopy—EDAX Sapphire spectrometer. An acceleration voltage of 25 kV and working distance of 10 mm were used. The SEM and EDS investigations were conducted on three randomly chosen areas.
- −
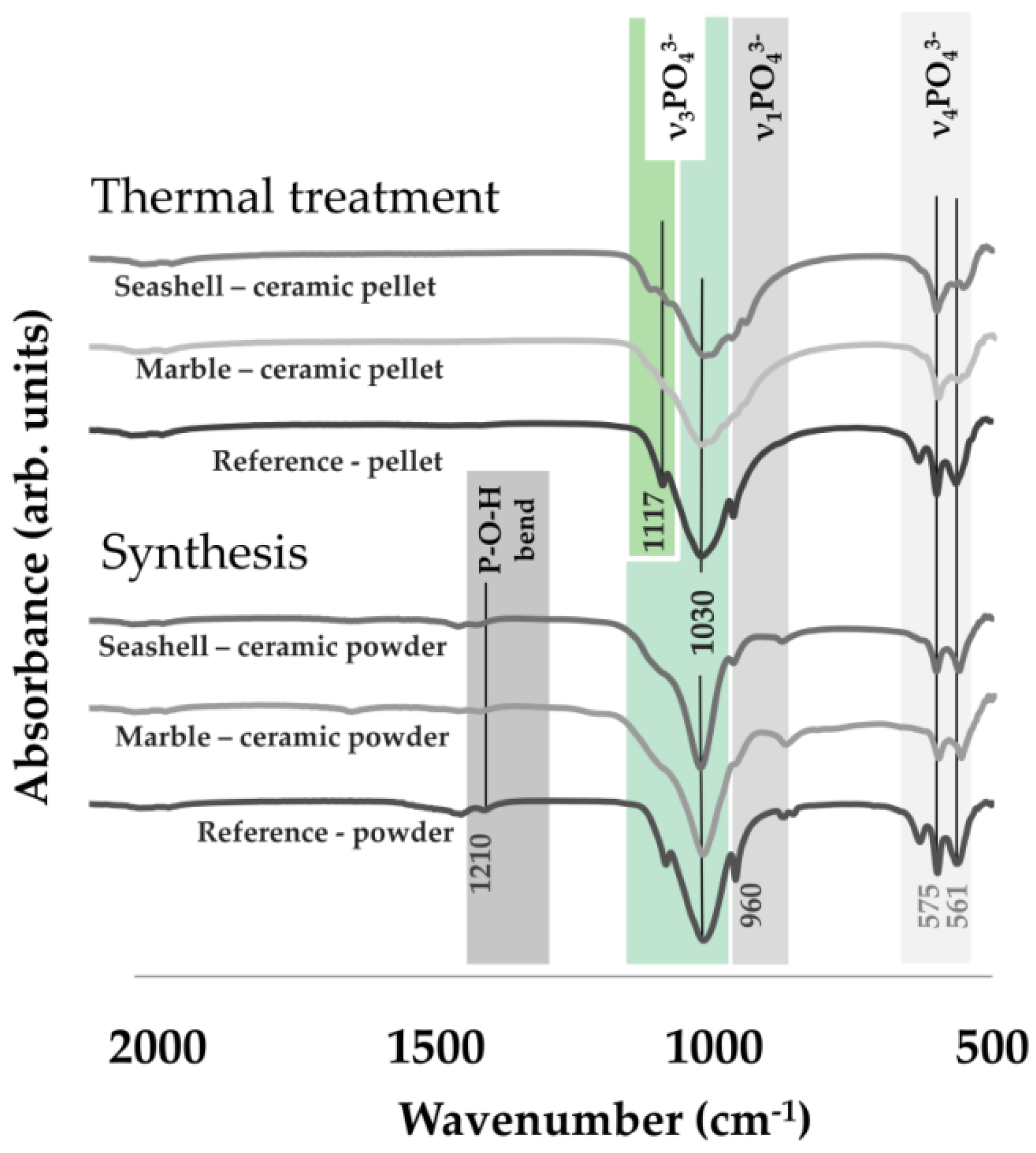
- Structural analyses: by (1) X ray diffraction, XRD—Bruker D8 (Bruker Corporation, Billerica, MA, USA). An Advance diffractometer was used equipped with a LynxEye detector, in Bragg-Brentano geometry, with Cu Kα (λ = 1.5418 Å) radiation with the scattered intensity being scanned in the 2θ range of 10–60°, with a step size of 0.04° and a dwell time of 1 s; and (2) Fourier Transform Infrared Spectroscopy: FT-IR were acquired on a Perkin Elmer Spectrum BX II spectrometer (PerkinElmer, Inc., Waltham, MA, USA), in attenuated total reflectance (ATR) mode (PikeMiracle head); the spectra being recorded over the range 4000–500 cm–1, at a resolution of 4 cm−1 and using 32 scans/experiment.
2.3. Cell Culture Experiments
2.3.1. Assessment of the Cellular Survival and Proliferation
2.3.2. Evaluation of MC3T3-E1 Cell Adhesion and Morphology
2.3.3. Measurement of the Pre-Osteoblast Differentiation
2.4. Statistical Analysis
3. Results and Discussion
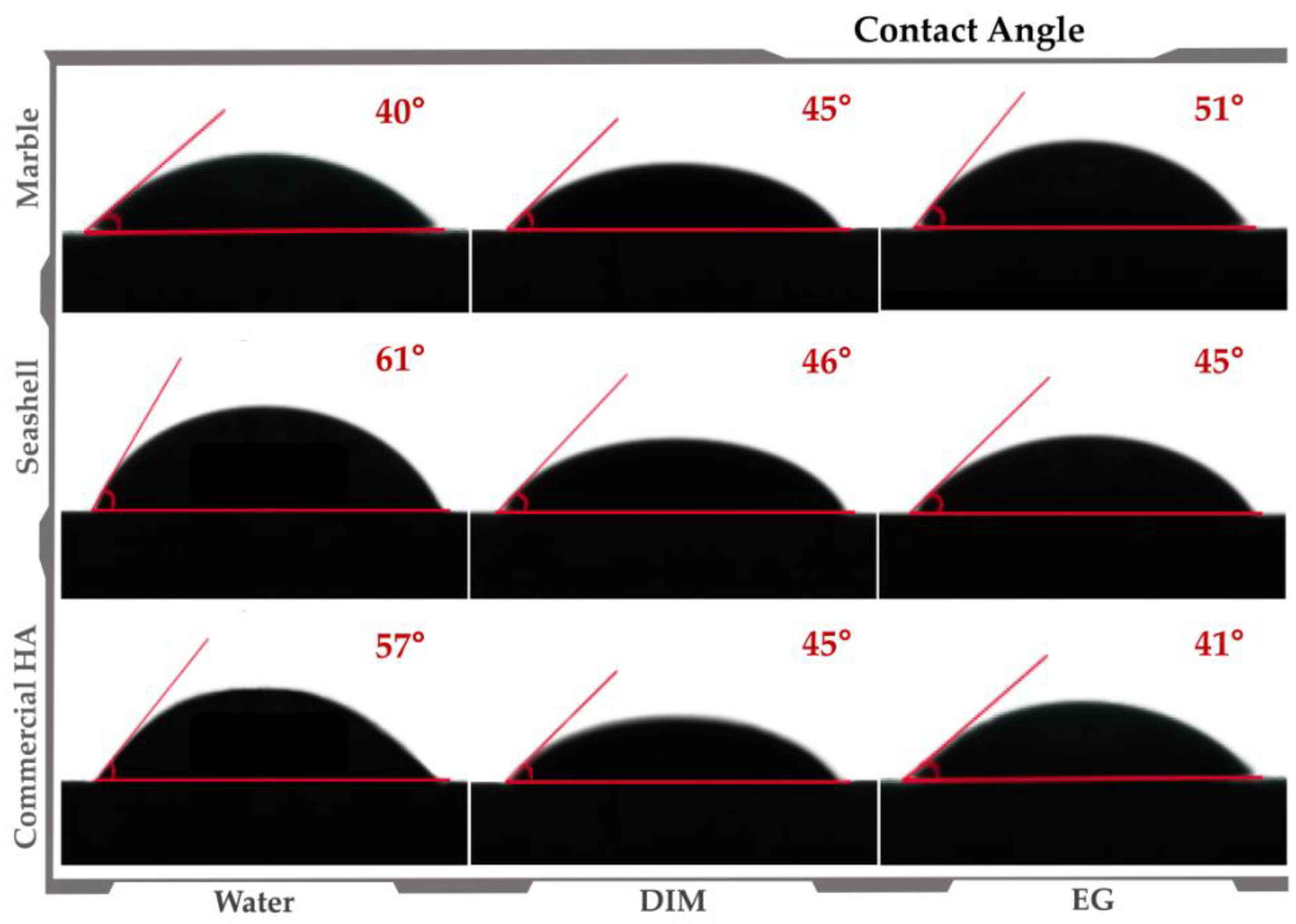
3.1. Materials’ Characterization
3.2. Pre-Osteoblast Cell Response to Developed Ceramics
3.2.1. Cellular Survival and Proliferation
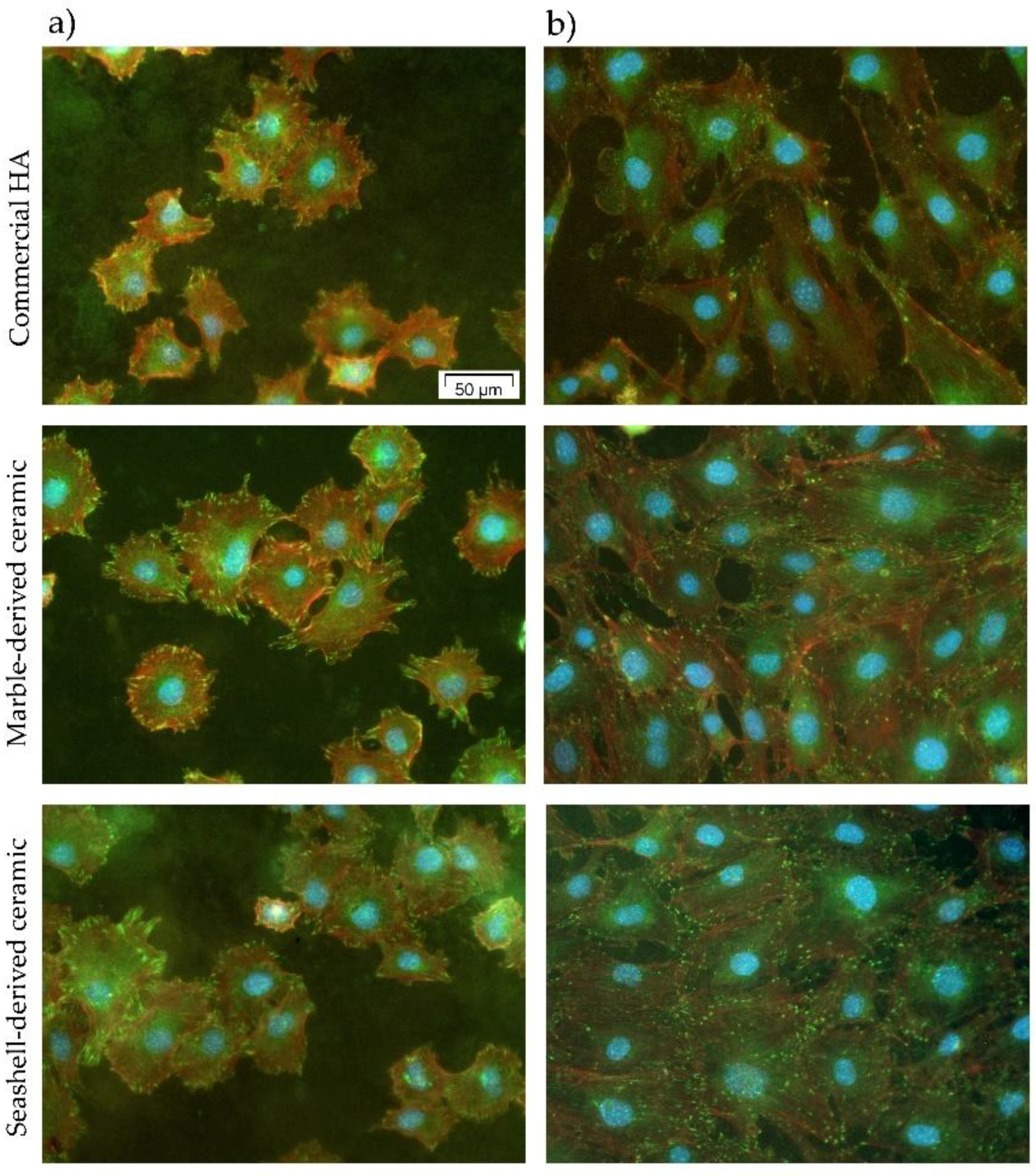
3.2.2. MC3T3-E1 Cell Adhesion and Morphological Features
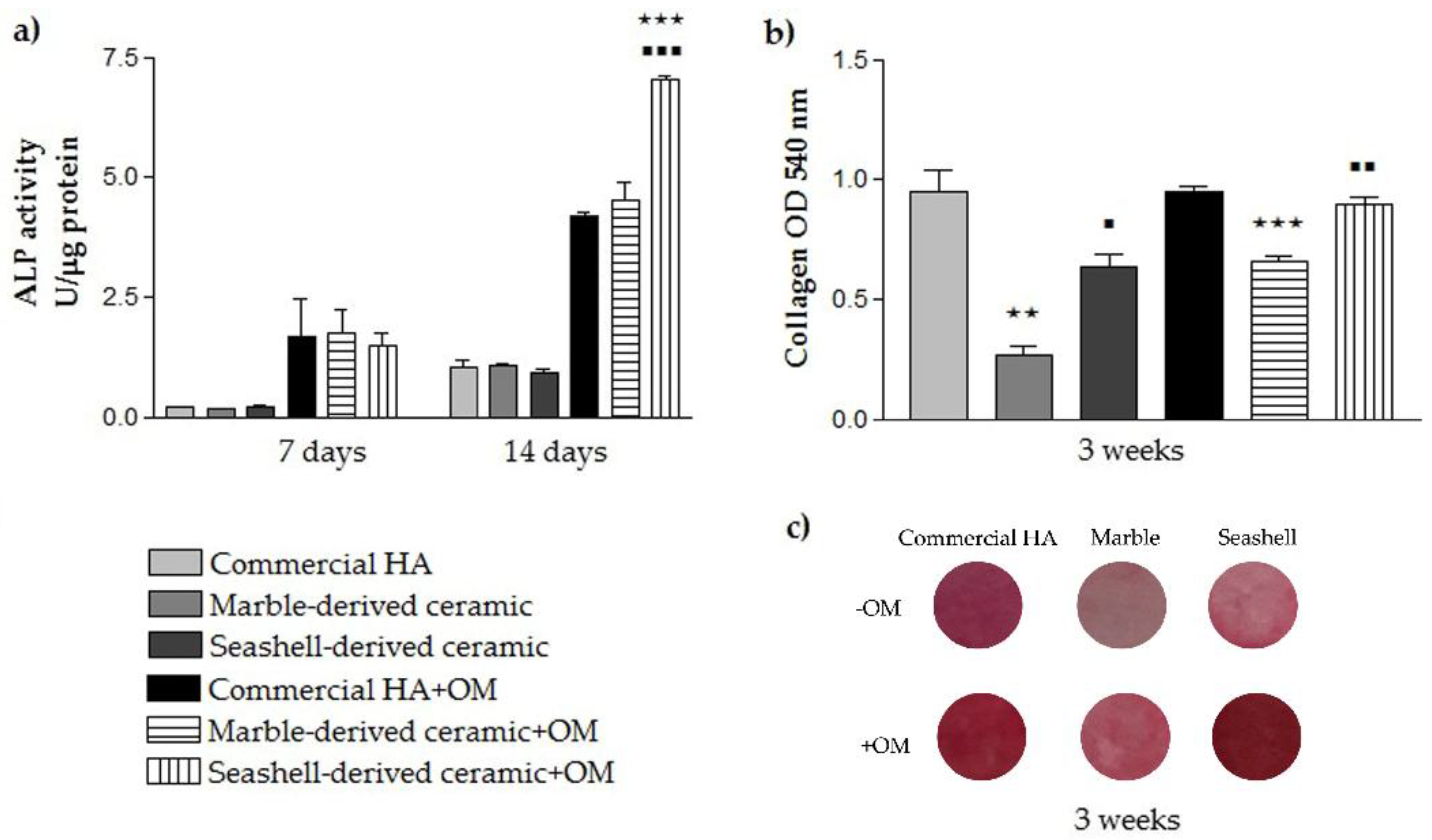
3.2.3. Pre-Osteoblast Differentiation Potential
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Roy, D.M.; Linnehan, S.K. Hydroxyapatite formed from coral skeletal carbonate by hydrothermal exchange. Nature 1974, 247, 220–222. [Google Scholar] [CrossRef] [PubMed]
- Miculescu, F.; Mocanu, A.C.; Maidaniuc, A.; Dascalu, C.A.; Miculescu, M.; Voicu, S.; Ciocoiu, R.C. Biomimetic calcium phosphates derived from marine and land bioresources. In Hydroxyapatite—Advances in Composite Nanomaterials, Biomedical Applications and Its Technological Facets; InTechOpen: London, UK, 2018; pp. 89–108. [Google Scholar]
- Miculescu, F.; Mocanu, A.C.; Stan, G.E.; Miculescu, M.; Maidaniuc, A.; Cîmpean, A.; Mitran, V.; Voicu, S.I.; Machedon-Pisu, T.; Ciocan, L.T. Influence of the modulated two-step synthesis of biogenic hydroxyapatite on biomimetic products’ surface. Appl. Surf. Sci. 2017, 438, 147–157. [Google Scholar] [CrossRef]
- Pujiyanto, E.; Widyo Laksono, P.; Triyono, J. Synthesis and characterization of hydroxyapatite powder from natural gypsum rock. Adv. Mater. Res. 2014, 893, 56–59. [Google Scholar] [CrossRef]
- Wu, S.-C.; Hsu, H.-C.; Wu, Y.-N.; Ho, W.-F. Hydroxyapatite synthesized from oyster shell powders by ball milling and heat treatment. Mater. Charact. 2011, 62, 1180–1187. [Google Scholar] [CrossRef]
- Miculescu, F.; Stan, G.; Ciocan, L.; Miculescu, M.; Berbecaru, A.; Antoniac, I. Cortical bone as resource for producing biomimetic materials for clinical use. Dig. J. Nanomater. Biostruct. 2012, 7, 1667–1677. [Google Scholar]
- Pal, A.; Maity, S.; Chabri, S.; Bera, S.; Chowdhury, A.R.; Das, M.; Sinha, A. Mechanochemical synthesis of nanocrystalline hydroxyapatite from mercenaria clam shells and phosphoric acid. Biomed. Phys. Eng. Express 2017, 3. [Google Scholar] [CrossRef]
- Bouler, J.; Pilet, P.; Gauthier, O.; Verron, E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017, 53, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Denry, I.; Kuhn, L.T. Design and characterization of calcium phosphate ceramic scaffolds for bone tissue engineering. Dental Mater. 2016, 32, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhao, L.; Liu, J.; Weir, M.D.; Zhou, X.; Xu, H.H. Bone tissue engineering via nanostructured calcium phosphate biomaterials and stem cells. Bone Res. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Sadowska, J.-M.; Guillem-Marti, J.; Montufar, E.B.; Espanol, M.; Ginebra, M.-P. Biomimetic versus sintered calcium phosphates: The in vitro behavior of osteoblasts and mesenchymal stem cells. Tissue Eng. Part A 2017, 23, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Joschek, S.; Nies, B.; Krotz, R.; Göpferich, A. Chemical and physicochemical characterization of porous hydroxyapatite ceramics made of natural bone. Biomaterials 2000, 21, 1645–1658. [Google Scholar] [CrossRef]
- Cozza, N.; Monte, F.; Bonani, W.; Aswath, P.; Motta, A.; Migliaresi, C. Bioactivity and mineralization of natural hydroxyapatite from cuttlefish bone and bioglass® co-sintered bioceramics. J. Tissue Eng. Regen. Med. 2018, 12, e1131–e1142. [Google Scholar] [CrossRef] [PubMed]
- Akram, M.; Ahmed, R.; Shakir, I.; Ibrahim, W.A.W.; Hussain, R. Extracting hydroxyapatite and its precursors from natural resources. J. Mater. Sci. 2014, 49, 1461–1475. [Google Scholar] [CrossRef]
- Abdulrahman, I.; Tijani, H.I.; Mohammed, B.A.; Saidu, H.; Yusuf, H.; Ndejiko Jibrin, M.; Mohammed, S. From garbage to biomaterials: An overview on egg shell based hydroxyapatite. J. Mater. 2014. [Google Scholar] [CrossRef]
- Łukaszewska-Kuska, M.; Krawczyk, P.; Martyla, A.; Hędzelek, W.; Dorocka-Bobkowska, B. Hydroxyapatite coating on titanium endosseous implants for improved osseointegration: Physical and chemical considerations. Adv. Clin. Exp. Med. 2018. [Google Scholar] [CrossRef]
- Ohe, M.; Moridaira, H.; Inami, S.; Takeuchi, D.; Nohara, Y.; Taneichi, H. Pedicle screws with a thin hydroxyapatite coating for improving fixation at the bone-implant interface in the osteoporotic spine: Experimental study in a porcine model. J. Neurosurg. Spine 2018, 28, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A. Bone repair in the twenty-first century: Biology, chemistry or engineering? Philos. Trans. Ser. A Math. Phys. Eng. Sci. 2004, 362, 2821–2850. [Google Scholar] [CrossRef] [PubMed]
- Hong, Z.; Zhang, P.; He, C.; Qiu, X.; Liu, A.; Chen, L.; Chen, X.; Jing, X. Nano-composite of poly (l-lactide) and surface grafted hydroxyapatite: Mechanical properties and biocompatibility. Biomaterials 2005, 26, 6296–6304. [Google Scholar] [CrossRef] [PubMed]
- Janus, A.M.; Faryna, M.; Haberko, K.; Rakowska, A.; Panz, T. Chemical and microstructural characterization of natural hydroxyapatite derived from pig bones. Microchim. Acta 2008, 161, 349–353. [Google Scholar] [CrossRef]
- Swetha, M.; Sahithi, K.; Moorthi, A.; Srinivasan, N.; Ramasamy, K.; Selvamurugan, N. Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int. J. Biol. Macromol. 2010, 47, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, H.; Myoui, A. Bone tissue engineering with porous hydroxyapatite ceramics. J. Artif. Organs 2005, 8, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Kubasiewicz-Ross, P.; Hadzik, J.; Seeliger, J.; Kozak, K.; Jurczyszyn, K.; Gerber, H.; Dominiak, M.; Kunert-Keil, C. New nano-hydroxyapatite in bone defect regeneration: A histological study in rats. Ann. Anat. 2017, 213, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, K.S.; Zhang, X.; Massie, J.B.; Wang, M.; Kim, C.W. Conversion of bulk seashells to biocompatible hydroxyapatite for bone implants. Acta Biomater. 2007, 3, 910–918. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, G.; Basu, B. A porous hydroxyapatite scaffold for bone tissue engineering: Physico-mechanical and biological evaluations. Ceram. Int. 2012, 38, 341–349. [Google Scholar] [CrossRef]
- Rathje, W. Zur kenntnis der phosphate i: Über hydroxylapatit. Bodenkd. Pflanzenernähr. 1939, 12, 121–128. [Google Scholar] [CrossRef]
- Miculescu, F.; Mocanu, A.-C.; Dascălu, C.A.; Maidaniuc, A.; Batalu, D.; Berbecaru, A.; Voicu, S.I.; Miculescu, M.; Thakur, V.K.; Ciocan, L.T. Facile synthesis and characterization of hydroxyapatite particles for high value nanocomposites and biomaterials. Vacuum 2017, 146, 614–622. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, F.; Voicu, S.I.; Andronescu, C.; Miculescu, M.; Matei, E.; Mocanu, A.C.; Pencea, I.; Csaki, I.; Machedon-Pisu, T. Induced wettability and surface-volume correlation of composition for bovine bone derived hydroxyapatite particles. Appl. Surf. Sci. 2017, 438, 158–166. [Google Scholar] [CrossRef]
- Maidaniuc, A.; Miculescu, M.; Voicu, S.; Ciocan, L.; Niculescu, M.; Corobea, M.; Rada, M.; Miculescu, F. Effect of micron sized silver particles concentration on the adhesion induced by sintering and antibacterial properties of hydroxyapatite microcomposites. J. Adhes. Sci. Technol. 2016, 30, 1829–1841. [Google Scholar] [CrossRef]
- Miculescu, F.; Maidaniuc, A.; Voicu, S.I.; Thakur, V.K.; Stan, G.E.; Ciocan, L. Progress in hydroxyapatite-starch based sustainable biomaterials for biomedical bone substitution applications. ACS Sustain. Chem. Eng. 2017, 5, 8491–8512. [Google Scholar] [CrossRef]
- Neacsu, P.; Staras, A.I.; Voicu, S.I.; Ionascu, I.; Soare, T.; Uzun, S.; Cojocaru, V.D.; Pandele, A.M.; Croitoru, S.M.; Miculescu, F. Characterization and in vitro and in vivo assessment of a novel cellulose acetate-coated mg-based alloy for orthopedic applications. Materials 2017, 10, 686. [Google Scholar] [CrossRef] [PubMed]
- Gordin, D.; Ion, R.; Vasilescu, C.; Drob, S.; Cimpean, A.; Gloriant, T. Potentiality of the “gum metal” titanium-based alloy for biomedical applications. Mater. Sci. Eng. C 2014, 44, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Hummert, T.W.; Dean, D.D.; Schwartz, Z. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 1996, 17, 137–146. [Google Scholar] [CrossRef]
- Quarles, L.D.; Yohay, D.A.; Lever, L.W.; Caton, R.; Wenstrup, R.J. Distinct proliferative and differentiated stages of murine mc3t3-e1 cells in culture: An in vitro model of osteoblast development. J. Bone Miner. Res. 1992, 7, 683–692. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, T.; Kawakami, M.; Kuribayashi, K.; Takenaka, T.; Minamide, A.; Tamaki, T. Effects of sintered bovine bone on cell proliferation, collagen synthesis, and osteoblastic expression in mc3t3-e1 osteoblast-like cells. J. Oorthop. Res. 1999, 17, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Aktuğ, S.L.; Durdu, S.; Yalçın, E.; Çavuşoğlu, K.; Usta, M. Bioactivity and biocompatibility of hydroxyapatite-based bioceramic coatings on zirconium by plasma electrolytic oxidation. Mater. Sci. Eng. C 2017, 71, 1020–1027. [Google Scholar] [CrossRef] [PubMed]
- Jarcho, M. Calcium phosphate ceramics as hard tissue prosthetics. Clin. Orthop. Relat. Res. 1981, 157, 259–278. [Google Scholar] [CrossRef]
- Jaramillo, C.D.; Rivera, J.A.; Echavarría, A.; O’byrne, J.; Congote, D.; Restrepo, L.F. Osteoconductive and osseointegration properties of a commercial hydroxyapatite compared to a synthetic product. Rev. Colomb. Cienc. Pecu. 2010, 23, 471–483. [Google Scholar]
- Do Prado Ribeiro, D.C.; de Abreu Figueira, L.; Mardegan Issa, J.P.; Dias Vecina, C.A.; JosÉDias, F.; Da Cunha, M.R. Study of the osteoconductive capacity of hydroxyapatite implanted into the femur of ovariectomized rats. Microsc. Res. Tech. 2012, 75, 133–137. [Google Scholar] [CrossRef] [PubMed]
- Denissen, H.; De Groot, K.; Makkes, P.C.; Van den Hooff, A.; Klopper, P. Tissue response to dense apatite implants in rats. J. Biomed. Mater. Res. Part A 1980, 14, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Carisey, A.; Ballestrem, C. Vinculin, an adapter protein in control of cell adhesion signalling. Eur. J. Cell Biol. 2011, 90, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K. Osteoblast adhesion on biomaterials. Biomaterials 2000, 21, 667–681. [Google Scholar] [CrossRef]
- Wozniak, M.A.; Modzelewska, K.; Kwong, L.; Keely, P.J. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta 2004, 1692, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Park, M.; Kim, H.; Lim, Y.; Chun, H.; Kim, H.; Moon, S. Ceramic bioactivity: Progresses, challenges and perspectives. Biomed. Mater. 2006, 1. [Google Scholar] [CrossRef] [PubMed]
- Boyan, B.D.; Lohmann, C.H.; Dean, D.D.; Sylvia, V.L.; Cochran, D.L.; Schwartz, Z. Mechanisms involved in osteoblast response to implant surface morphology. Annu. Rev. Mater. Res. 2001, 31, 357–371. [Google Scholar] [CrossRef]
- Annaz, B.; Hing, K.; Kayser, M.; Buckland, T.; Di Silvio, L. An ultrastructural study of cellular response to variation in porosity in phase-pure hydroxyapatite. J. Microsc. 2004, 216, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Arima, Y.; Iwata, H. Effect of wettability and surface functional groups on protein adsorption and cell adhesion using well-defined mixed self-assembled monolayers. Biomaterials 2007, 28, 3074–3082. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Ha, H.J.; Ko, Y.K.; Yoon, S.J.; Rhee, J.M.; Kim, M.S.; Lee, H.B.; Khang, G. Correlation of proliferation, morphology and biological responses of fibroblasts on LDPE with different surface wettability. J. Biomater. Sci. Polym. Ed. 2007, 18, 609–622. [Google Scholar] [CrossRef] [PubMed]



| Sample Type | O (at. %) | Mg (at. %) | P (at. %) | Ca (at. %) | Ca/P ratio | Compressive Strength (N/mm2) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) | (B) | (A) | (B) | (A) | (B) | (A) | (B) | (A) | (B) | ||
| Marble | 55.10 | 54.04 | 0.58 | 1.28 | 17.71 | 16.65 | 26.61 | 26.64 | 1.50 | 1.60 | 2.37 |
| Seashell | 60.76 | 55.99 | - | - | 15.51 | 15.81 | 23.73 | 25.61 | 1.53 | 1.62 | 4.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitran, V.; Ion, R.; Miculescu, F.; Necula, M.G.; Mocanu, A.-C.; Stan, G.E.; Antoniac, I.V.; Cimpean, A. Osteoblast Cell Response to Naturally Derived Calcium Phosphate-Based Materials. Materials 2018, 11, 1097. https://doi.org/10.3390/ma11071097
Mitran V, Ion R, Miculescu F, Necula MG, Mocanu A-C, Stan GE, Antoniac IV, Cimpean A. Osteoblast Cell Response to Naturally Derived Calcium Phosphate-Based Materials. Materials. 2018; 11(7):1097. https://doi.org/10.3390/ma11071097
Chicago/Turabian StyleMitran, Valentina, Raluca Ion, Florin Miculescu, Madalina Georgiana Necula, Aura-Catalina Mocanu, George E. Stan, Iulian Vasile Antoniac, and Anisoara Cimpean. 2018. "Osteoblast Cell Response to Naturally Derived Calcium Phosphate-Based Materials" Materials 11, no. 7: 1097. https://doi.org/10.3390/ma11071097
APA StyleMitran, V., Ion, R., Miculescu, F., Necula, M. G., Mocanu, A.-C., Stan, G. E., Antoniac, I. V., & Cimpean, A. (2018). Osteoblast Cell Response to Naturally Derived Calcium Phosphate-Based Materials. Materials, 11(7), 1097. https://doi.org/10.3390/ma11071097










