Titanium Oxide (TiO2)/Polymethylmethacrylate (PMMA) Denture Base Nanocomposites: Mechanical, Viscoelastic and Antibacterial Behavior
Abstract
1. Introduction
2. Materials and Methods
Antibacterial Adhesion Activity
3. Results and Discussions
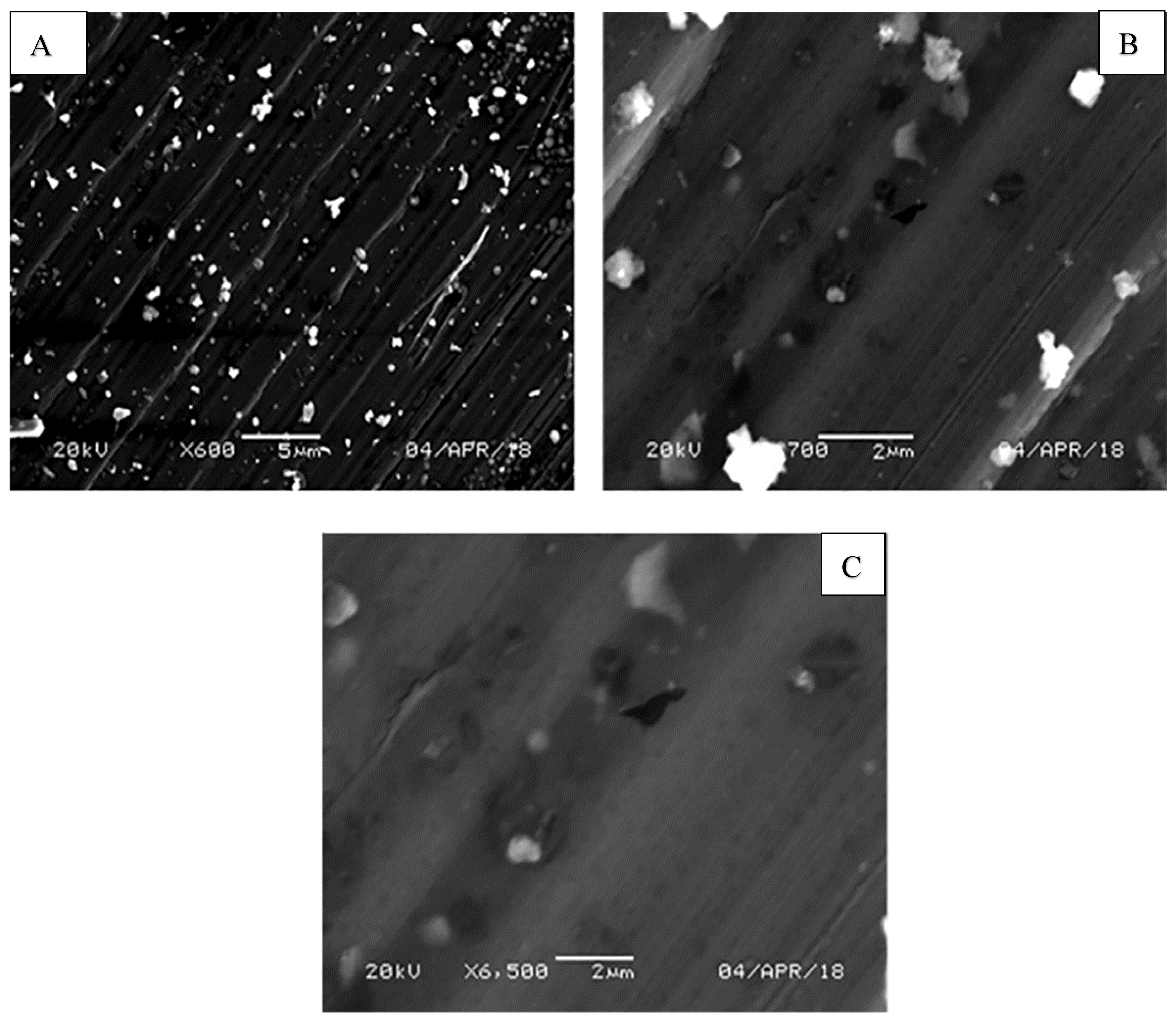
3.1. Fracture Surface Morphology
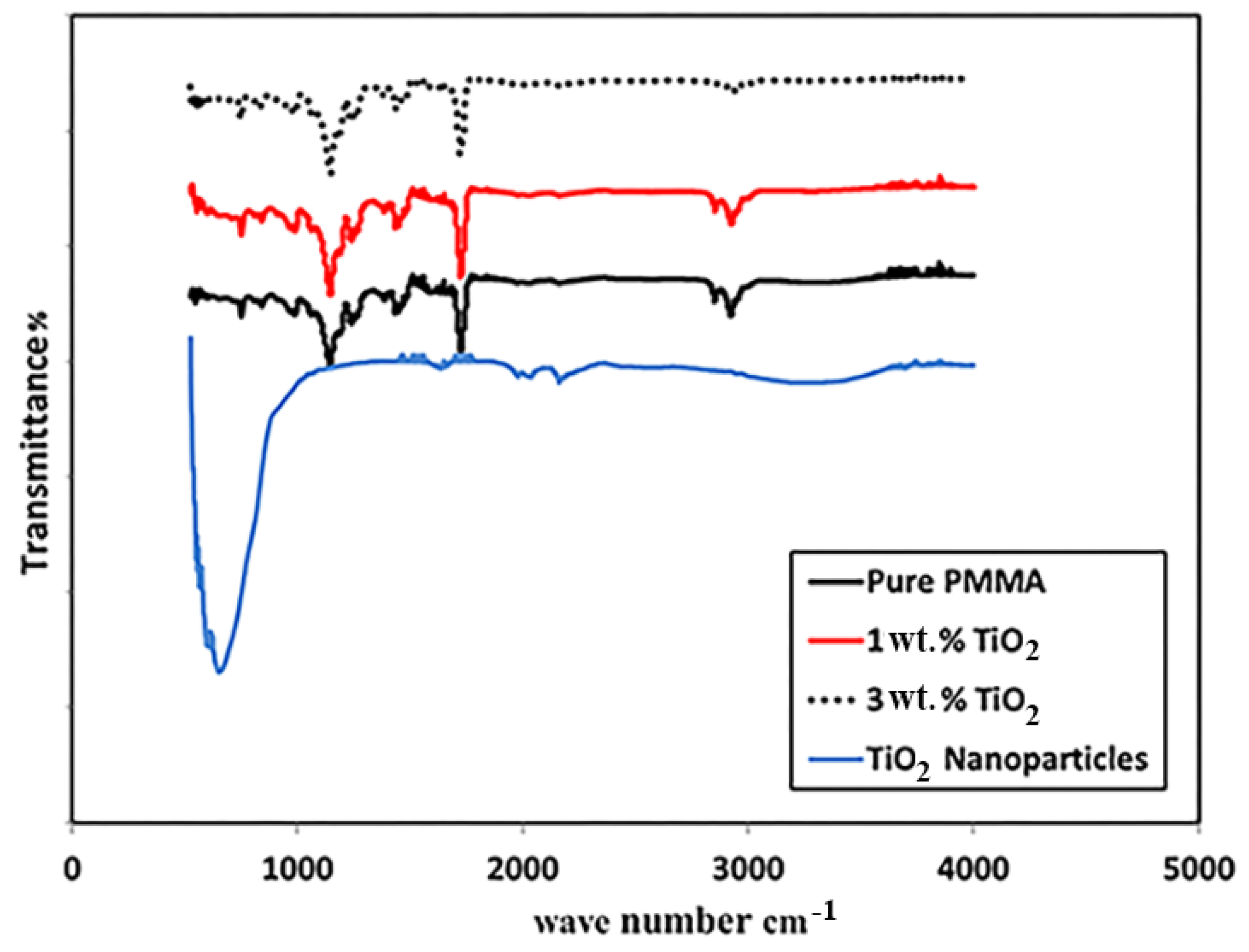
3.2. Fourier Transform Infrared Spectroscopy
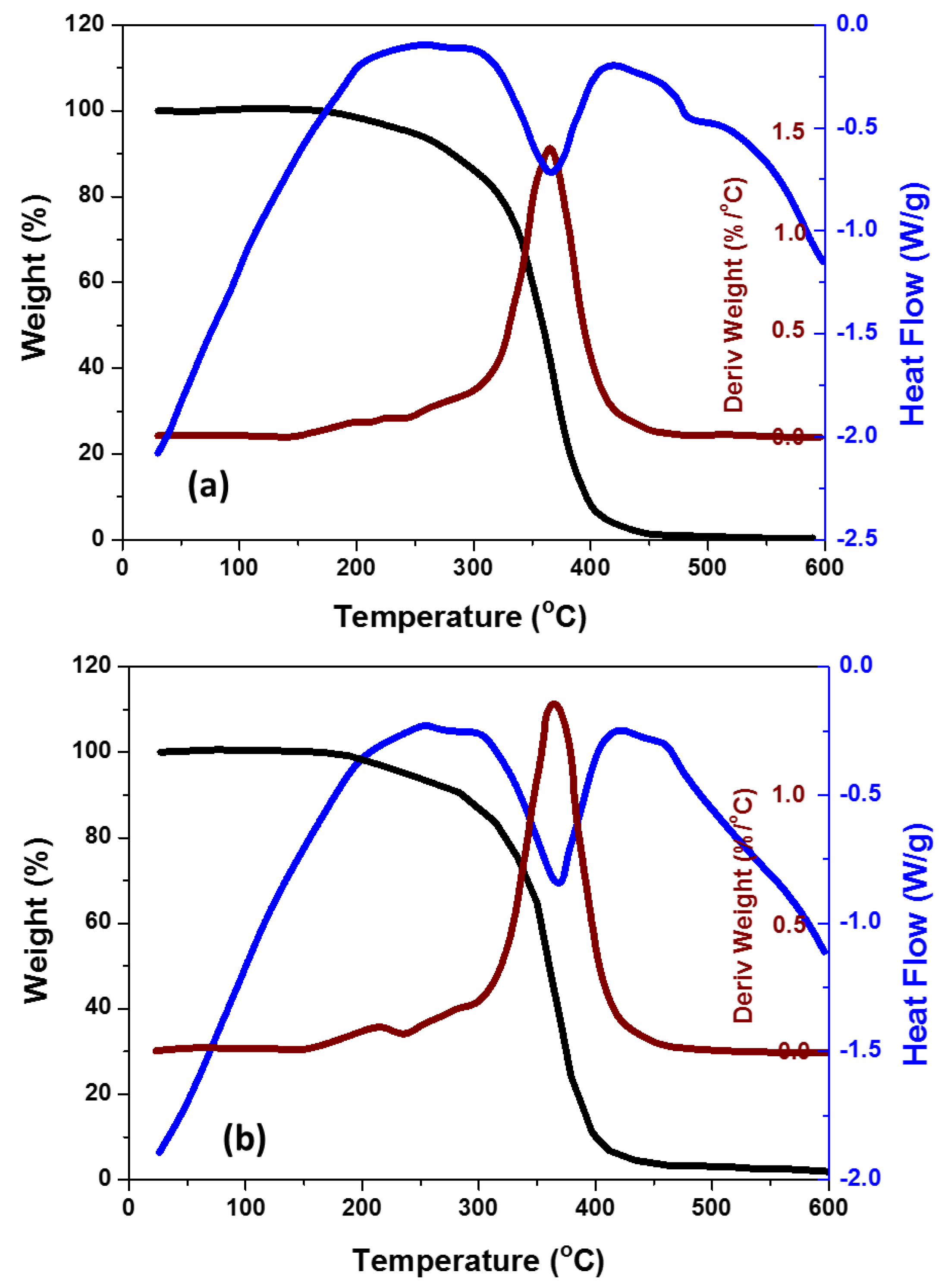
3.3. Thermal Behavior
3.4. Nanomechanical Behavior
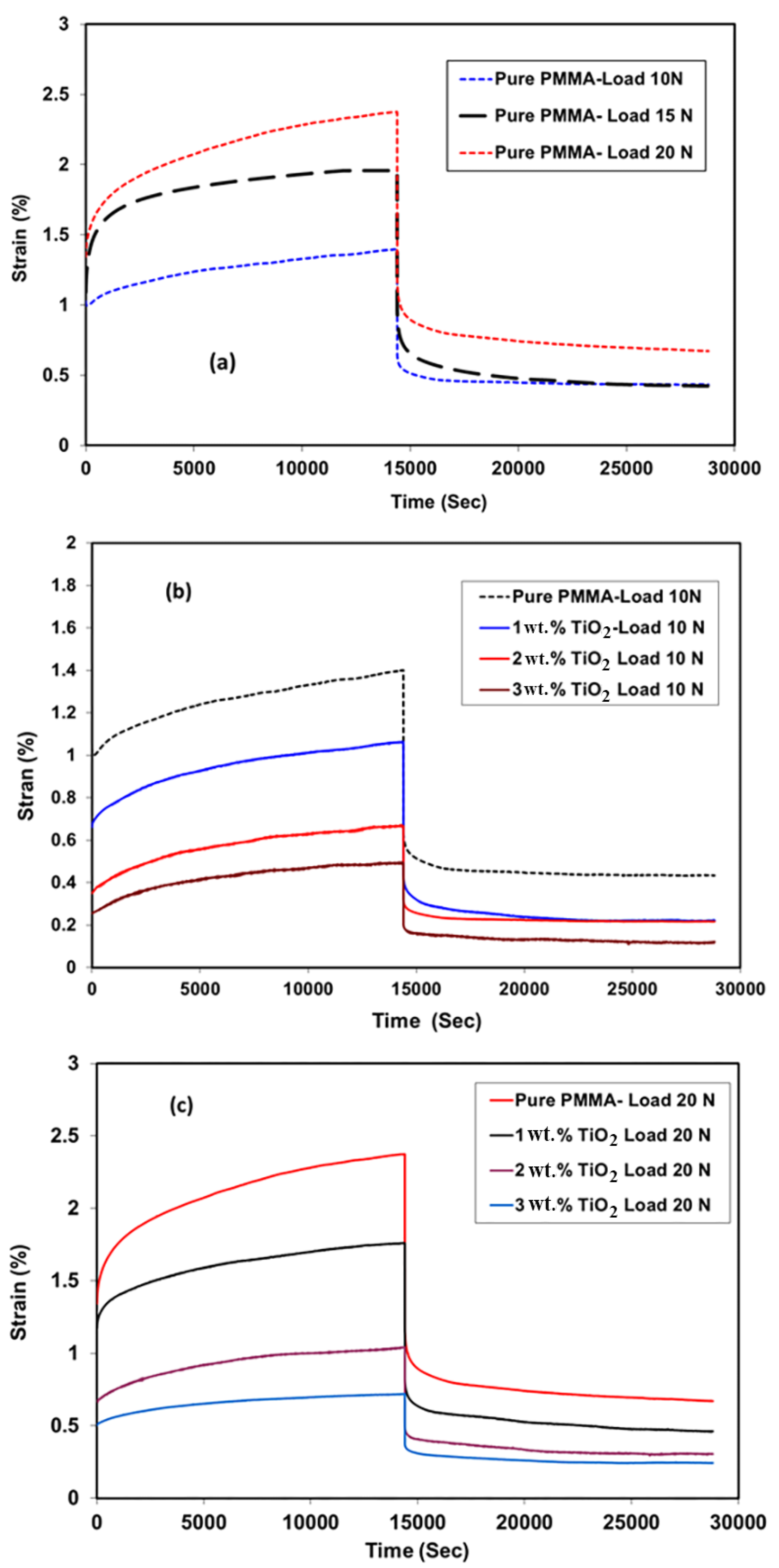
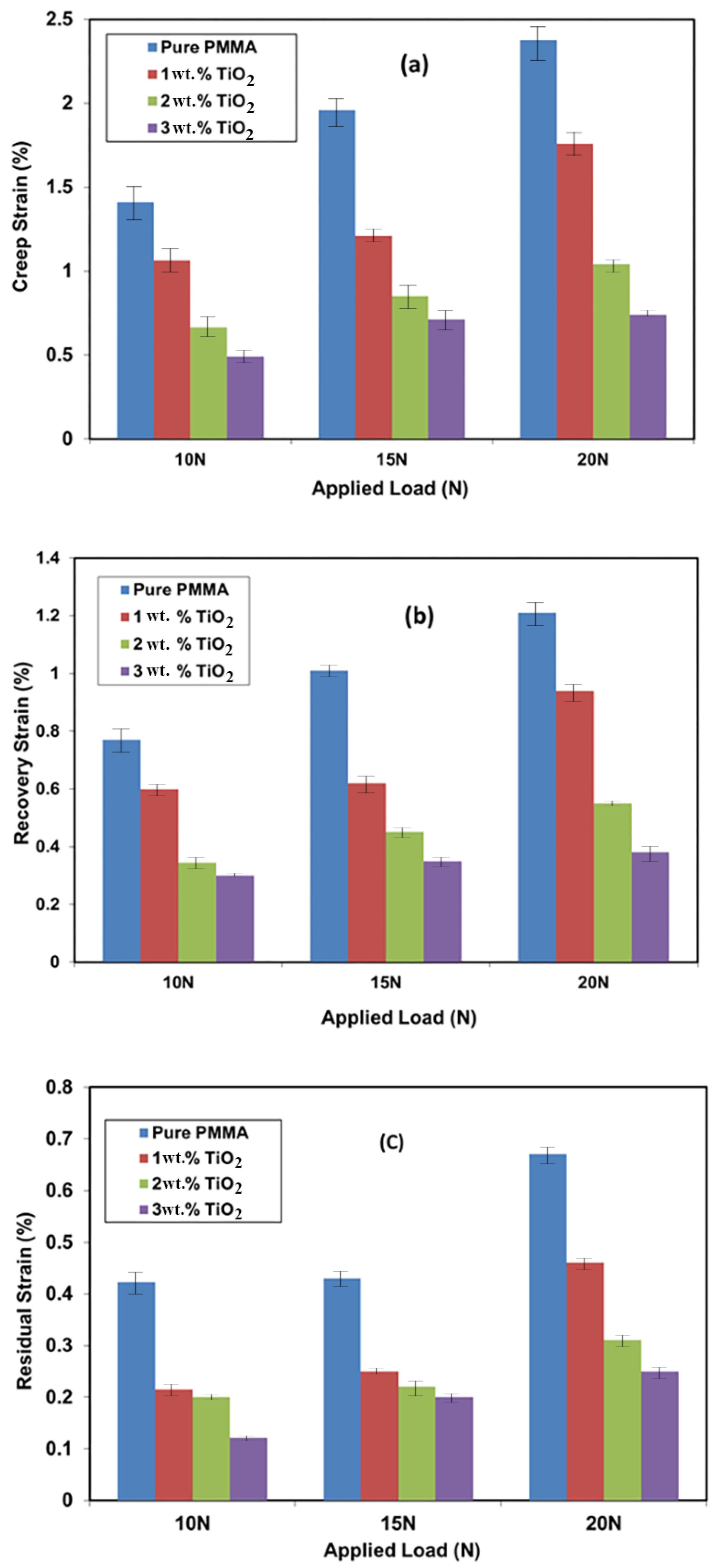
3.5. Creep Recovery
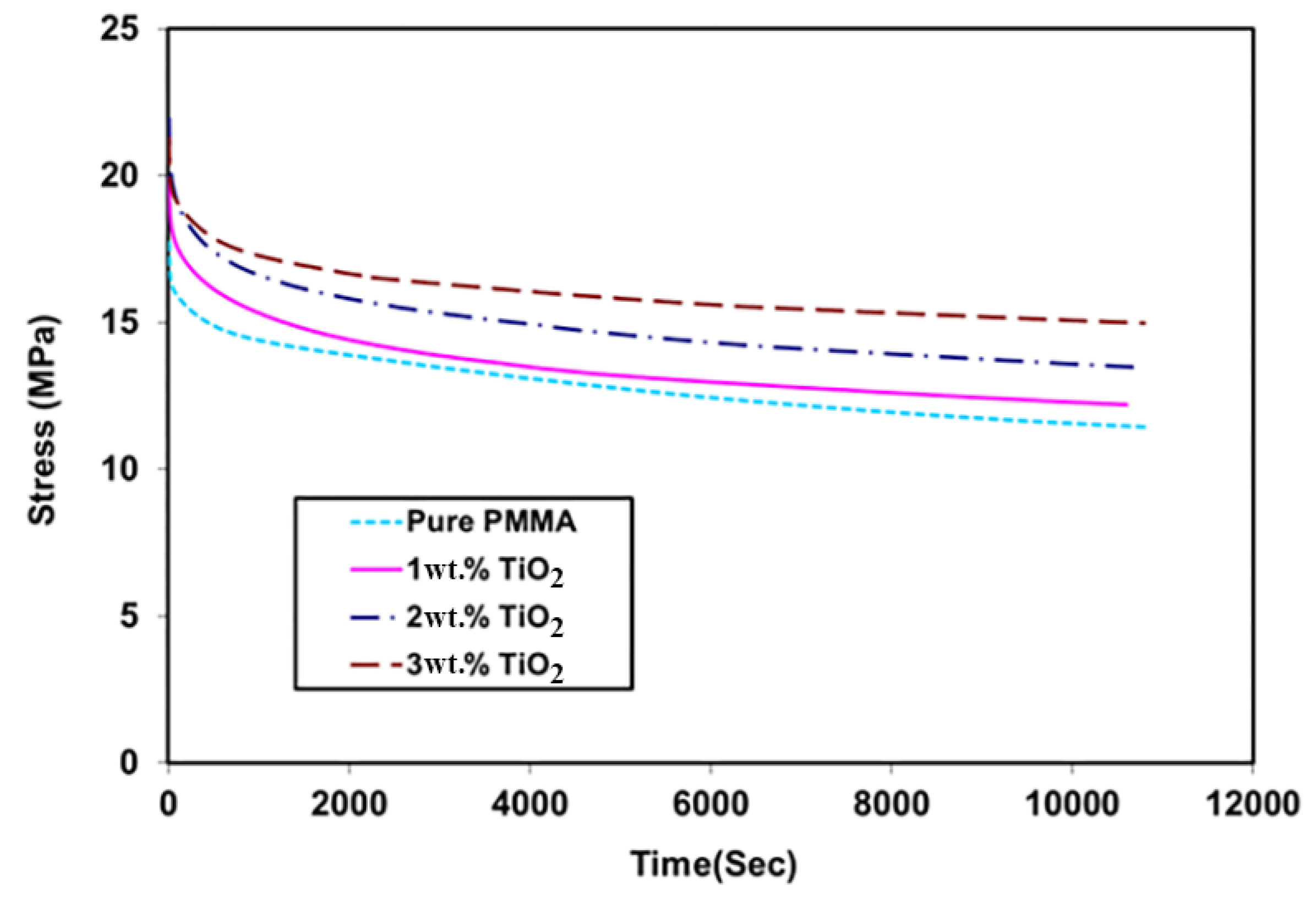
3.6. Relaxation Test
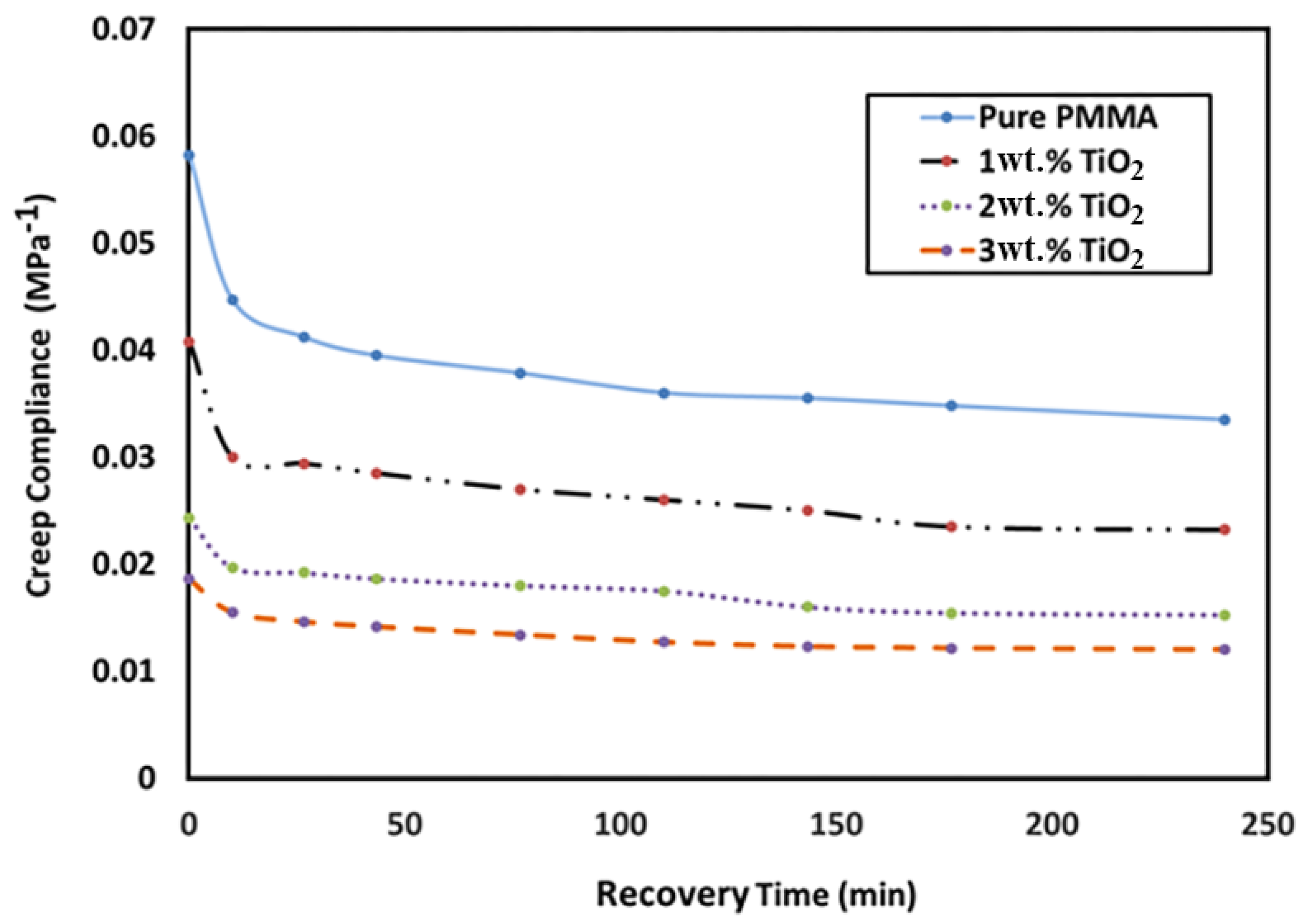
3.7. Creep Compliance
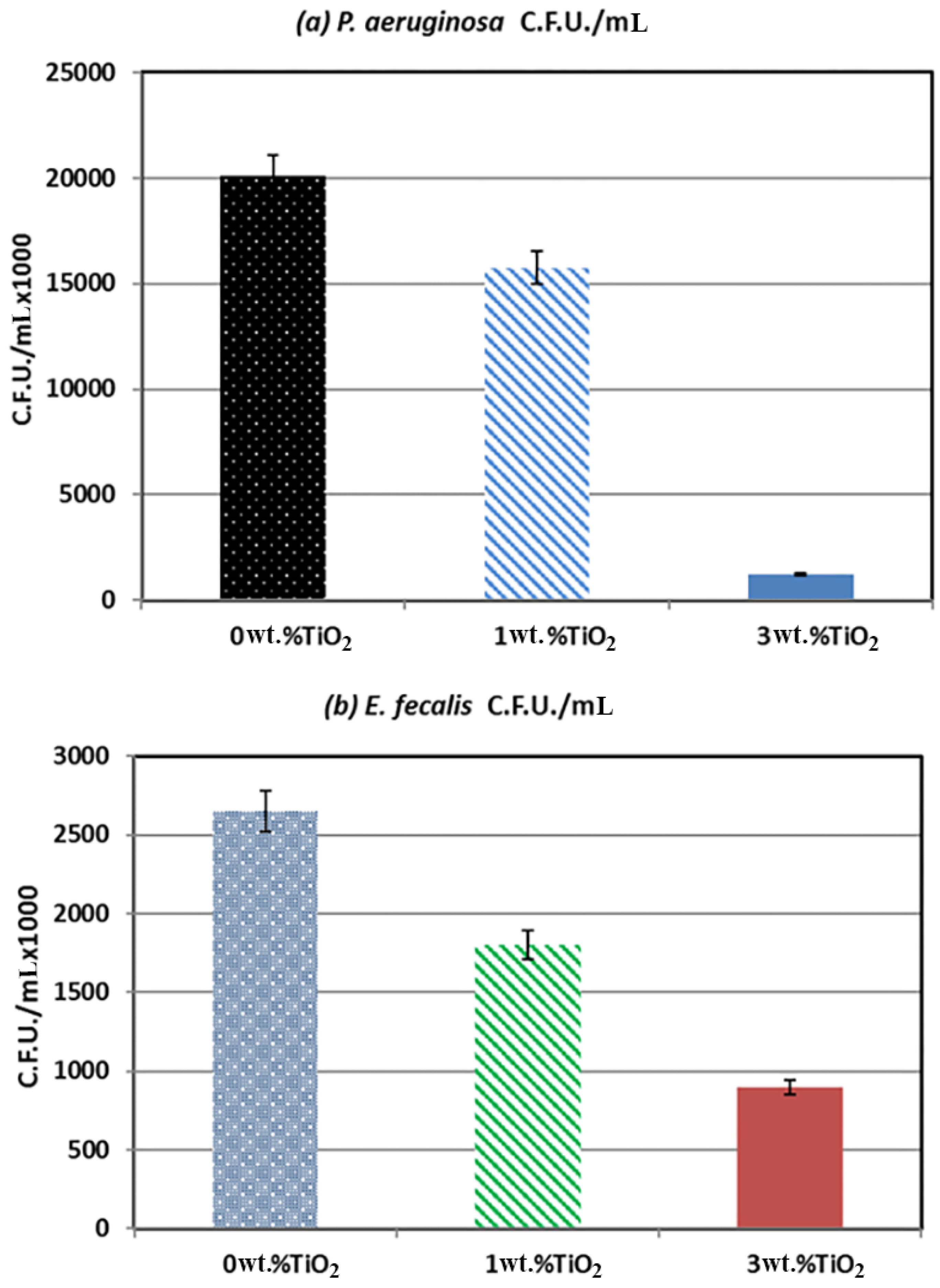
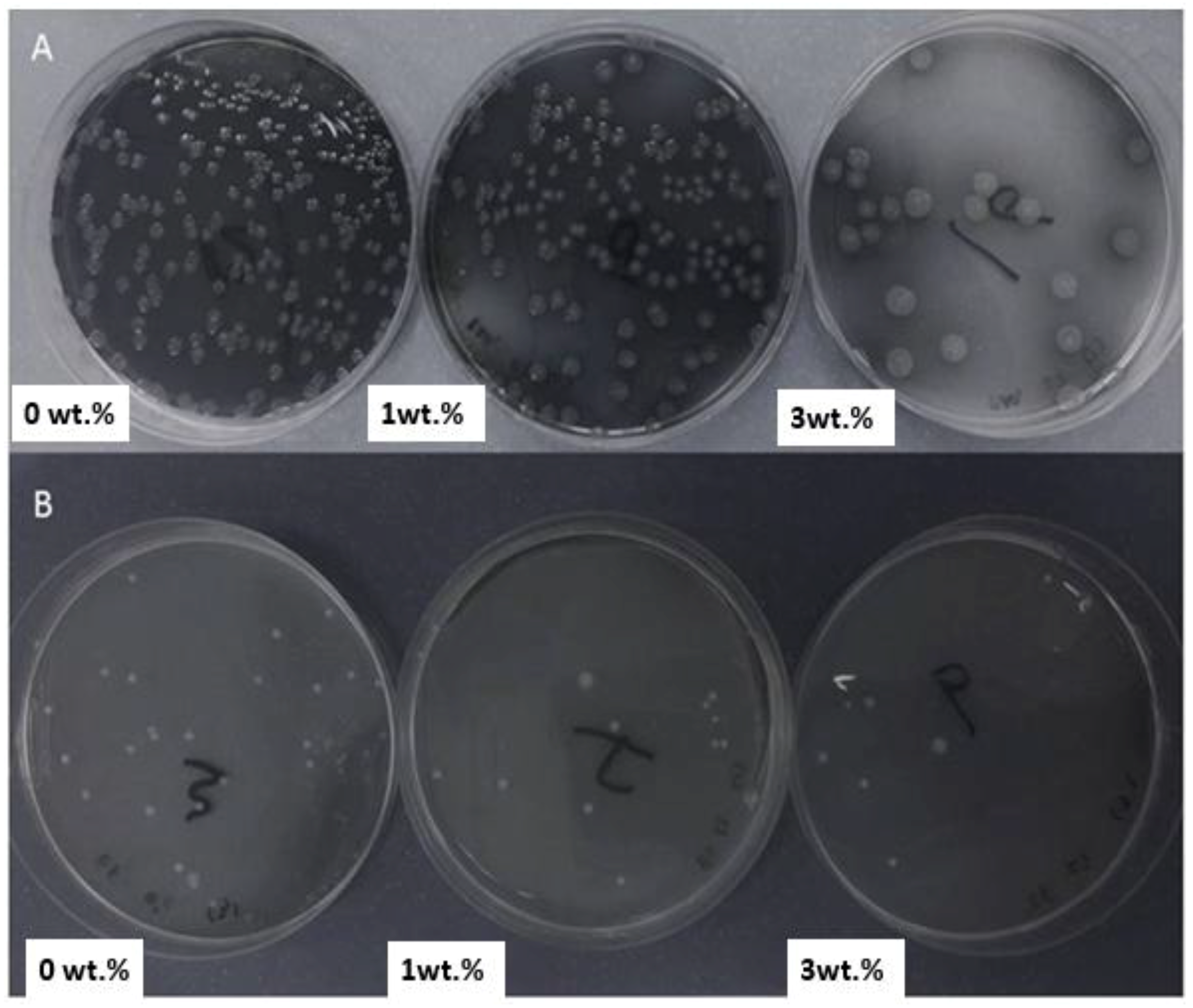
4. Antibacterial Adhesion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pires-de-Souza, F.D.C.; Panzeri, H.; Vieira, M.A.; Garcia, L.D.F.R.; Consani, S. Impact and fracture resistance of an experimental acrylic polymer with elastomer in different proportions. Mater. Res. 2009, 12, 415–418. [Google Scholar] [CrossRef]
- Rakhshan, V. Marginal integrity of provisional resin restoration materials: A review of the literature. Saudi J. Dent. Res. 2015, 6, 33–40. [Google Scholar] [CrossRef]
- Vallo, C.; Abraham, G.; Cuadrado, T.; San Román, J. Influence of cross-linked PMMA beads on the mechanical behavior of self-curing acrylic cements. J. Biomed. Mater. Res. 2004, 70, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Yadav, N.S.; Elkawash, H. Flexural strength of denture base resin reinforced with aluminum oxide and processed by different processing techniques. J. Adv. Oral Res. 2011, 2, 33–36. [Google Scholar]
- Han, Z.; Zhu, B.; Chen, R.; Huang, Z.; Zhu, C.; Zhang, X. Effect of silver-supported materials on the mechanical and antibacterial properties of reinforced acrylic resin composites. Mater. Des. (1980–2015) 2015, 65, 1245–1252. [Google Scholar] [CrossRef]
- Dhir, G.; Berzins, D.W.; Dhuru, V.B.; Periathamby, A.R.; Dentino, A. Physical properties of denture base resins potentially resistant to Candida adhesion. J. Prosthodont. 2007, 16, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Nam, K.Y.; Lee, C.H.; Lee, C.J. Antifungal and physical characteristics of modified denture base acrylic incorporated with silver nanoparticles. Gerodontology 2012, 29, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Shibata, T.; Hamada, N.; Kimoto, K.; Sawada, T.; Sawada, T.; Kumada, H.; Umemoto, T.; Toyoda, M. Antifungal effect of acrylic resin containing apatite-coated TiO2 photocatalyst. Dent. Mater. J. 2007, 26, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Lessa, F.C.R.; Enoki, C.; Ito, I.Y.; Faria, G.; Matsumoto, M.A.N.; Nelson-Filho, P. In-vivo evaluation of the bacterial contamination and disinfection of acrylic baseplates of removable orthodontic appliances. Am. J. Orthod. Dentofacial Orthop. 2007, 131, 705.e11–705.e17. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Chen, Y.; Leng, F.; Huang, L.; Wang, Z.; Tian, W. Recent advances on surface modification of halloysite nanotubes for multifunctional applications. Appl. Sci. 2017, 7, 1215. [Google Scholar] [CrossRef]
- Cavallaro, G.; Danilushkina, A.A.; Evtugyn, V.G.; Lazzara, G.; Milioto, S.; Parisi, F.; Rozhina, E.V.; Fakhrullin, R.F. Halloysite nanotubes: Controlled access and release by smart gates. Nanomaterials 2017, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Makaremi, M.; Pasbakhsh, P.; Cavallaro, G.; Lazzara, G.; Aw, Y.A.; Lee, S.M.; Milioto, S. Effect of morphology and size of halloysite nanotubes on functional pectin bionanocomposites for food packaging applications. ACS Appl. Mater. Interfaces 2017, 9, 17476–17488. [Google Scholar] [CrossRef] [PubMed]
- Pant, H.R.; Pandeya, D.R.; Nam, K.T.; Baek, W.I.; Hong, S.T.; Kim, H.Y. Photocatalytic and antibacterial properties of a TiO2/nylon-6 electrospun nanocomposite mat containing silver nanoparticles. J. Hazard. Mater. 2011, 189, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Li, Y.; Hu, J.; Li, F.; Tian, J.; Xue, B.; Zhan, P.; Shen, W.; Li, B. Effect of Titanium Dioxide Nanoparticles on Silkworm's Innate Immunity and Resistance to Bacillus bombyseptieus. Sci. Adv. Mater. 2016, 8, 1512–1522. [Google Scholar] [CrossRef]
- Reijnders, L. The release of TiO2 and SiO2 nanoparticles from nanocomposites. Polym. Degrad. Stable 2009, 94, 873–876. [Google Scholar] [CrossRef]
- Chatterjee, A. Properties improvement of PMMA using nano TiO2. J. Appl. Polym. Sci. 2010, 118, 2890–2897. [Google Scholar] [CrossRef]
- Chatterjee, A. Effect of nanoTiO2 addition on poly (methyl methacrylate): An exciting nanocomposite. J. Appl. Polym. Sci. 2010, 116, 3396–3407. [Google Scholar]
- Yang, P.; Tang, Y.; Yang, H.; Wu, Y. Thermal conductivity reconstruction at TiO2/ZnO multilayer nanoscale interface structure. Sci. Adv. Mater. 2014, 6, 1986–1992. [Google Scholar] [CrossRef]
- Anwar, M.; Danish, R.; Ahmed, F.; Koo, B.H. Pressure dependent synthesis and enhanced photocatalytic activity of TiO2 nano-structures. Nanosci. Nanotechnol. Lett. 2016, 8, 778–781. [Google Scholar] [CrossRef]
- Sodagar, A.; Bahador, A.; Khalil, S.; Shahroudi, A.S.; Kassaee, M.Z. The effect of TiO2 and SiO2 nanoparticles on flexural strength of poly (methyl methacrylate) acrylic resins. J. Prosthodont. Res. 2013, 57, 15–19. [Google Scholar] [CrossRef] [PubMed]
- El-Zaher, N.A.; Melegy, M.S.; Guirguis, O.W. Thermal and structural analyses of PMMA/TiO2 nanoparticles composites. Nat. Sci. 2014, 6, 859–870. [Google Scholar]
- Khaled, S.; Sui, R.; Charpentier, P.A.; Rizkalla, A.S. Synthesis of TiO2-PMMA nanocomposite: Using methacrylic acid as a coupling agent. Langmuir 2007, 23, 3988–3995. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, F.H.; Mourad, A.H.; Barton, D. UV irradiation and aging effects on nanoscale mechanical properties of ultra high molecular weight polyethylene for biomedical implants. Plast. Rubber Compos. 2008, 37, 346–352. [Google Scholar] [CrossRef]
- Albarrag, A.M.; Alothman, O.Y.; Elsharawy, M.A.; Fayez Al Rez, M.; Fouad, H.; Hashem, M.; Ansari, S. Effect of nigella sativa extracts on candida species adhesion to acrylic denture base material and on nanomechanical properties. Sci. Adv. Mater. 2017, 9, 775–781. [Google Scholar] [CrossRef]
- Mourad, A.H.; Fouad, H.; Elleithy, R. Impact of some environmental conditions on the tensile, creep-recovery, relaxation, melting and crystallinity behaviour of UHMWPE-GUR 410-medical grade. Mater. Des. 2009, 30, 4112–4119. [Google Scholar] [CrossRef]
- Alothman, O.Y.; Fouad, H.; Al-Zahrani, S.; Eshra, A.; Al Rez, M.F.; Ansari, S. Thermal, creep-recovery and viscoelastic behavior of high density polyethylene/hydroxyapatite nano particles for bone substitutes: Effects of gamma radiation. Biomed. Eng. Online 2014, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Fouad, H.; Elleithy, R.; Al-Zahrani, S.; Ali, M.A. Characterization and processing of high density polyethylene/carbon nano-composites. Mater. Des. 2011, 32, 1974. [Google Scholar]
- Fouad, H.; Elleithy, R. High density polyethylene/graphite nano-composites for total hip joint replacements: Processing and in vitro characterization. J. Mech. Behav. Biomed. Mater. 2011, 4, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.Y.; Zhu, B.S.; Wang, X.F.; Lu, Q.H. Cytotoxicity of titanium dioxide nanoparticles in mouse fibroblast cells. Chem. Res. Toxicol. 2008, 21, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Ciofu, O.; Johansen, H.K.; Song, Z.J.; Moser, C.; Jensen, P.Ø.; Molin, S.; Givskov, M.; Tolker-Nielsen, T.; Bjarnsholt, T. The clinical impact of bacterial biofilms. Int. J. Oral Sci. 2011, 3, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Hengzhuang, W.; Wu, H.; Ciofu, O.; Song, Z.; Høiby, N. Pharmacokinetics/pharmacodynamics of colistin and imipenem on mucoid and nonmucoid Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 2011, 55, 4469–4474. [Google Scholar] [CrossRef] [PubMed]
- Hengzhuang, W.; Wu, H.; Ciofu, O.; Song, Z.; Høiby, N. In vivo pharmacokinetics/pharmacodynamics of colistin and imipenem in Pseudomonas aeruginosa biofilm infection. Antimicrob. Agents Chemother. 2012, 56, 2683–2690. [Google Scholar] [CrossRef] [PubMed]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Besinis, A.; Hadi, S.D.; Le, H.R.; Tredwin, C.; Handy, R.D. Antibacterial activity and biofilm inhibition by surface modified titanium alloy medical implants following application of silver, titanium dioxide and hydroxyapatite nanocoatings. Nanotoxicology 2017, 11, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Chu, P.K.; Zhang, Y.; Wu, Z. Antibacterial coatings on titanium implants. J. Biomed. Mater. Res. Part B Appl. Biomater. 2009, 91, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Wei, S.S.; Hu, S.Q.; Tang, J.X. Preparation of TiO2/Ag colloids with ultraviolet resistance and antibacterial property using short chain polyethylene glycol. J. Hazard. Mater. 2009, 172, 716–720. [Google Scholar] [CrossRef] [PubMed]
- Heinlaan, M.; Ivask, A.; Blinova, I.; Dubourguier, H.C.; Kahru, A. Toxicity of nanosized and bulk ZnO, CuO and TiO2 to bacteria Vibrio fischeri and crustaceans Daphnia magna and Thamnocephalus platyurus. Chemosphere 2008, 71, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Fujishima, A.; Zhang, X.; Tryk, D.A. TiO2 photocatalysis and related surface phenomena. Surf. Sci. Rep. 2008, 63, 515–582. [Google Scholar] [CrossRef]
- Kohen, R.; Nyska, A. Invited review: Oxidation of biological systems: Oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol. Pathol. 2002, 6, 620–650. [Google Scholar] [CrossRef] [PubMed]
- Rikans, L.E.; Hornbrook, K.R. Lipid peroxidation, antioxidant protection and aging. Biochim. Biophys. Acta Mol. Basis Dis. 1997, 1362, 116–127. [Google Scholar] [CrossRef]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Long, T.C.; Tajuba, J.; Sama, P.; Saleh, N.; Swartz, C.; Parker, J.; Hester, S.; Lowry, G.V.; Veronesi, B. Nanosize titanium dioxide stimulates reactive oxygen species in brain microglia and damages neurons in vitro. Environ Health Perspect. 2007, 115, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.X.; Chen, C.Y.; Liu, Y.; Jiao, F.; Li, W.; Lao, F.; Li, Y.; Li, B.; Ge, C.; Zhou, G.; et al. Potential neurological lesion after nasal instillation of TiO2 nanoparticles in the anatase and rutile crystal phases. Toxicol. Lett. 2008, 183, 72–80. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrahlah, A.; Fouad, H.; Hashem, M.; Niazy, A.A.; AlBadah, A. Titanium Oxide (TiO2)/Polymethylmethacrylate (PMMA) Denture Base Nanocomposites: Mechanical, Viscoelastic and Antibacterial Behavior. Materials 2018, 11, 1096. https://doi.org/10.3390/ma11071096
Alrahlah A, Fouad H, Hashem M, Niazy AA, AlBadah A. Titanium Oxide (TiO2)/Polymethylmethacrylate (PMMA) Denture Base Nanocomposites: Mechanical, Viscoelastic and Antibacterial Behavior. Materials. 2018; 11(7):1096. https://doi.org/10.3390/ma11071096
Chicago/Turabian StyleAlrahlah, Ali, H. Fouad, Mohamed Hashem, Abdurahman A. Niazy, and Abdulhakim AlBadah. 2018. "Titanium Oxide (TiO2)/Polymethylmethacrylate (PMMA) Denture Base Nanocomposites: Mechanical, Viscoelastic and Antibacterial Behavior" Materials 11, no. 7: 1096. https://doi.org/10.3390/ma11071096
APA StyleAlrahlah, A., Fouad, H., Hashem, M., Niazy, A. A., & AlBadah, A. (2018). Titanium Oxide (TiO2)/Polymethylmethacrylate (PMMA) Denture Base Nanocomposites: Mechanical, Viscoelastic and Antibacterial Behavior. Materials, 11(7), 1096. https://doi.org/10.3390/ma11071096




