Effect of Acetic Acid Concentration on Pore Structure for Mesoporous Bioactive Glass during Spray Pyrolysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Characterization
3. Results
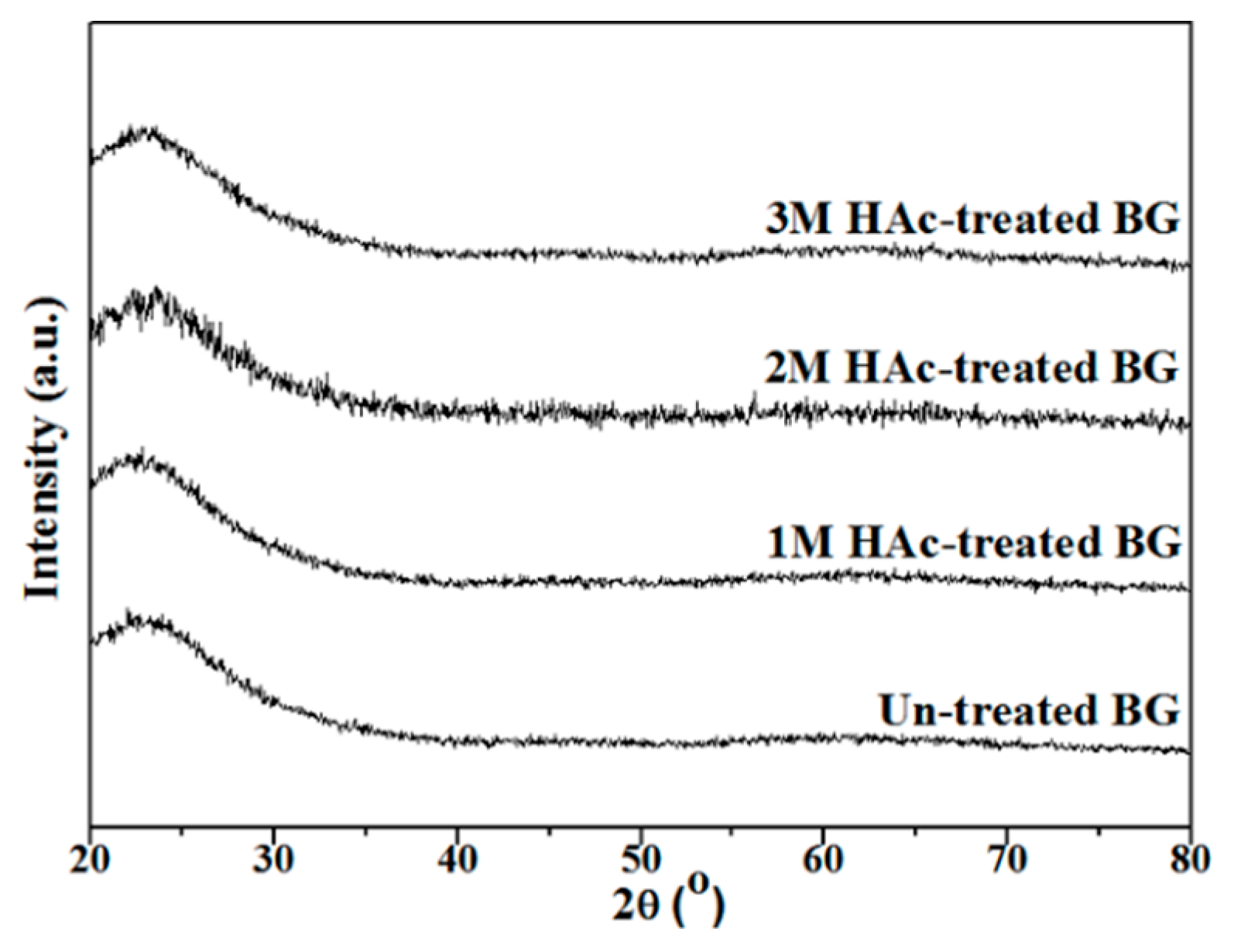
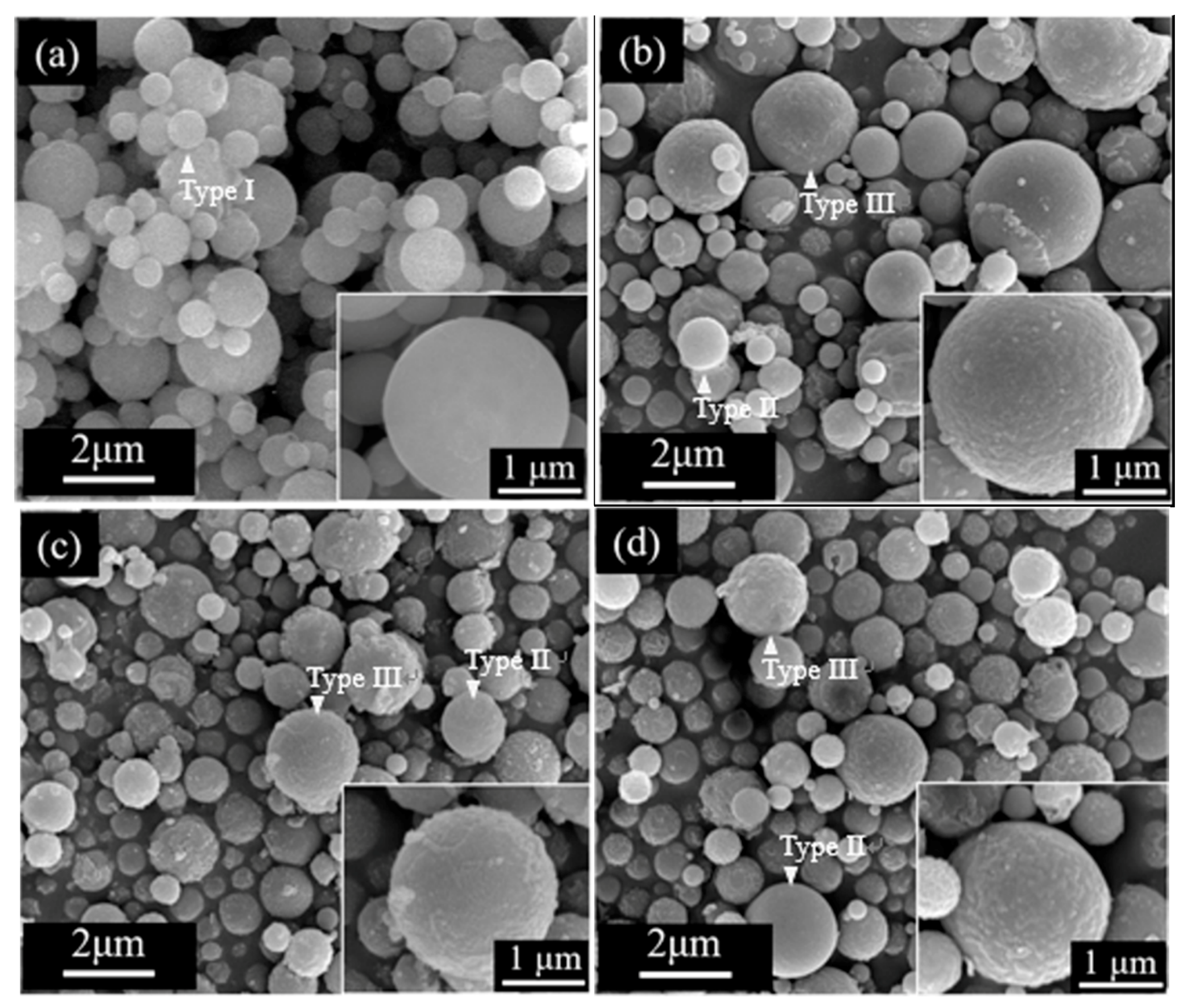
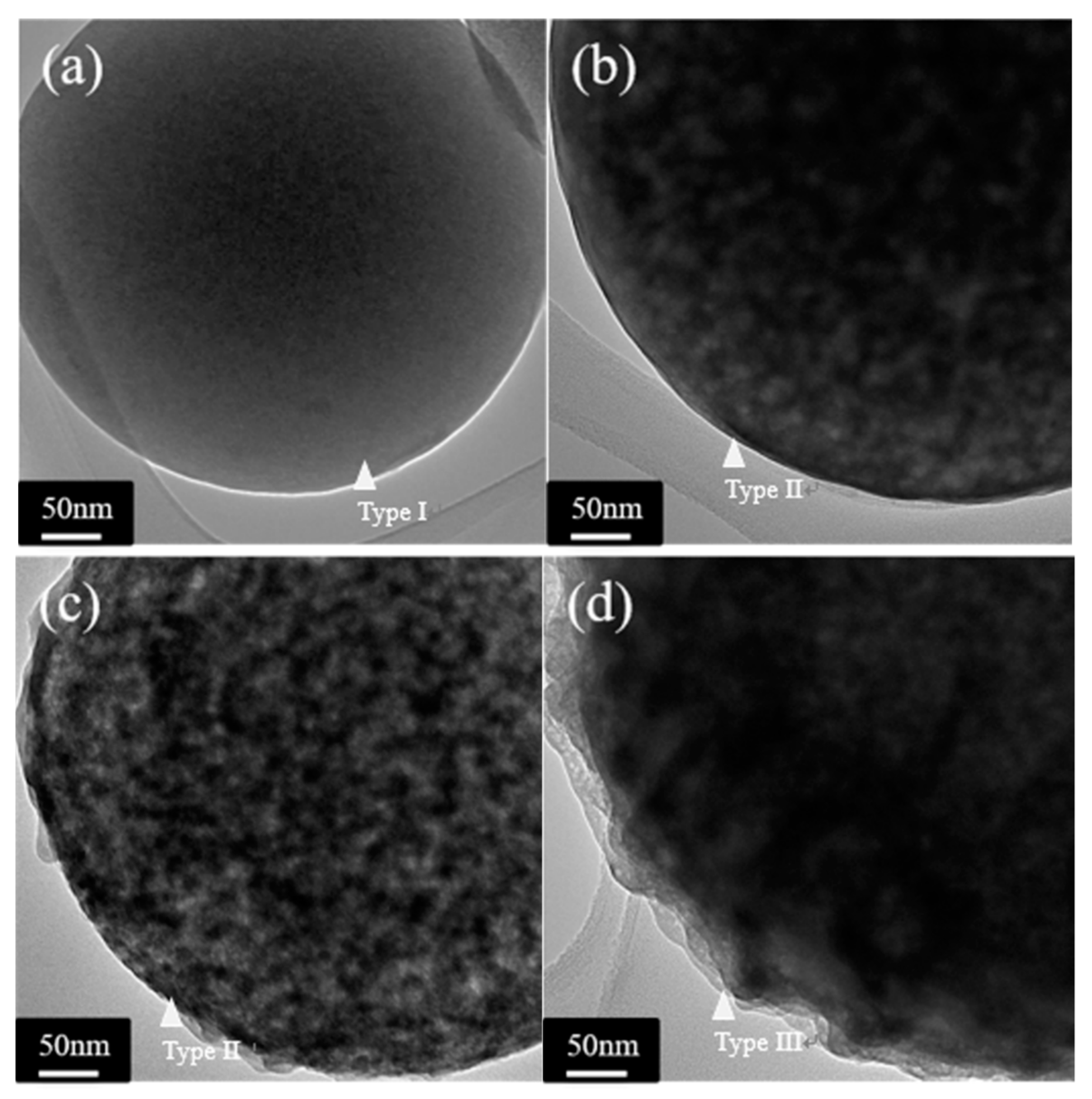
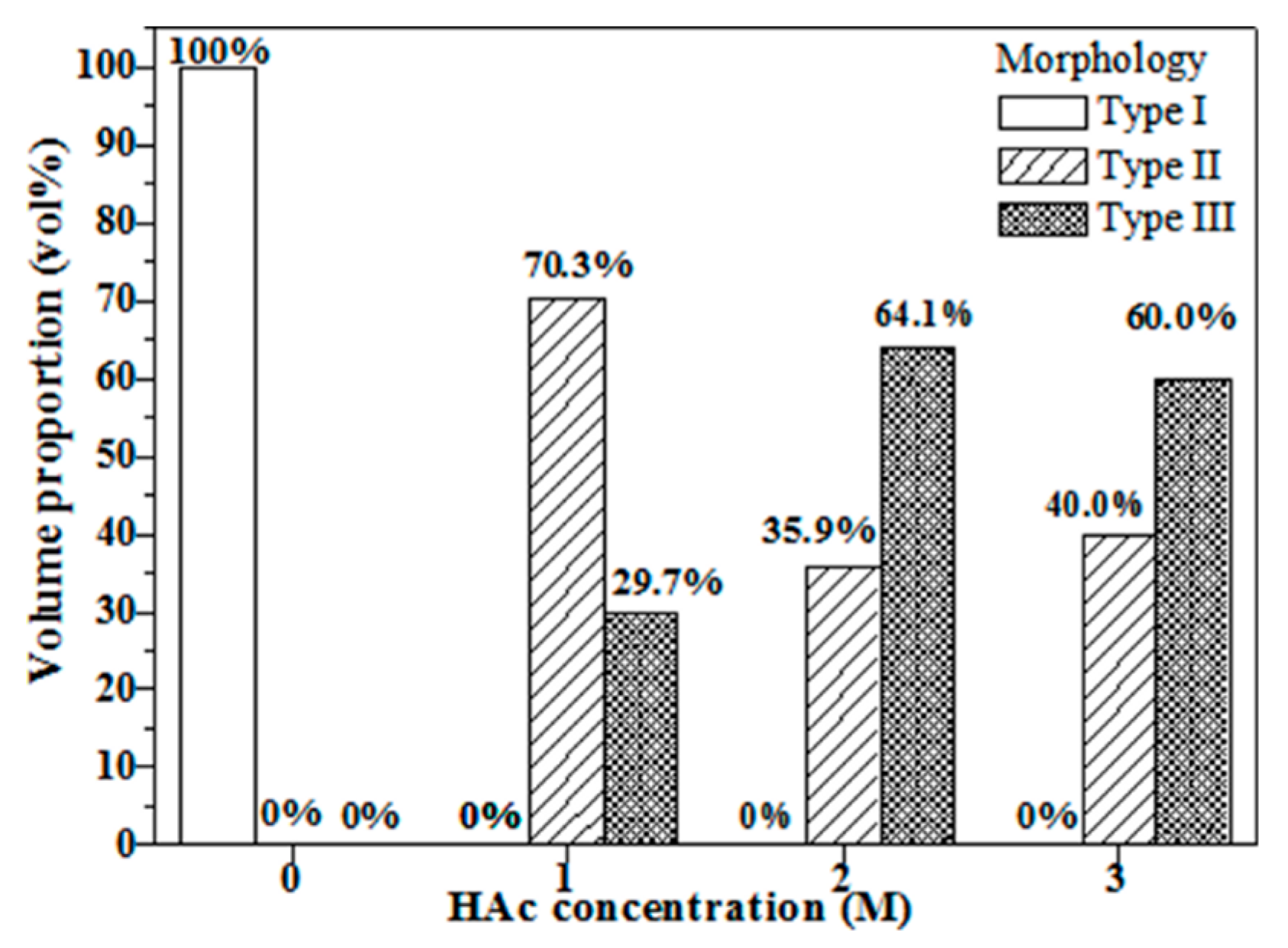
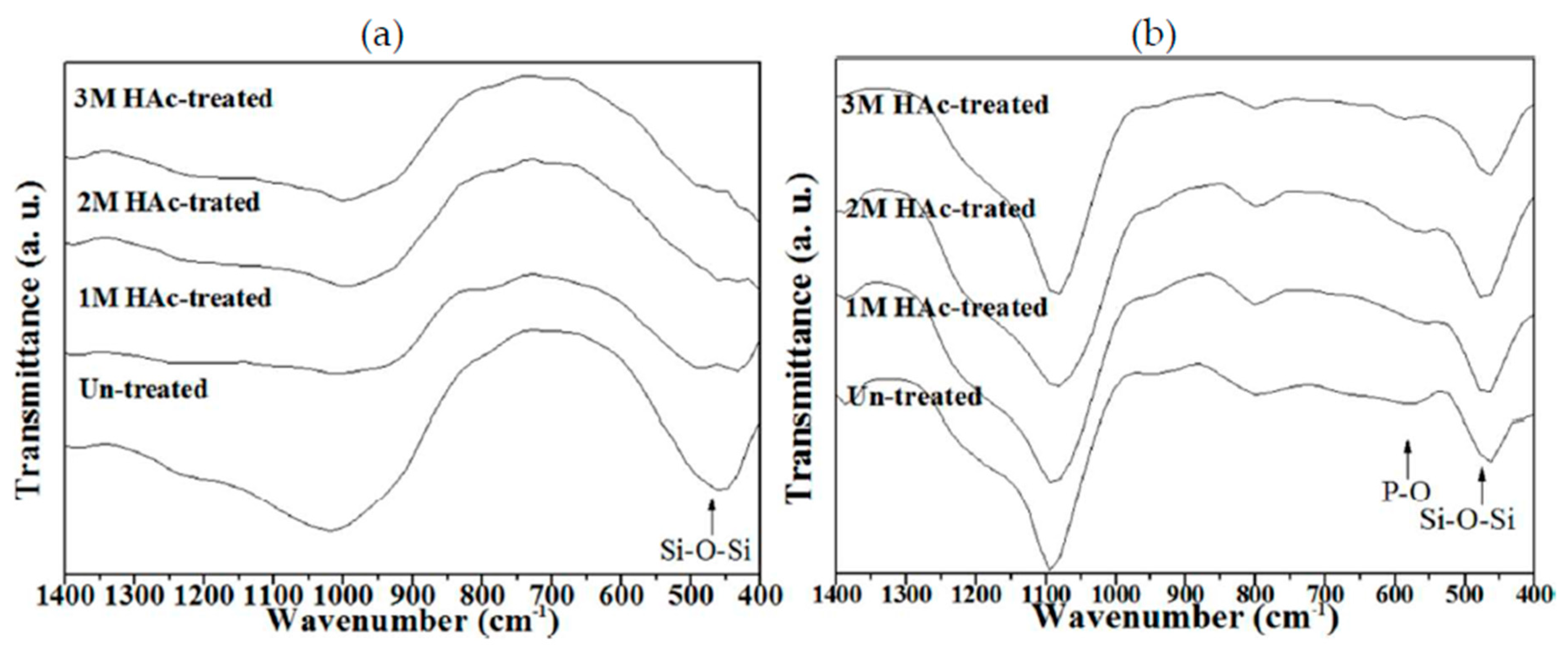
3.1. Crystallographic Structure and Morphology
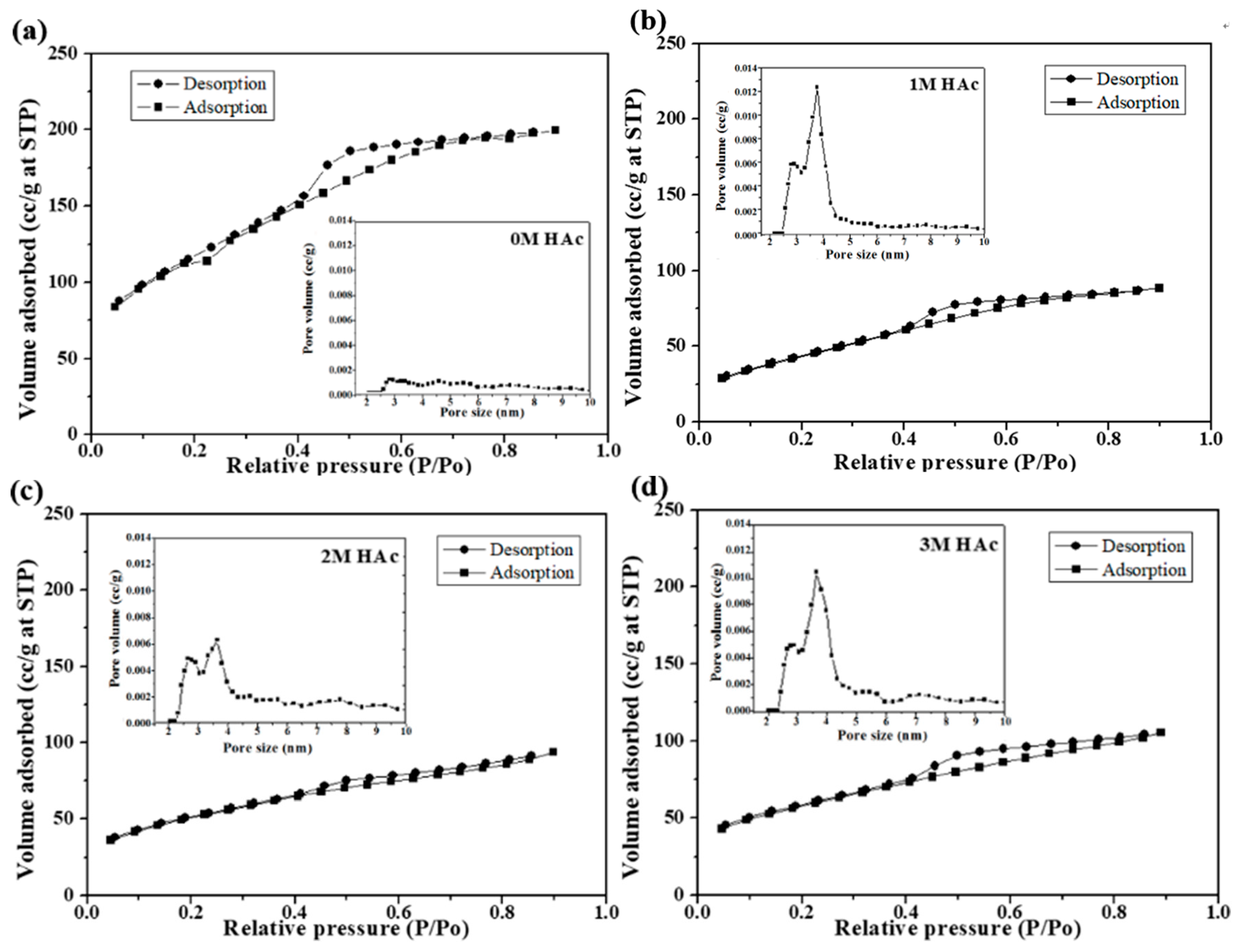
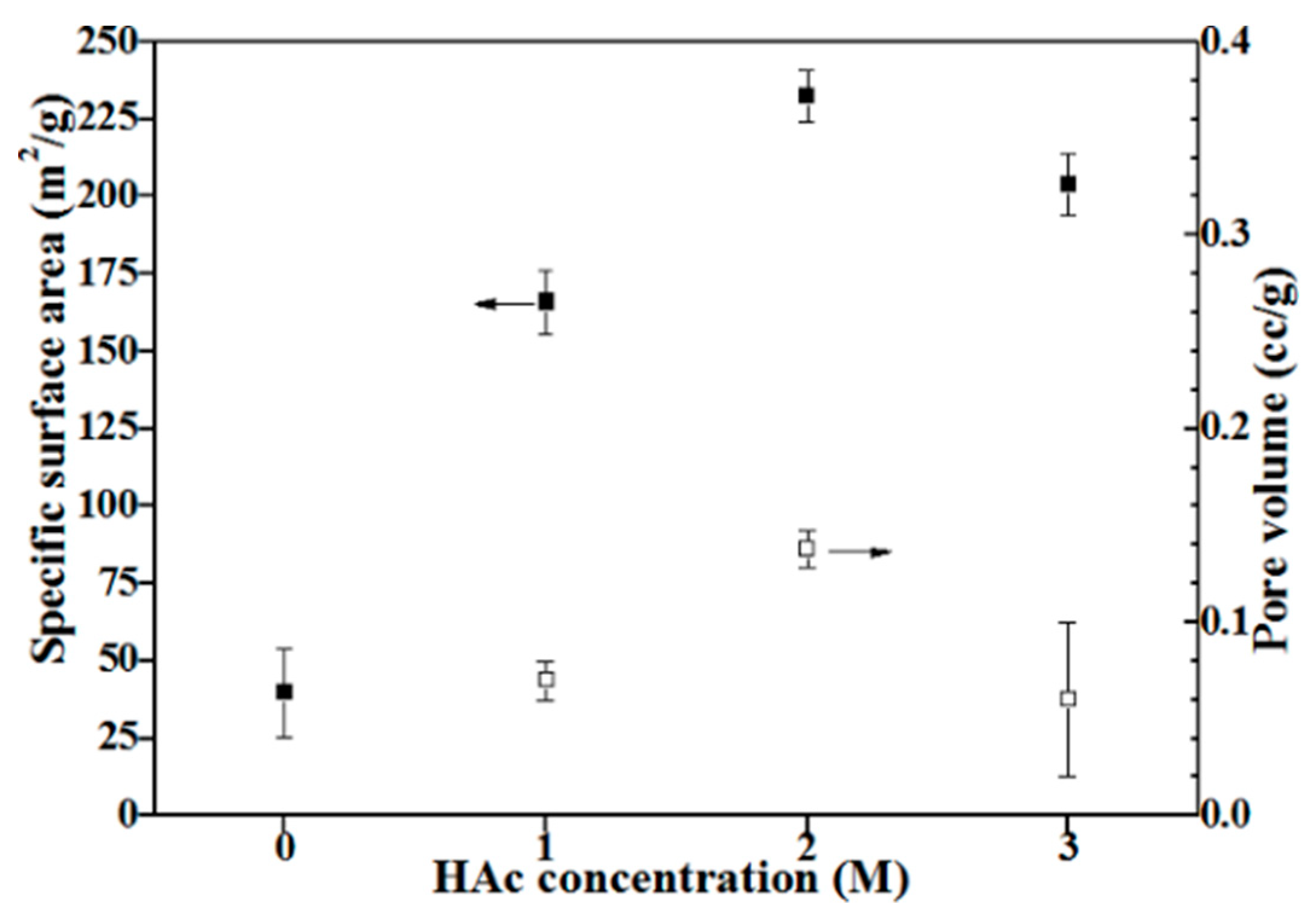
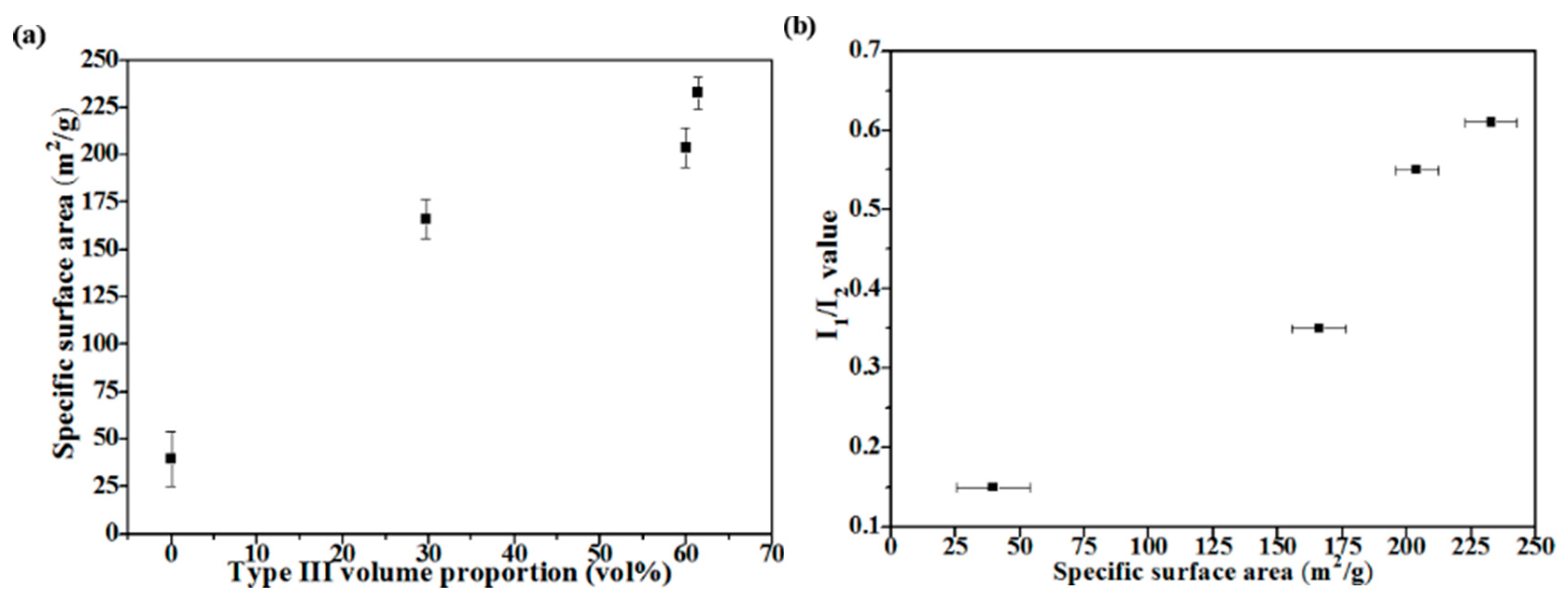
3.2. Particle Sizes and Specific Surface Areas
3.3. In Vitro Bioactivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Rahaman, M.N.; Day, D.E.; Bal, B.S.; Fu, Q.; Jung, S.B.; Bonewald, L.F.; Tomsia, A.P. Bioactive glass in tissue engineering. Acta Biomater. 2011, 7, 2355–2373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, B.; Chen, X.F.; Wang, Y.J.; Zhao, N.R.; Du, C.; Fang, L.M. Synthesis and in vitro bioactivity of novel mesoporous hollow bioactive glass microspheres. Mater. Lett. 2009, 63, 1719–1721. [Google Scholar] [CrossRef]
- Hench, L.L. The story of bioglass. J. Mater. Sci. Mater. Med. 2006, 11, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Chang, J. Preparation, in vitro bioactivity and drug release property of well-ordered mesoporous 58S bioactive glass. J. Non-Cryst. Solids 2008, 354, 1338–1341. [Google Scholar] [CrossRef]
- Mačković, M.; Hoppe, A.; Detsch, R.; Mohn, D.; Stark, W.; Spiecker, E.; Boccaccini, A. Bioactive glass (type 45S5) nanoparticles: In vitro reactivity on nanoscale and biocompatibility. J. Nanopart. Res. 2012, 14, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Clark, A.; Hench, L.L. An investigation of bioactive glass powders by sol-gel processing. J. Appl. Biomater. 1991, 2, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Yu, C.; Zhou, X.; Tang, J.; Zhao, D. Highly ordered mesoporous bioactive glasses with superior in vitro bone-forming bioactivities. Angew. Chem. Int. Ed. 2004, 43, 5980–5984. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.-J.; Wu, Y.-Y.; Chen, C.-Y.; Yu, C.-Y. Morphology and formation mechanism of ceria nanoparticles by spray pyrolysis. J. Nanopart. Res. 2012, 14, 879. [Google Scholar] [CrossRef]
- Chou, Y.J.; Hong, B.J.; Lin, Y.C.; Wang, C.Y.; Shih, S.J. The correlation of pore size and bioactivity of spray-pyrolyzed mesoporous bioactive glasses. Materials 2017, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Lei, B.; Chen, X.; Wang, Y.; Zhao, N.; Du, C.; Fang, L. Synthesis and bioactive properties of macroporous nanoscale SiO2–CaO–P2O5 bioactive glass. J. Non-Cryst. Solids 2009, 355, 2678–2681. [Google Scholar] [CrossRef]
- Vallet-Regí, M. Ceramics for medical applications. J. Chem. Soc. Dalton Trans. 2001, 2, 97–108. [Google Scholar] [CrossRef]
- Shih, S.-J.; Lin, Y.-C.; Panjaitan, L.V.P.; Sari, D.R.M. The Correlation of Surfactant Concentrations on the Properties of Mesoporous Bioactive Glass. Materials 2016, 9, 58. [Google Scholar] [CrossRef] [PubMed]
- Bush, A.; Gabriel, R. The lungs in uraemia: A review. J. R. Soc. Med. 1985, 78, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Siahpoosh, S.M.; Salahi, E.; Hessari, F.A.; Mobasherpour, I. Facile synthesis of γ-alumina nanoparticles via the sol-gel method in presence of various solvents. Sigma J. Eng. Natl. Sci. 2017, 35, 441–456. [Google Scholar]
- Shih, C.; Chen, H.; Huang, L.; Lu, P.; Chang, H.; Chang, I. Synthesis and in vitro bioactivity of mesoporous bioactive glass scaffolds. Mater. Sci. Eng. 2010, 30, 657–663. [Google Scholar] [CrossRef]
- López-Delgado, A.; Cano, E.; Bastidas, J.; López, F. A laboratory study of the effect of acetic acid vapor on atmospheric copper corrosion. J. Electrochem. Soc. 1998, 145, 4140–4147. [Google Scholar] [CrossRef]
- Alexandridis, P.; Hatton, T.A. Poly (ethylene oxide)-poly (propylene oxide)-poly (ethylene oxide) block copolymer surfactants in aqueous solutions and at interfaces: Thermodynamics, structure, dynamics, and modeling. Colloids Surf. A Physicochem. Eng. Aspects 1995, 96, 1–46. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Dollimore, D.; Heal, G. An improved method for the calculation of pore size distribution from adsorption data. J. Chem. Technol. Biotechnol. 1964, 14, 109–114. [Google Scholar] [CrossRef]
- Shih, S.-J.; Chou, Y.-J.; Panjaitan, L.V.P. Synthesis and characterization of spray pyrolyzed mesoporous bioactive glass. Ceram. Int. 2013, 39, 8773–8779. [Google Scholar] [CrossRef]
- Kokubo, T.; Kushitani, H.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Solutions able to reproduce in vivo surface-structure changes in bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 721–734. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Hench, L.L. Bioceramics: From concept to clinic. J. Am. Ceram. Soc. 1991, 74, 1487–1510. [Google Scholar] [CrossRef]
- Kay, M.I.; Young, R.; Posner, A. Crystal structure of hydroxyapatite. Nature 1964, 204, 1050. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.-J.; Chou, Y.-J.; Chen, C.Y.; Lin, C.-K. One-step synthesis and characterization of nanosized bioactive glass. J. Med. Biol. Eng. 2014, 34, 18–23. [Google Scholar] [CrossRef]
- Djelloul, A.; Bouzid, K.; Guerrab, F. Role of substrate temperature on the structural and morphological properties of ZnO thin films deposited by ultrasonic spray pyrolysis. Turk. J. Phys. 2008, 32, 49–58. [Google Scholar]
- Arenas, L.T.; Simm, C.W.; Gushikem, Y.; Dias, S.L.; Moro, C.C.; Costa, T.M.; Benvenutti, E.V. Synthesis of silica xerogels with high surface area using acetic acid as catalyst. J. Braz. Chem. Soc. 2007, 18, 886–890. [Google Scholar] [CrossRef] [Green Version]
- Shih, S.-J.; Tzeng, W.-L. Manipulation of morphology of strontium titanate particles by spray pyrolysis. Powder Technol. 2014, 264, 291–297. [Google Scholar] [CrossRef]
- Yan, X.; Huang, X.; Yu, C.; Deng, H.; Wang, Y.; Zhang, Z.; Qiao, S.; Lu, G.; Zhao, D. The in-vitro bioactivity of mesoporous bioactive glasses. Biomaterials 2006, 27, 3396–3403. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.-J.; Chou, Y.-J.; Borisenko, K. Preparation method: Structure–bioactivity correlation in mesoporous bioactive glass. J. Nanopart. Res. 2013, 15, 1763. [Google Scholar] [CrossRef]
- Shih, S.J.; Sari, D.R.M.; Lin, Y.C. Influence of chemical composition on the bioactivity of spray pyrolyzed mesoporous bioactive glass. Int. J. Appl. Ceram. Technol. 2016, 13, 787–794. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, B.-J.; Hsiao, C.-W.; Bakare, F.F.; Sun, J.-T.; Shih, S.-J. Effect of Acetic Acid Concentration on Pore Structure for Mesoporous Bioactive Glass during Spray Pyrolysis. Materials 2018, 11, 963. https://doi.org/10.3390/ma11060963
Hong B-J, Hsiao C-W, Bakare FF, Sun J-T, Shih S-J. Effect of Acetic Acid Concentration on Pore Structure for Mesoporous Bioactive Glass during Spray Pyrolysis. Materials. 2018; 11(6):963. https://doi.org/10.3390/ma11060963
Chicago/Turabian StyleHong, Bo-Jiang, Chih-Wei Hsiao, Fufa Fetene Bakare, Jung-Ting Sun, and Shao-Ju Shih. 2018. "Effect of Acetic Acid Concentration on Pore Structure for Mesoporous Bioactive Glass during Spray Pyrolysis" Materials 11, no. 6: 963. https://doi.org/10.3390/ma11060963
APA StyleHong, B.-J., Hsiao, C.-W., Bakare, F. F., Sun, J.-T., & Shih, S.-J. (2018). Effect of Acetic Acid Concentration on Pore Structure for Mesoporous Bioactive Glass during Spray Pyrolysis. Materials, 11(6), 963. https://doi.org/10.3390/ma11060963




