Nanoscale Zero-Valent Iron Decorated on Bentonite/Graphene Oxide for Removal of Copper Ions from Aqueous Solution
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Graphene Oxide/Bentonite Decorated Nano-Iron (GO-B-nZVI)
2.3. Characterization of Materials
2.4. Removal Efficiency of Copper Ions
3. Results and Discussion
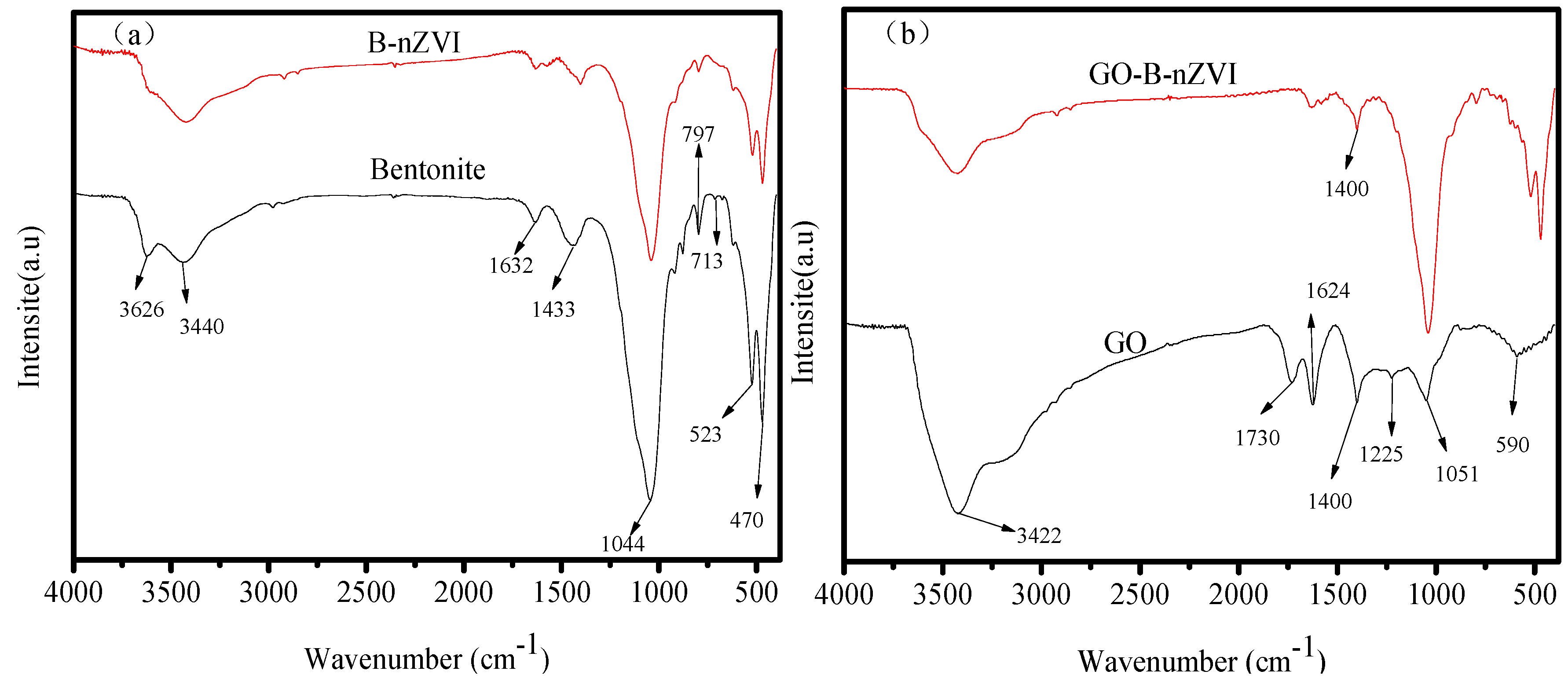
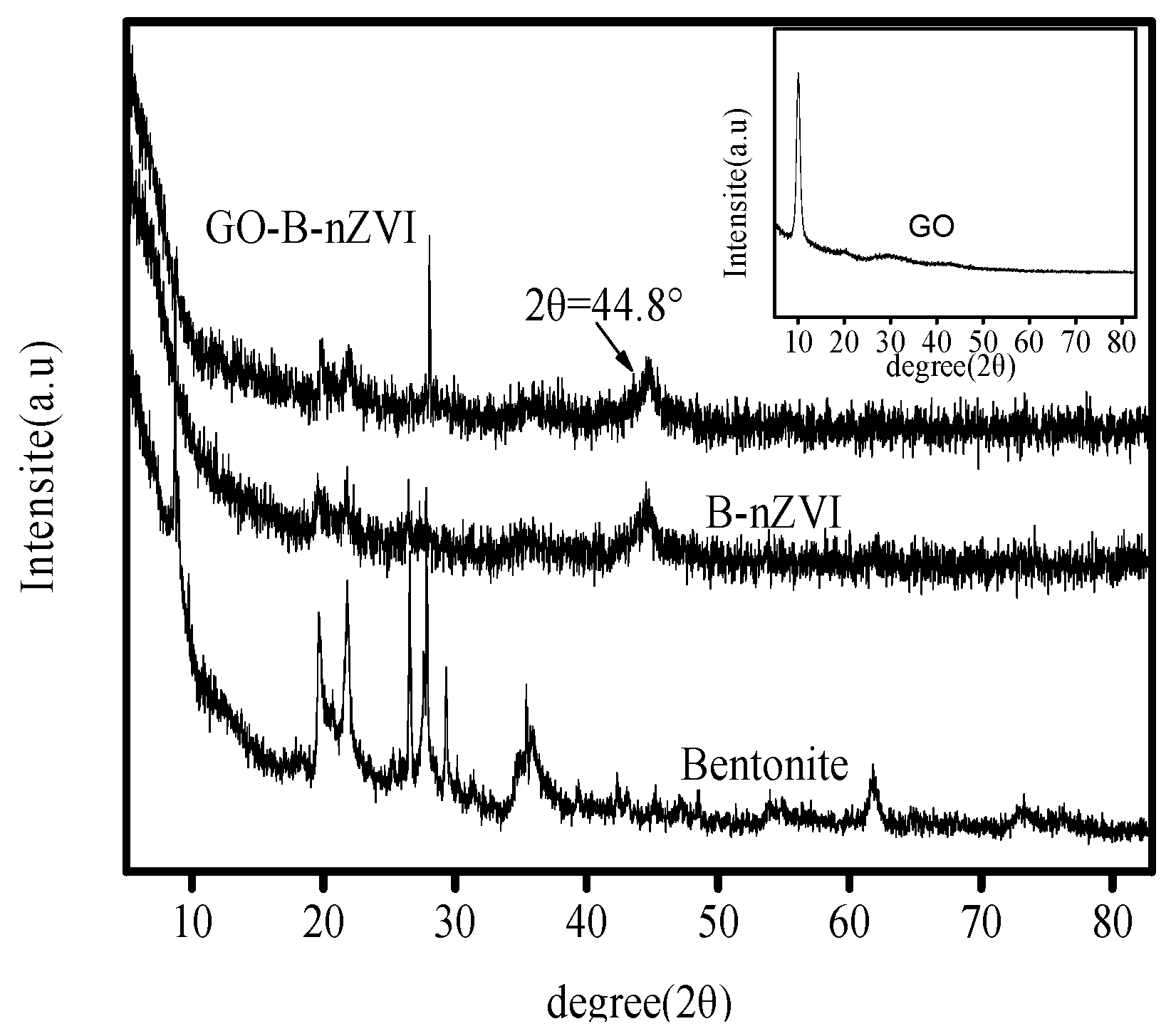
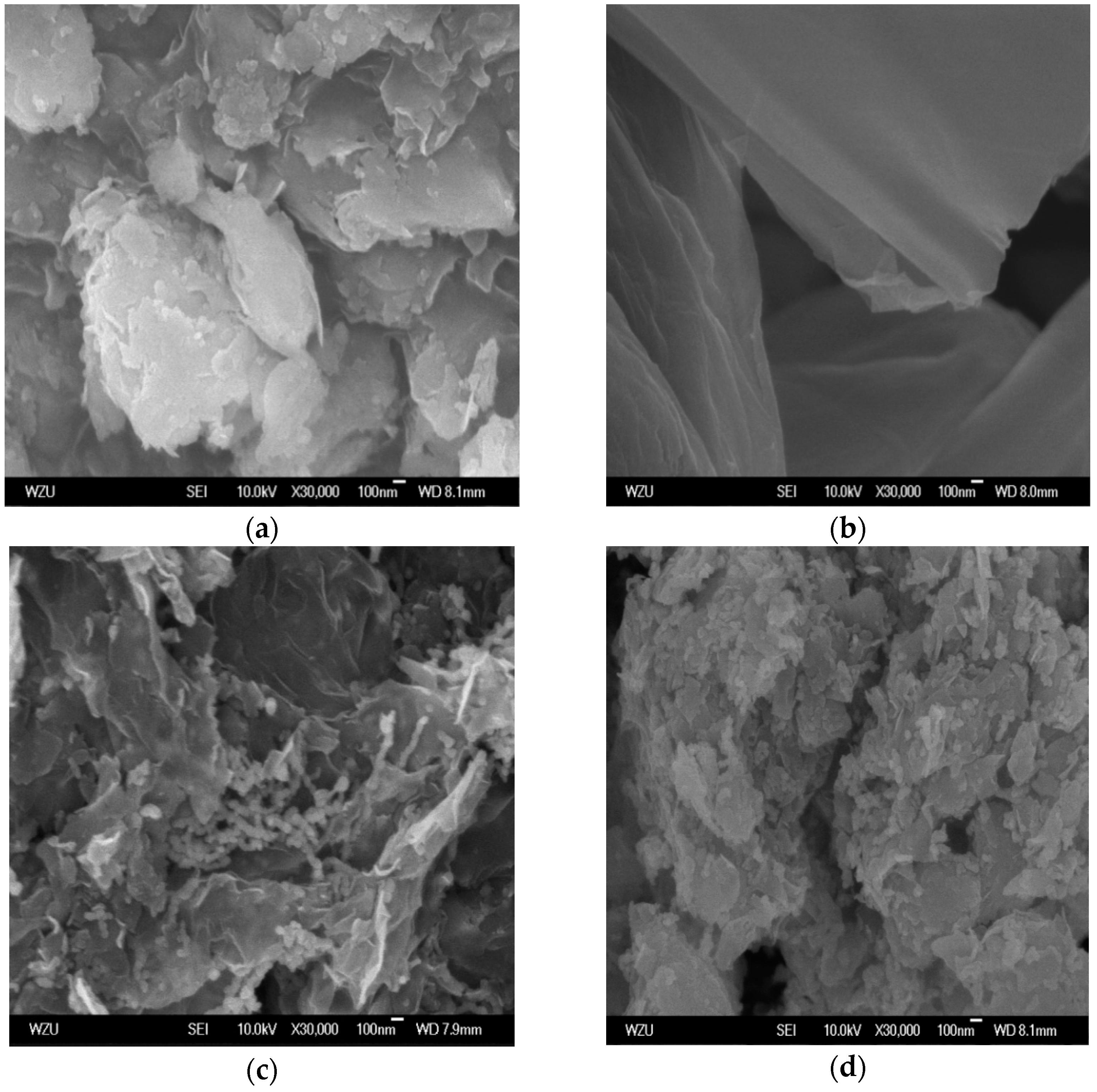
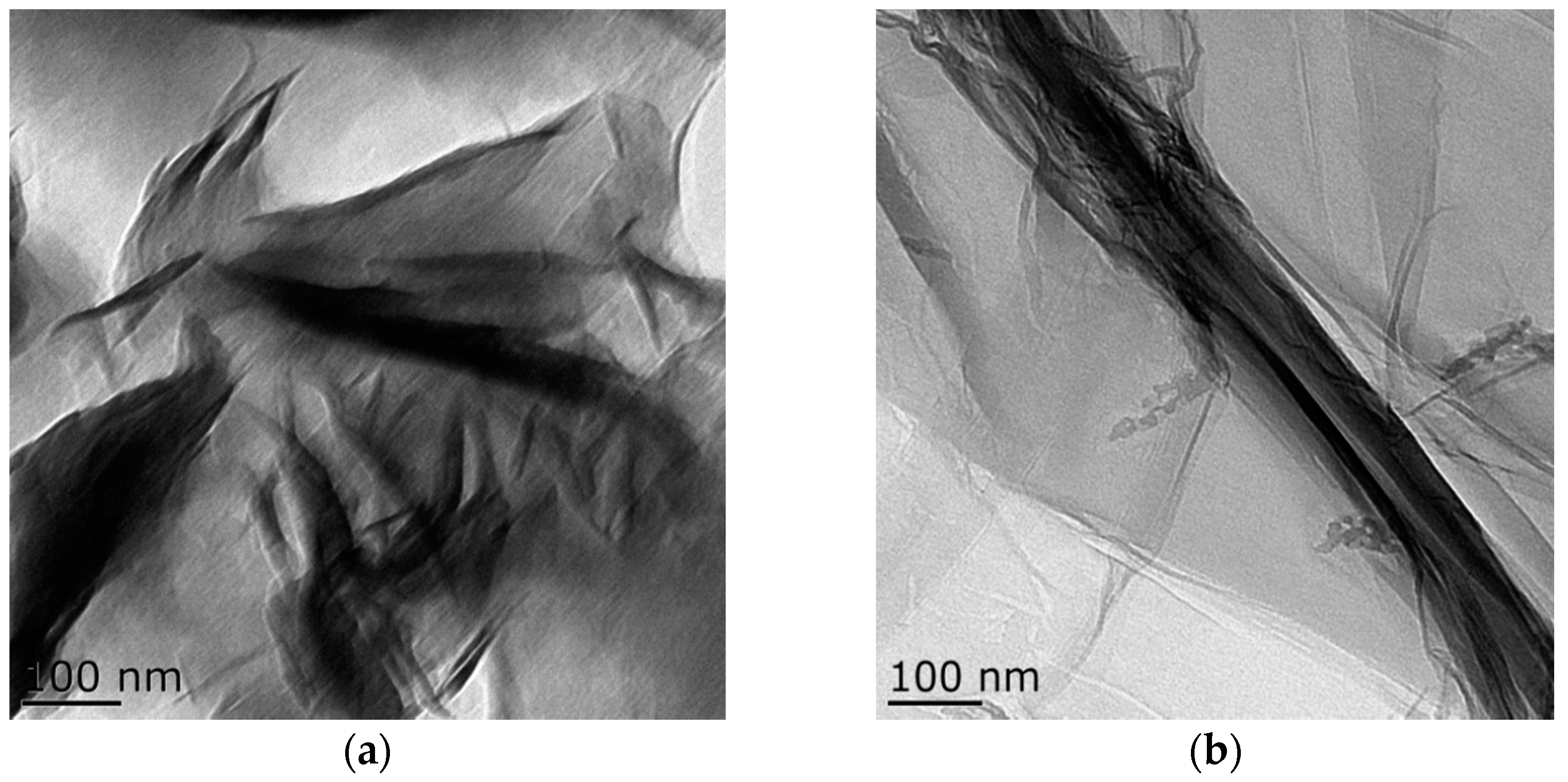
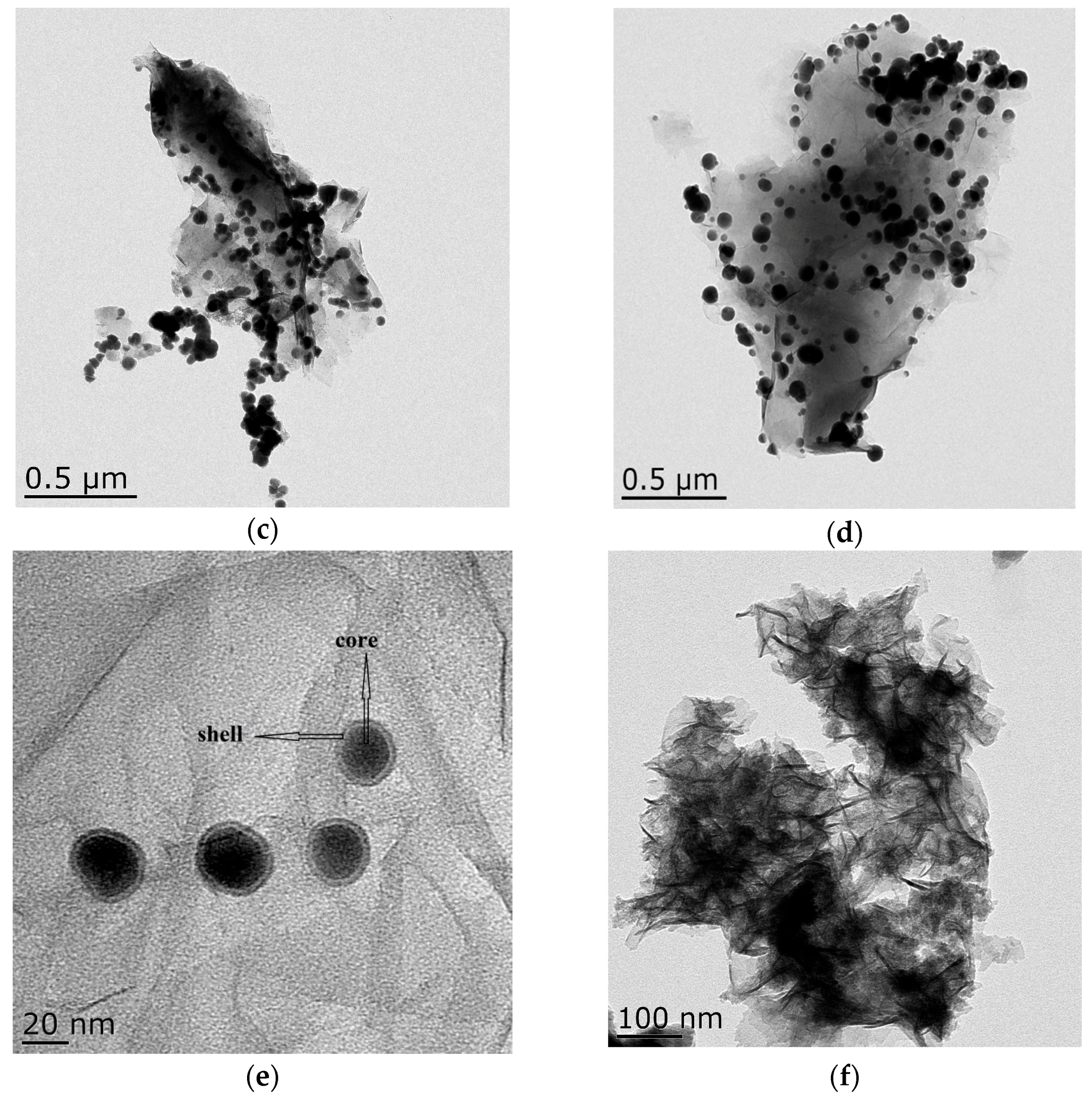
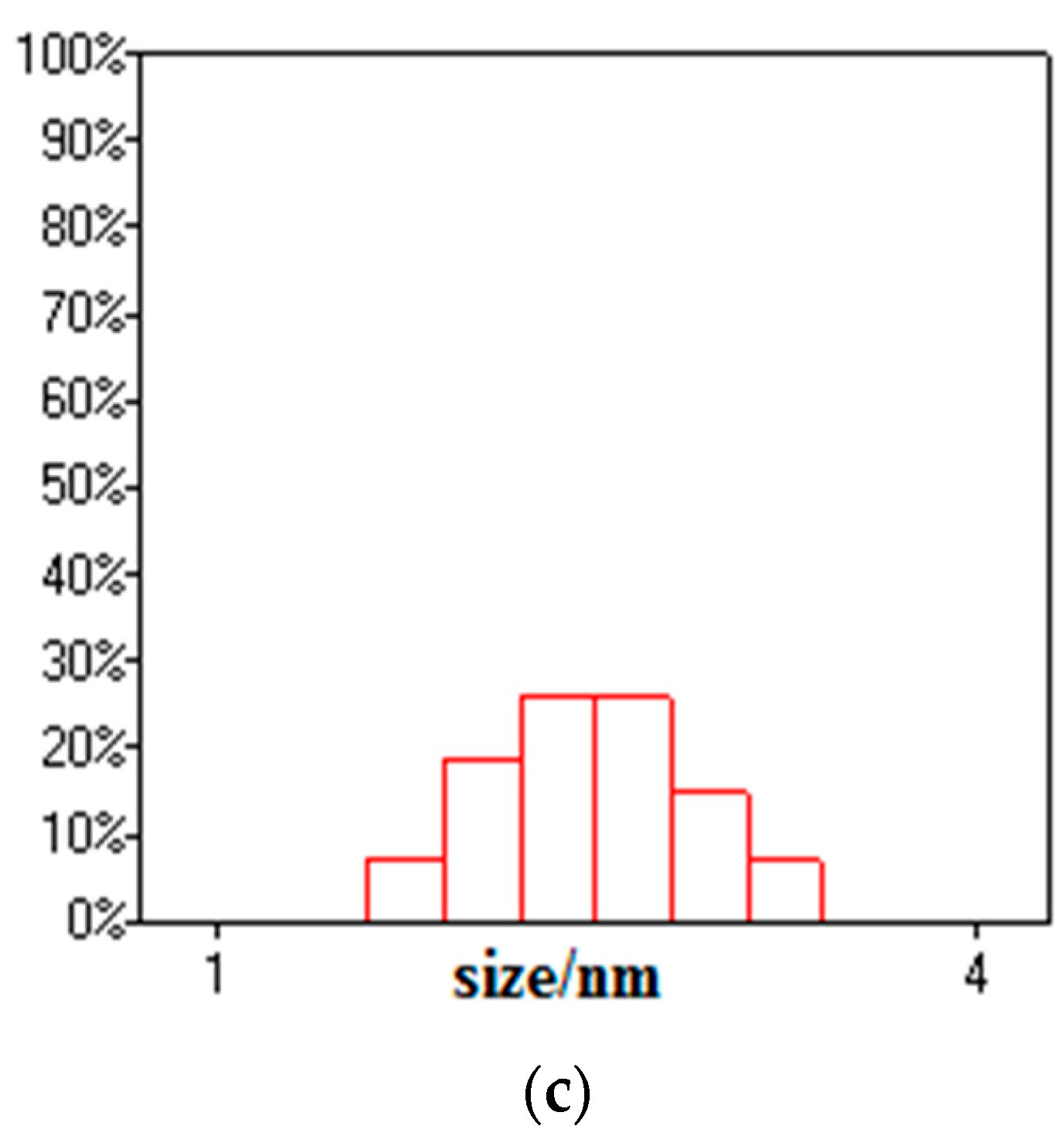
3.1. Composition and Microstructure of Synthesized Materials
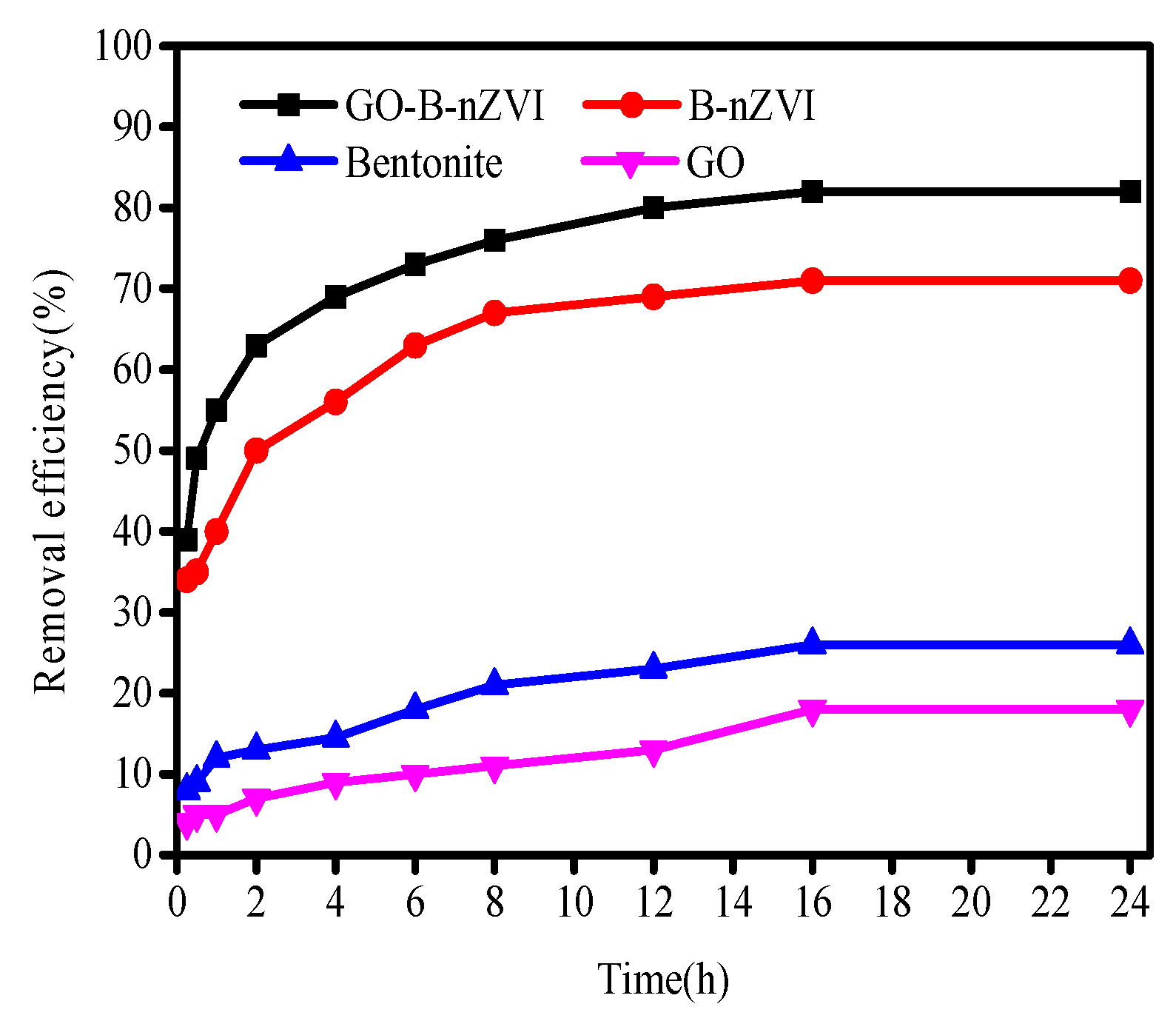
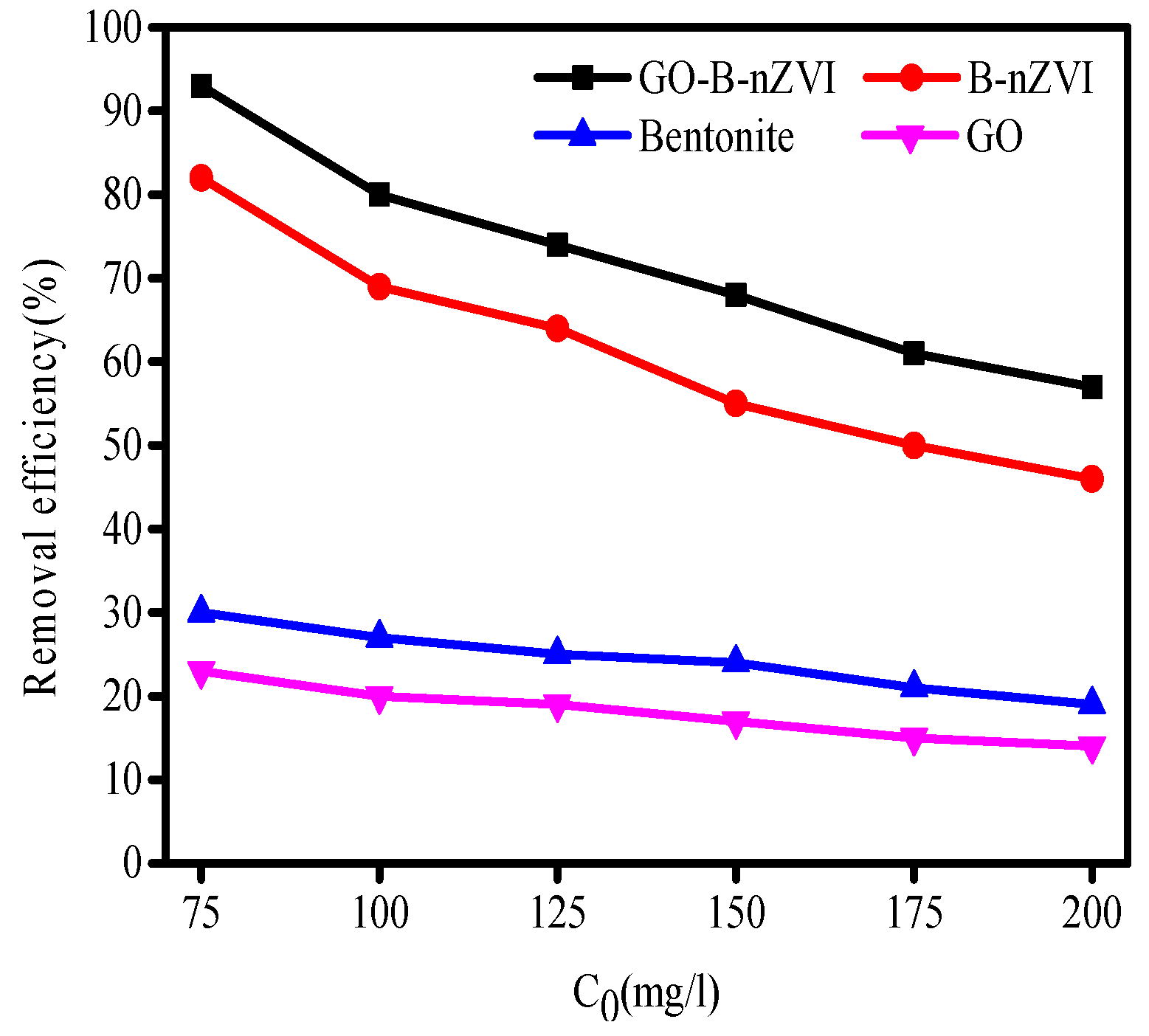
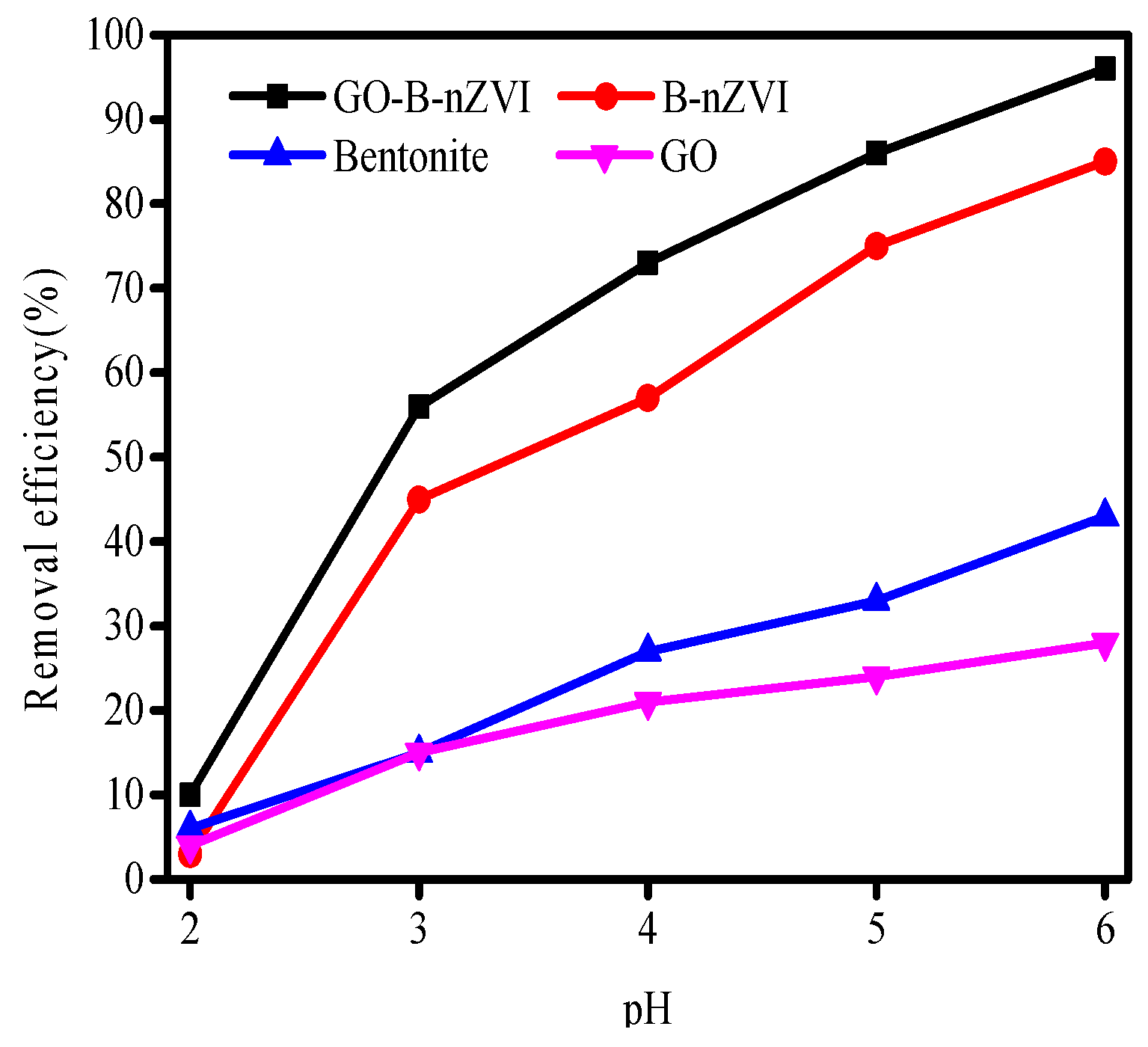
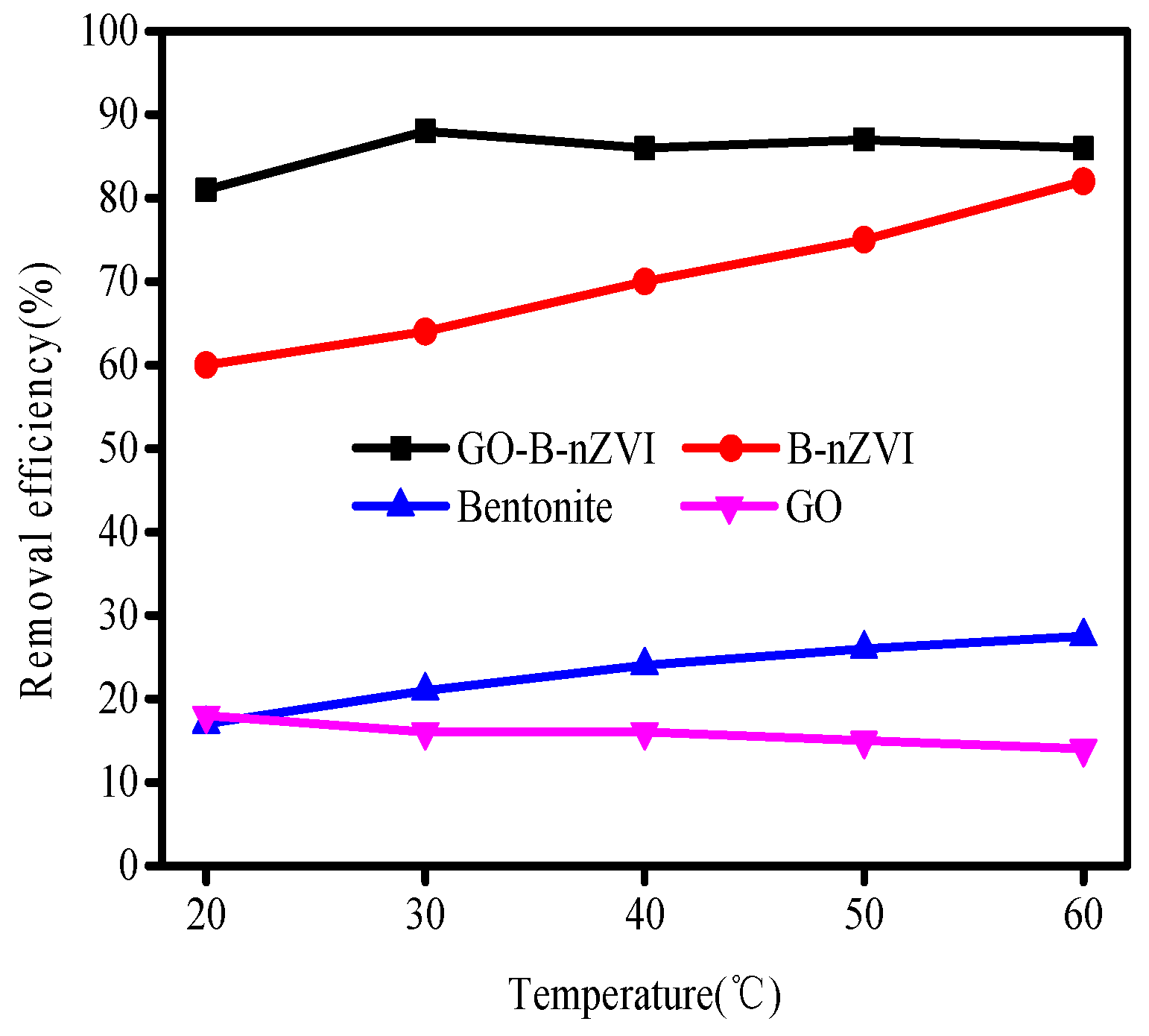
3.2. The Effect of Time, pH, Temperature, and Concentration of Copper Ions on Removal Efficiency
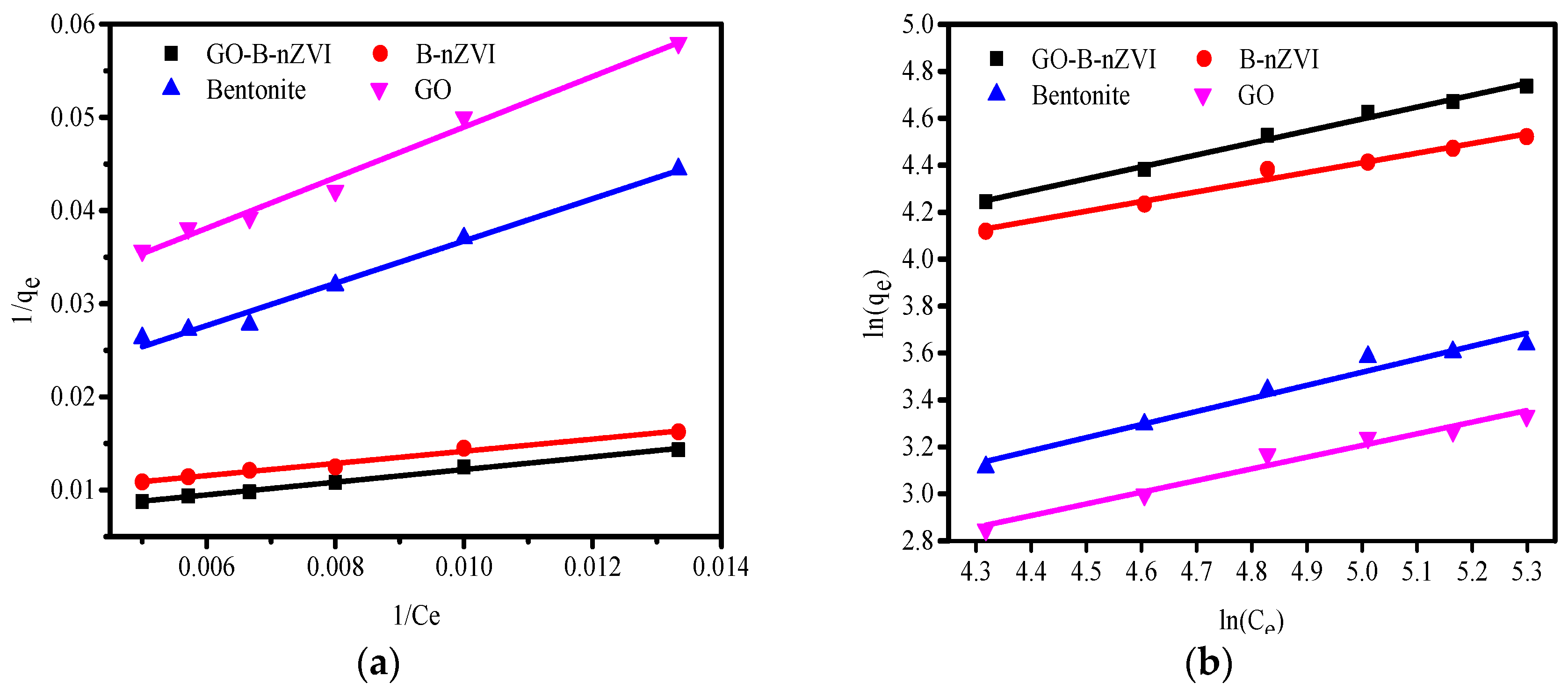
3.3. Adsorption Kinetics and Adsorption Isotherms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, X.F. Research on environmental hazards and countermeasures of copper ions. Land Nat. Resour. Res. 2015, 1, 55–57. [Google Scholar]
- Iftekhar, S.; Srivastava, V.; Mika Sillanpää, M. Synthesis and application of LDH intercalated cellulose nanocomposite for separation of rare earth elements (REEs). Chem. Eng. J. 2017, 309, 130–139. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Mika Sillanpää, M. Enrichment of lanthanides in aqueous system by cellulose based silica nanocomposite. Chem. Eng. J. 2017, 320, 151–159. [Google Scholar] [CrossRef]
- Leupin, O.X.; Hug, S.J.; Badruzzaman, A.B.M. Arsenic removal from Bangladesh tube well water with filter columns containing zerovalent iron filings and sand. Environ. Sci. Technol. 2005, 39, 8032–8037. [Google Scholar] [CrossRef] [PubMed]
- Iftekhar, S.; Srivastava, V.; Ramasamya, D.L.; Naseera, W.A.; Mika Sillanpää, M. A novel approach for synthesis of exfoliated biopolymeric-LDH hybrid nanocomposites via in-stiu coprecipitation with gum Arabic: Application towards REEs recovery. Chem. Eng. J. 2018, 347, 398–406. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Alba Casas, A.; Mika Sillanpa, M. Synthesis of novel GA-g-PAM/SiO2 nanocomposite for the recovery of rare earth elements (REE) ions from aqueous solution. J. Clean. Prod. 2018, 170, 251–259. [Google Scholar] [CrossRef]
- Iftekhar, S.; Srivastava, V.; Hammoudaa, S.B.; Sillanpää, M. Fabrication of novel metal ion imprinted xanthan gum-layered double hydroxide nanocomposite for adsorption of rare earth elements. Carbohydr. Polym. 2018, 194, 274–284. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Liu, W.; Cai, Z.Q.; Han, C.B.; Qian, T.W.; Zhao, D.Y. An overview of preparation and applications of stabilized zero-valent iron nanoparticles for soil and groundwater remediation. Water Res. 2016, 100, 245–266. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.G.; Zong, M.Z.; Yu, Y.; Kong, X.R.; Du, Q.; Liao, Q.J.H. Removal of chromium (VI) from water using nanoscale zerovalent iron particles supported on herb-residue biochar. J. Environ. Manag. 2017, 197, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Shao, J.C.; Cai, X.Q.; Yu, X.N. Research on preparation of nanoscale iron supported by bentonite and its remediation of lead ions. Dig. J. Nanomater. Biostruct. 2018, 13, 31–38. [Google Scholar]
- Liu, T.Y.; Yang, Y.L.; Wang, Z.L.; Sun, Y.Q. Remediation of arsenic(III) from aqueous solutions using improved nanoscale zero-valent iron on pumice. Chem. Eng. J. 2016, 288, 739–744. [Google Scholar] [CrossRef]
- Su, H.J.; Fang, Z.Q.; Tsang, P.E.; Zheng, L.C.; Cheng, W.; Fang, J.Z.; Zhao, D.Y. Remediation of hexavalent chromium contaminated soil by biochar-supported zero-valent iron nanoparticles. J. Hazard. Mater. 2016, 318, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Ezzatahmadi, N.; Ayoko, G.A.; Millar, G.J.; Speight, R.; Yan, C.; Li, J.H.; Li, S.Z.; Zhu, J.X.; Xi, Y.F. Clay-supported nanoscale zero-valent iron composite materials for the remediation of contaminated aqueous solutions: A review. Chem. Eng. J. 2017, 312, 336–350. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Lei, C.; Khan, E.; Chen, S.S.; Tsang, D.C.W.; Ok, Y.S.; Lin, D.H.; Feng, Y.J.; Li, X.D. Nanoscale zero-valent iron for metal/metalloid removal from model hydraulic fracturing wastewater. Chemosphere 2017, 176, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Boparai, H.K.; Joseph, M.; O’Carroll, D.M. Kinetics and thermodynamics of cadmium ion removal by adsorption onto nano zerovalent iron particles. J. Hazard. Mater. 2011, 1986, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Miranda, N.A.; Baltazar, S.E.; García, A.; Romero, A.H.; Rubio, M.A.; Altbir, D. Lead removal by nano- scale zero valent iron: surface analysis and pH effect. Mater. Res. Bull. 2014, 59, 341–348. [Google Scholar] [CrossRef]
- Soliemanzadeh, A.; Fekri, M. The application of green tea extract to prepare bentonite-supported nanoscale zero-valent iron and its performance on removal of Cr(VI): Effect of relative parameters and soil experiments. Micropor. Mesopor. Mater. 2017, 239, 60–69. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Terrones, M.; Botello-Méndez, A.R.; Campos-Delgado, J.; López-Urías, F.; Vega-Cantú, Y.I.; Rodríguez-Macías, F.J.; Elías, A.L.; Muñoz-Sandoval, E.; Cano-Márquez, A.G.; Charlier, J.; et al. Graphene and graphite nanoribbons: Morphology, properties, synthesis, defects and applications. Nano Today 2010, 5, 351–372. [Google Scholar] [CrossRef]
- Tabak, A.; Afsin, B.; Caglar, B.; Koksal, E. Characterization and pillaring of a Turkish bentonite 7(Resadiye). J. Colloid Interface Sci. 2007, 313, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.Z.; Shu-Yan, X.U.; Zhang, W.L.; Meng, L.X. Preparation and properties of graphene and polylactic acid composites. Pack. Eng. 2016, 37, 7–11. [Google Scholar]
- Raghubanshi, H.; Shalate, M.N.; Ntaote, D.S.; Charity, W.D.; Eliazer, B.N.; Ezekiel, D.D.; Rajiv, K.P.; Rajiv, P. Synthesis of graphene oxide and its application for the adsorption of Pb+2 from aqueous solution. J. Ind. Eng. Chem. 2017, 47, 169–178. [Google Scholar] [CrossRef]
- Shang, Y.; Li, T.; Yin, Y.; Li, H.; Dang, A.; Zhang, L.; Chen, X.; Zhang, Y.; Xiong, C.; Zhao, T. Effect of the graphene derived from thermal reduction within matrix on the performance of graphene/poly (methyl methacrylate) composites. J. Anal. Appl. Pyrol. 2016, 120, 215–221. [Google Scholar] [CrossRef]
- Jiao, C.; Cheng, Y.; Fan, W.; Li, J. Synthesis of agar-stabilized nanoscale zero-valent iron particles and removal study of hexavalent chromium. Environ. Sci. Technol. 2015, 12, 1603–1612. [Google Scholar] [CrossRef]
- Diao, Z.H.; Xu, X.R.; Chen, H.; Jiang, D.; Yang, Y.X.; Kong, L.J.; Sun, Y.X.; Hu, Y.X.; Hao, Q.W.; Liu, L. Simultaneous removal of Cr(VI) and phenol by persulfate activated with bentonite-supported nanoscale zero-valent iron: Reactivity and mechanism. J. Hazard. Mater. 2016, 316, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.X.; Jin, X.Y.; Chen, Z.L.; Mallavarapu, M.; Ravendra, N. Removal of methyl orange from aqueous solution using bentonite-supported nanoscale zero-valent iron. J. Colloid Interface Sci. 2011, 363, 603–607. [Google Scholar] [CrossRef] [PubMed]
- Jabeen, H.; Kemp, K.C.; Chandra, V. Synthesis of nano zerovalent iron nanoparticles-Graphene composite for the treatment of lead contaminated water. J. Environ. Manag. 2013, 130, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Shahwan, T.; Üzüm, Ç.; Eroğlu, A.E.; Lieberwirth, I. Synthesis and characterization of bentonite/iron nanoparticles and their application as adsorbent of cobalt ions. Appl. Clay Sci. 2009, 47, 257–262. [Google Scholar] [CrossRef]
- Kurahashi, T.; Oda, K.; Sugimoto, M.; Ogura, T.; Fujii, H. Trigonal-bipyramidal geometry induced by an external water ligand in a sterically hindered iron salen complex, related to the active site of protocatechuate 3,4-dioxygenase. Inorg. Chem. 2006, 45, 7709–7721. [Google Scholar] [CrossRef] [PubMed]
- Üzüm, Ç.; Shahwan, T.; Eroğlu, A.E.; Lieberwirth, I.; Scott, T.B.; Hallam, K.R. Application of zero-valent iron nanoparticles for the removal of aqueous Co2+ ions under various experimental conditions. Chem. Eng. J. 2008, 144, 213–220. [Google Scholar] [CrossRef]
- Fu, R.B.; Yang, Y.P.; Xu, Z.; Zhang, X.; Guo, X.P.; Bi, D.S. The removal of chromium (VI) and lead (II) from groundwater using sepiolite-supported nanoscale zero-valent iron (S-NZVI). Chemosphere 2015, 138, 726–734. [Google Scholar] [CrossRef] [PubMed]
- Nurmi, J.T.; Tratnyek, P.G.; Sarathy, V.; Bear, D.R.; Amonette, J.E.; Peacher, K.; Wang, C.; Linehan, J.C.; Matson, D.W.; Penn, R.L.; et al. Characterization and properties of metallic iron nanoparticles: spectroscopy, electrochemistry, and kinetics. Environ. Sci. Technol. 2005, 39, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Q.; Zhang, W.X. Sequestration of metal cations with zerovalent iron nanoparticles—a study with high resolution X-ray photoelectron spectroscopy (HR-XPS). J. Phys. Chem. C 2007, 111, 6939–6946. [Google Scholar] [CrossRef]
- Sherman, M.; Ponder, G.; Darab, T.E.M. Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ. Sci. Technol. 2000, 34, 2564–2569. [Google Scholar]
- Tiberg, C.; Kumpiene, J.; Gustafsson, J.P.; Marsz, A.; Persson, I.; Mench, M.; Kleja, D.B. Immobilization of Cu and As in two contaminated soils with zerovalent iron e Long-term performance and mechanisms. Appl. Geochem. 2016, 67, 144–152. [Google Scholar] [CrossRef]
- Ho, Y.S. Review of second-order models for adsorption systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V. Adsorption kinetics and isotherms for the removal of rhodamine B dye and Pb2+ ions from aqueous solutions by a hybrid ion-exchanger. Arab. J. Chem. 2016, in press. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, W.; Mao, Y.; Zhang, L.; Cheng, J.; Gong, M.; Zhao, H.B.; Dai, L.M.; Zhang, S.; Zhao, Q. Adsorption behavior of copper ions from aqueous solution onto graphene oxide-CdS composite. Chem. Eng. J. 2015, 259, 603–610. [Google Scholar] [CrossRef]
- Agarwal, A.K.; Kadu, M.S.; Pandhurnekar, C.P.; Muthreja, I.L. Brunauer-Emmett-Teller (B.E.T.), Langmuir and Freundlich isotherm studies for the adsorption of nickel ions onto coal fly ash. Asian J. Water Environ. Pollut. 2016, 13, 49–53. [Google Scholar] [CrossRef]


| SiO2 | Al2O3 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O |
|---|---|---|---|---|---|---|---|
| 61.62 | 14.18 | 2.45 | 0.4 | 1.77 | 2.33 | 2.13 | 1.1 |
| Samples | Surface Area (m2·g−1) | Average Pore Diameter (nm) |
|---|---|---|
| Bentonite | 32.4 | 10.57 |
| GO | 53.80 | 18.75 |
| B-nZVI | 42.88 | 11.56 |
| GO-B-nZVI | 47.32 | 12.06 |
| Absorbents | qe,exp/(mg·g−1) | Pseudo-First-Order Kinetic Model | Pseudo-Second-Order Kinetic Model | ||||
|---|---|---|---|---|---|---|---|
| k1 | qe,cal/(mg·g−1) | R2 | k2·h−1 | qe,cal/(mg·g−1) | R2 | ||
| bentonite | 26 | 0.1496 | 18.18 | 0.980 | 0.0207 | 27.74 | 0.982 |
| GO | 18 | 0.0851 | 13.61 | 0.985 | 0.0172 | 19.56 | 0.928 |
| B-nZVI | 71 | 0.2580 | 38.71 | 0.988 | 0.0190 | 73.37 | 0.999 |
| GO-B-nZVI | 82 | 0.2404 | 36.86 | 0.983 | 0.0211 | 83.89 | 0.999 |
| Absorbents | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| qm/(mg·g−1) | KL/(L·mg−1) | R2 | KF | n | R2 | |
| Bentonite | 71.2 | 0.0062 | 0.985 | 2.0813 | 1.795 | 0.961 |
| GO | 45.9 | 0.0080 | 0.985 | 2.046 | 2.008 | 0.967 |
| B-nZVI | 130.7 | 0.0117 | 0.984 | 10.465 | 2.427 | 0.976 |
| GO-B-nZVI | 184.5 | 0.0080 | 0.993 | 7.766 | 1.965 | 0.996 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shao, J.; Yu, X.; Zhou, M.; Cai, X.; Yu, C. Nanoscale Zero-Valent Iron Decorated on Bentonite/Graphene Oxide for Removal of Copper Ions from Aqueous Solution. Materials 2018, 11, 945. https://doi.org/10.3390/ma11060945
Shao J, Yu X, Zhou M, Cai X, Yu C. Nanoscale Zero-Valent Iron Decorated on Bentonite/Graphene Oxide for Removal of Copper Ions from Aqueous Solution. Materials. 2018; 11(6):945. https://doi.org/10.3390/ma11060945
Chicago/Turabian StyleShao, Jicheng, Xiaoniu Yu, Min Zhou, Xiaoqing Cai, and Chuang Yu. 2018. "Nanoscale Zero-Valent Iron Decorated on Bentonite/Graphene Oxide for Removal of Copper Ions from Aqueous Solution" Materials 11, no. 6: 945. https://doi.org/10.3390/ma11060945
APA StyleShao, J., Yu, X., Zhou, M., Cai, X., & Yu, C. (2018). Nanoscale Zero-Valent Iron Decorated on Bentonite/Graphene Oxide for Removal of Copper Ions from Aqueous Solution. Materials, 11(6), 945. https://doi.org/10.3390/ma11060945





