Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles
Abstract
1. Introduction
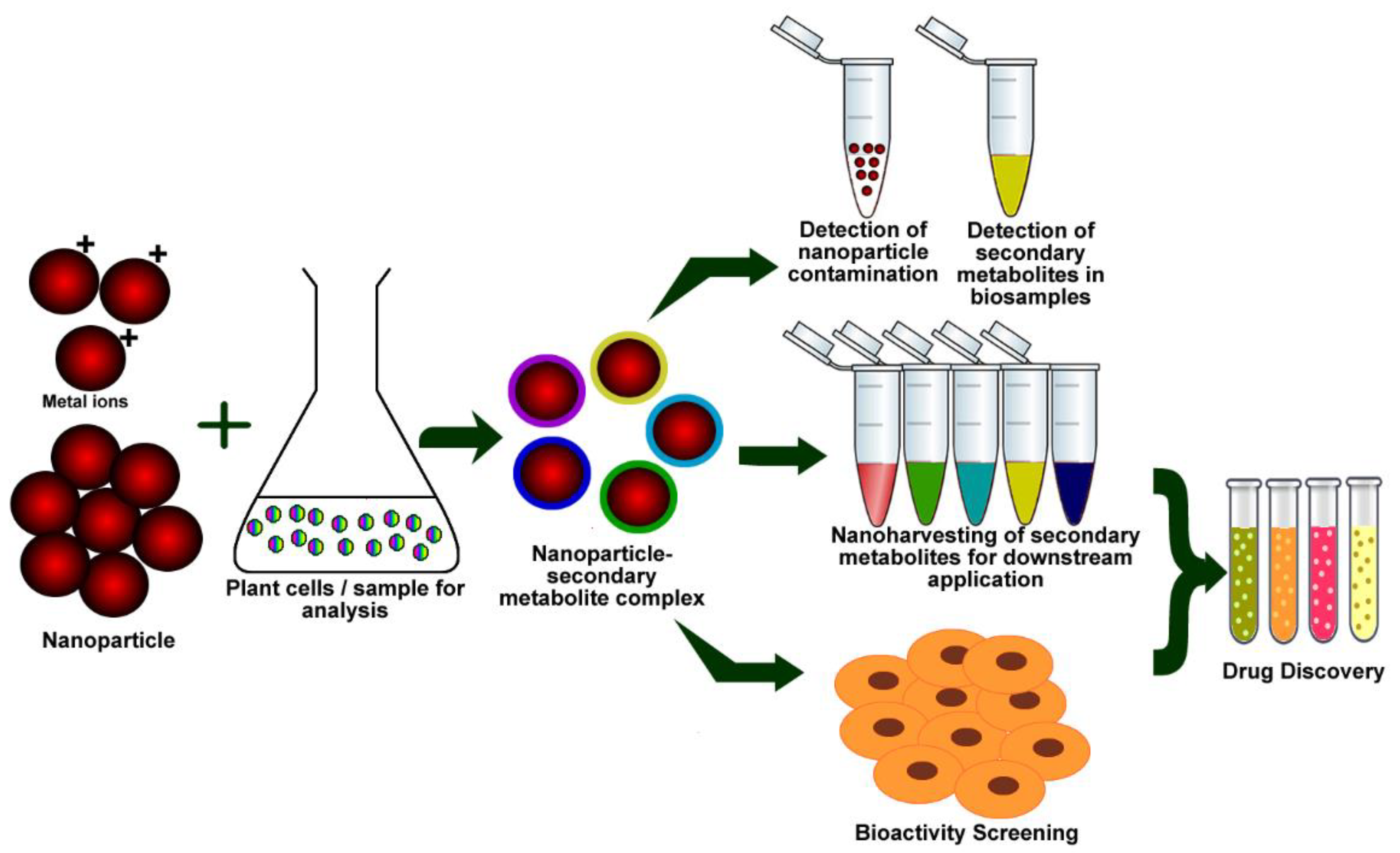
2. Mechanism of Green Synthesis of NPs by Plants
3. Mechanism of Green Synthesis of NPs by Plant Extracts
4. Secondary Metabolites in Plant Extract-Mediated Green Synthesis of NPs
5. Flavonoids are the Major Contributors of Green Synthesis of NPs
6. Green-Synthesized NPs are Highly Bioactive and Biocompatible
7. Potential Applications of Green-Synthesized NPs
8. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Agarwal, H.; Venkat Kumar, S.; Rajeshkumar, S. A review on green synthesis of zinc oxide nanoparticles—An eco-friendly approach. Resour. Effic. Technol. 2017, 3, 406–413. [Google Scholar] [CrossRef]
- Prasad, R.; Bhattacharyya, A.; Nguyen, Q.D. Nanotechnology in sustainable agriculture: Recent developments, challenges, and perspectives. Front. Microbiol. 2017, 8, 1014. [Google Scholar] [CrossRef] [PubMed]
- Dimkpa, C.O.; Bindraban, P.S. Nanofertilizers: New Products for the Industry? J. Agric. Food Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, A.M.; Balaji, K.; Girilal, M.; Yadav, R.; Kalaichelvan, P.T.; Venketesan, R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: A study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Graña, S.; Perez-Ameneiro, M.; Vecino, X.; Pastoriza-Santos, I.; Perez-Juste, J.; Cruz, J.; Moldes, A. Biogenic synthesis of metal nanoparticles using a biosurfactant extracted from corn and their antimicrobial properties. Nanomaterials 2017, 7, 139. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, K.B.; Sakthivel, N. Biological synthesis of metal nanoparticles by microbes. Adv. Colloid Interface Sci. 2010, 156, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Gardea-Torresdey, J.L.; Parsons, J.G.; Gomez, E.; Peralta-Videa, J.; Troiani, H.E.; Santiago, P.; Yacaman, M.J. Formation and growth of Au nanoparticles inside live alfalfa plants. Nano Lett. 2002, 2, 397–401. [Google Scholar] [CrossRef]
- Gardea-Torresdey, J.L.; Gomez, E.; Peralta-Videa, J.R.; Parsons, J.G.; Troiani, H.; Jose-Yacaman, M. Alfalfa sprouts: A natural source for the synthesis of silver nanoparticles. Langmuir 2003, 19, 1357–1361. [Google Scholar] [CrossRef]
- Harris, A.T.; Bali, R. On the formation and extent of uptake of silver nanoparticles by live plants. J. Nanopart. Res. 2008, 10, 691–695. [Google Scholar] [CrossRef]
- Haverkamp, R.G.; Marshall, A.T. The mechanism of metal nanoparticle formation in plants: Limits on accumulation. J. Nanopart. Res. 2009, 11, 1453–1463. [Google Scholar] [CrossRef]
- Roh, K.-H.; Kwak, B.-K.; Kim, J.-B.; Lee, K.-R.; Kim, H.-U.; Kim, S.-H. The influence of silver thiosulfate and thidiazuron on shoot regeneration from cotyledon explants of Brassica napus. J. Plant Biotechnol. 2012, 39, 133–139. [Google Scholar] [CrossRef]
- Kumari, R.; Singh, J.S.; Singh, D.P. Biogenic synthesis and spatial distribution of silver nanoparticles in the legume mungbean plant (Vigna radiata L.). Plant Physiol. Biochem. 2017, 110, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Vineet, K.; Kumar, Y.S. Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar]
- Marslin, G.; Selvakesavan, R.K.; Franklin, G.; Sarmento, B.; Dias, A.C. Antimicrobial activity of cream incorporated with silver nanoparticles biosynthesized from Withania somnifera. Int. J. Nanomed. 2015, 10, 5955–5963. [Google Scholar]
- Stan, M.; Lung, I.; Soran, M.-L.; Leostean, C.; Popa, A.; Stefan, M.; Lazar, M.D.; Opris, O.; Silipas, T.-D.; Porav, A.S. Removal of antibiotics from aqueous solutions by green synthesized magnetite nanoparticles with selected agro-waste extracts. Proc. Saf. Environ. Prot. 2017, 107, 357–372. [Google Scholar] [CrossRef]
- Kanchi, S.; Kumar, G.; Lo, A.-Y.; Tseng, C.-M.; Chen, S.-K.; Lin, C.-Y.; Chin, T.-S. Exploitation of de-oiled jatropha waste for gold nanoparticles synthesis: A green approach. Arab. J. Chem. 2018, 11, 247–255. [Google Scholar] [CrossRef]
- Gunalan, S.; Sivaraj, R.; Rajendran, V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog. Nat. Sci. Mater. Int. 2012, 22, 693–700. [Google Scholar] [CrossRef]
- Shaik, M.; Albalawi, G.; Khan, S.; Khan, M.; Adil, S.; Kuniyil, M.; Al-Warthan, A.; Siddiqui, M.; Alkhathlan, H.; Khan, M. “Miswak” based green synthesis of silver nanoparticles: Evaluation and comparison of their microbicidal activities with the chemical synthesis. Molecules 2016, 21, 1478. [Google Scholar] [CrossRef] [PubMed]
- Sudhasree, S.; Shakila Banu, A.; Brindha, P.; Kurian, G.A. Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity. Toxicol. Environ. Chem. 2014, 96, 743–754. [Google Scholar] [CrossRef]
- Bala, N.; Saha, S.; Chakraborty, M.; Maiti, M.; Das, S.; Basu, R.; Nandy, P. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: Effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015, 5, 4993–5003. [Google Scholar] [CrossRef]
- Singh, J.; Mehta, A.; Rawat, M.; Basu, S. Green synthesis of silver nanoparticles using sun dried tulsi leaves and its catalytic application for 4-Nitrophenol reduction. J. Environ. Chem. Eng. 2018, 6, 1468–1474. [Google Scholar] [CrossRef]
- Yuan, C.-G.; Huo, C.; Gui, B.; Liu, P.; Zhang, C. Green synthesis of silver nanoparticles using Chenopodium aristatum L. stem extract and their catalytic/antibacterial activities. J. Clust. Sci. 2017, 28, 1319–1333. [Google Scholar] [CrossRef]
- Anjum, N.A.; Hasanuzzaman, M.; Hossain, M.A.; Thangavel, P.; Roychoudhury, A.; Gill, S.S.; Rodrigo, M.A.M.; Adam, V.; Fujita, M.; Kizek, R.; et al. Jacks of metal/metalloid chelation trade in plants—An overview. Front. Plant Sci. 2015, 6, 192. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.-Y.; Ahammed, G.J.; Qi, Z.-Y. Responses of Plant Proteins to Heavy Metal Stress—A Review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef] [PubMed]
- Simão, B.N.; Cristiano, S.; Alexandra, S.; Viviana, M.; Manuel, A.; Hernâni, G.; Fernanda, F. An efficient antioxidant system and heavy metal exclusion from leaves make Solanum cheesmaniae more tolerant to Cu than its cultivated counterpart. Food Energy Secur. 2017, 6, 123–133. [Google Scholar]
- Marslin, G.; Sheeba, C.J.; Franklin, G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017, 8, 832. [Google Scholar] [CrossRef] [PubMed]
- Franklin, G.; Conceição, L.F.R.; Kombrink, E.; Dias, A.C.P. Xanthone biosynthesis in Hypericum perforatum cells provides antioxidant and antimicrobial protection upon biotic stress. Phytochemistry 2009, 70, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Parihar, P.; Singh, R.; Singh, V.P.; Prasad, S.M. Heavy metal tolerance in plants: Role of transcriptomics, proteomics, metabolomics, and ionomics. Front. Plant Sci. 2016, 6, 1143. [Google Scholar] [CrossRef] [PubMed]
- Pham, H.N.; Michalet, S.; Bodillis, J.; Nguyen, T.D.; Nguyen, T.K.O.; Le, T.P.Q.; Haddad, M.; Nazaret, S.; Dijoux-Franca, M.-G. Impact of metal stress on the production of secondary metabolites in Pteris vittata L. and associated rhizosphere bacterial communities. Environ. Sci. Pollut. Res. 2017, 24, 16735–16750. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Dı́az, J.; Bernal, A.; Pomar, F.; Merino, F. Induction of shikimate dehydrogenase and peroxidase in pepper (Capsicum annuum L.) seedlings in response to copper stress and its relation to lignification. Plant Sci. 2001, 161, 179–188. [Google Scholar] [CrossRef]
- Jun, M.; Fu, H.Y.; Hong, J.; Wan, X.; Yang, C.S.; Ho, C.T. Comparison of antioxidant activities of isoflavones from kudzu root (Pueraria lobata Ohwi). J. Food Sci. 2003, 68, 2117–2122. [Google Scholar] [CrossRef]
- Cherrak, S.A.; Mokhtari-Soulimane, N.; Berroukeche, F.; Bensenane, B.; Cherbonnel, A.; Merzouk, H.; Elhabiri, M. In vitro antioxidant versus metal ion chelating properties of flavonoids: A structure-activity investigation. PLoS ONE 2016, 11, e0165575. [Google Scholar] [CrossRef] [PubMed]
- Matile, P. Das toxische Kompartiment der Pflanzenzelle. Naturwissenschaften 1984, 71, 18–24. [Google Scholar] [CrossRef]
- Zhao, J. Flavonoid transport mechanisms: How to go, and with whom. Trends Plant Sci. 2015, 20, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Marinova, K.; Pourcel, L.; Weder, B.; Schwarz, M.; Barron, D.; Routaboul, J.-M.; Debeaujon, I.; Klein, M. The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 2007, 19, 2023. [Google Scholar] [CrossRef] [PubMed]
- Manceau, A.; Nagy, K.L.; Marcus, M.A.; Lanson, M.; Geoffroy, N.; Jacquet, T.; Kirpichtchikova, T. Formation of metallic copper nanoparticles at the soil−root interface. Environ. Sci. Technol. 2008, 42, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Makarov, V.V.; Love, A.J.; Sinitsyna, O.V.; Makarova, S.S.; Yaminsky, I.V.; Taliansky, M.E.; Kalinina, N.O. “Green” nanotechnologies: Synthesis of metal nanoparticles dsing plants. Acta Nat. 2014, 6, 35–44. [Google Scholar]
- Smitha, S.L.; Philip, D.; Gopchandran, K.G. Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2009, 74, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.M.H.; Ismail, E.H.; El-Magdoub, F. Biosynthesis of Au nanoparticles using olive leaf extract: 1st Nano Updates. Arab. J. Chem. 2012, 5, 431–437. [Google Scholar] [CrossRef]
- MubarakAli, D.; Thajuddin, N.; Jeganathan, K.; Gunasekaran, M. Plant extract mediated synthesis of silver and gold nanoparticles and its antibacterial activity against clinically isolated pathogens. Colloids Surf. B Biointerfaces 2011, 85, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Suman, T.Y.; Radhika Rajasree, S.R.; Ramkumar, R.; Rajthilak, C.; Perumal, P. The Green synthesis of gold nanoparticles using an aqueous root extract of Morinda citrifolia L. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 118, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Gole, A.; Dash, C.; Ramakrishnan, V.; Sainkar, S.R.; Mandale, A.B.; Rao, M.; Sastry, M. Pepsin-gold colloid conjugates: Preparation, characterization, and enzymatic activity. Langmuir 2001, 17, 1674–1679. [Google Scholar] [CrossRef]
- Vanaja, M.; Annadurai, G. Coleus aromaticus leaf extract mediated synthesis of silver nanoparticles and its bactericidal activity. Appl. Nanosci. 2013, 3, 217–223. [Google Scholar] [CrossRef]
- Prasad, R.; Swamy, V.S. Antibacterial activity of silver nanoparticles synthesized by bark extract of Syzygium cumini. J. Nanoparticles 2013, 2013, 6. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green synthesis of silver nanoparticles using Azadirachta indica aqueous leaf extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Kaviya, S.; Santhanalakshmi, J.; Viswanathan, B.; Muthumary, J.; Srinivasan, K. Biosynthesis of silver nanoparticles using Citrus sinensis peel extract and its antibacterial activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 79, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Velmurugan, P.; Anbalagan, K.; Manosathyadevan, M.; Lee, K.J.; Cho, M.; Lee, S.M.; Park, J.H.; Oh, S.G.; Bang, K.S.; Oh, B.T. Green synthesis of silver and gold nanoparticles using Zingiber officinale root extract and antibacterial activity of silver nanoparticles against food pathogens. Bioprocess Biosyst. Eng. 2014, 37, 1935–1943. [Google Scholar] [CrossRef] [PubMed]
- Ramteke, C.; Chakrabarti, T.; Sarangi, B.K.; Pandey, R.-A. Synthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. J. Chem. 2013, 2013, 7. [Google Scholar] [CrossRef]
- Dinesh, S.; Karthikeyan, S.; Arumugam, P. Biosynthesis of silver nanoparticles from Glycyrrhiza glabra root extract. Arch Appl. Sci. Res. 2012, 4, 178–187. [Google Scholar]
- Chahardoli, A.; Karimi, N.; Fattahi, A. Biosynthesis, characterization, antimicrobial and cytotoxic effects of silver nanoparticles using Nigella arvensis seed extract. Iran. J. Pharm. Res. 2017, 16, 1167–1175. [Google Scholar] [PubMed]
- Ghosh, S.; Patil, S.; Ahire, M.; Kitture, R.; Kale, S.; Pardesi, K.; Cameotra, S.S.; Bellare, J.; Dhavale, D.D.; Jabgunde, A.; et al. Synthesis of silver nanoparticles using Dioscorea bulbifera tuber extract and evaluation of its synergistic potential in combination with antimicrobial agents. Int. J. Nanomed. 2012, 7, 483–496. [Google Scholar]
- Ajitha, B.; Ashok Kumar Reddy, Y.; Shameer, S.; Rajesh, K.M.; Suneetha, Y.; Sreedhara Reddy, P. Lantana camara leaf extract mediated silver nanoparticles: Antibacterial, green catalyst. J. Photochem. Photobiol. B 2015, 149, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Thakore, S.; Rathore, P.S.; Jadeja, R.N.; Thounaojam, M.; Devkar, R.V. Sunflower oil mediated biomimetic synthesis and cytotoxicity of monodisperse hexagonal silver nanoparticles. Mater. Sci. Eng. C 2014, 44, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Ghaedi, M.; Yousefinejad, M.; Safarpoor, M.; Khafri, H.Z.; Purkait, M.K. Rosmarinus officinalis leaf extract mediated green synthesis of silver nanoparticles and investigation of its antimicrobial properties. J. Ind. Eng. Chem. 2015, 31, 167–172. [Google Scholar] [CrossRef]
- Kiran Kumar, H.A.; Mandal, B.K.; Mohan Kumar, K.; Maddinedi, S. b.; Sai Kumar, T.; Madhiyazhagan, P.; Ghosh, A.R. Antimicrobial and antioxidant activities of Mimusops elengi seed extract mediated isotropic silver nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 130, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, S.C.; Yadav, S.K. Syzygium cumini leaf and seed extract mediated biosynthesis of silver nanoparticles and their characterization. J. Chem. Technol. Biotechnol. 2010, 85, 1301–1309. [Google Scholar] [CrossRef]
- Jain, S.; Mehata, M.S. Medicinal plant leaf extract and pure flavonoid mediated green synthesis of silver nanoparticles and their enhanced antibacterial property. Sci. Rep. 2017, 7, 15867. [Google Scholar] [CrossRef] [PubMed]
- Krishnaraj, C.; Jagan, E.G.; Rajasekar, S.; Selvakumar, P.; Kalaichelvan, P.T.; Mohan, N. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf. B Biointerfaces 2010, 76, 50–56. [Google Scholar] [CrossRef] [PubMed]
- LÜ, F.; Gao, Y.; Huang, J.; Sun, D.; Li, Q. Roles of biomolecules in the biosynthesis of silver nanoparticles: Case of Gardenia jasminoides extract. Chin. J. Chem. Eng. 2014, 22, 706–712. [Google Scholar] [CrossRef]
- Cruz, D.; Fale, P.L.; Mourato, A.; Vaz, P.D.; Serralheiro, M.L.; Lino, A.R. Preparation and physicochemical characterization of Ag nanoparticles biosynthesized by Lippia citriodora (Lemon Verbena). Colloids Surf. B Biointerfaces 2010, 81, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rashmi, V.; Sanjay, K.R. Green synthesis, characterisation and bioactivity of plant-mediated silver nanoparticles using Decalepis hamiltonii root extract. IET Nanobiotechnol. 2017, 11, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Elumalai, D.; Kaleena, P.K.; Ashok, K.; Suresh, A.; Hemavathi, M. Green synthesis of silver nanoparticle using Achyranthes aspera and its larvicidal activity against three major mosquito vectors. Eng. Agric. Environ. Food 2016, 9, 1–8. [Google Scholar] [CrossRef]
- Suman, T.Y.; Rajasree, S.R.R.; Jayaseelan, C.; Mary, R.R.; Gayathri, S.; Aranganathan, L.; Remya, R.R. GC-MS analysis of bioactive components and biosynthesis of silver nanoparticles using Hybanthus enneaspermus at room temperature evaluation of their stability and its larvicidal activity. Environ. Sci. Pollut. Res. 2016, 23, 2705–2714. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, N.; Sharma, S.; Singh, V.N.; Shamsi, S.F.; Fatma, A.; Mehta, B.R. Biosynthesis of Silver Nanoparticles from Desmodium triflorum: A Novel Approach Towards Weed Utilization. Biotechnol. Res. Int. 2011, 2011, 454090. [Google Scholar] [CrossRef] [PubMed]
- Nabikhan, A.; Kandasamy, K.; Raj, A.; Alikunhi, N.M. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf. B Biointerfaces 2010, 79, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Sengottaiyan, A.; Mythili, R.; Selvankumar, T.; Aravinthan, A.; Kamala-Kannan, S.; Manoharan, K.; Thiyagarajan, P.; Govarthanan, M.; Kim, J.-H. Green synthesis of silver nanoparticles using Solanum indicum L. and their antibacterial, splenocyte cytotoxic potentials. Res. Chem. Int. 2016, 42, 3095–3103. [Google Scholar] [CrossRef]
- Mukunthan, K.S.; Balaji, S. Cashew apple juice (Anacardium occidentale L.) speeds up the synthesis of silver nanoparticles. Int. J. Green Nanotechnol. 2012, 4, 71–79. [Google Scholar] [CrossRef]
- Dipankar, C.; Murugan, S. The green synthesis, characterization and evaluation of the biological activities of silver nanoparticles synthesized from Iresine herbstii leaf aqueous extracts. Colloids Surf. B Biointerfaces 2012, 98, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Geethalakshmi, R.; Sarada, D.V. Synthesis of plant-mediated silver nanoparticles using Trianthema decandra extract and evaluation of their antimicrobial activities. Int. J. Eng. Sci. Technol. 2010, 2, 970–975. [Google Scholar]
- Kumar, K.R.; Nattuthurai, N.; Gopinath, P.; Mariappan, T. Synthesis of eco-friendly silver nanoparticles from Morinda tinctoria leaf extract and its larvicidal activity against Culex quinquefasciatus. Parasitol. Res. 2015, 114, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Banala, R.R.; Nagati, V.B.; Karnati, P.R. Green synthesis and characterization of Carica papaya leaf extract coated silver nanoparticles through X-ray diffraction, electron microscopy and evaluation of bactericidal properties. Saudi J. Biol. Sci. 2015, 22, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Vivek, R.; Thangam, R.; Muthuchelian, K.; Gunasekaran, P.; Kaveri, K.; Kannan, S. Green biosynthesis of silver nanoparticles from Annona squamosa leaf extract and its in vitro cytotoxic effect on MCF-7 cells. Process Biochem. 2012, 47, 2405–2410. [Google Scholar] [CrossRef]
- Geethalakshmi, R.; Sarada, D.V.L. Characterization and antimicrobial activity of gold and silver nanoparticles synthesized using saponin isolated from Trianthema decandra L. Ind. Crops Products 2013, 51, 107–115. [Google Scholar]
- Jagajjanani Rao, K.; Paria, S. Green synthesis of silver nanoparticles from aqueous Aegle marmelos leaf extract. Mater. Res. Bull. 2013, 48, 628–634. [Google Scholar] [CrossRef]
- Dubey, S.P.; Lahtinen, M.; Sillanpää, M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf. A Physicochem. Eng. Asp. 2010, 364, 34–41. [Google Scholar] [CrossRef]
- Nayak, D.; Ashe, S.; Rauta, P.R.; Nayak, B. Biosynthesis, characterisation and antimicrobial activity of silver nanoparticles using Hibiscus rosa-sinensis petals extracts. IET Nanobiotechnol. 2015, 9, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Im, A.R.; Han, L.; Kim, E.R.; Kim, J.; Kim, Y.S.; Park, Y. Enhanced antibacterial activities of Leonuri herba extracts containing silver nanoparticles. Phytother. Res. 2012, 26, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Tang, R.-C. Facile and eco-friendly fabrication of AgNPs coated silk for antibacterial and antioxidant textiles using honeysuckle extract. J. Photochem. Photobiol. B Biol. 2018, 178, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Li, W.-H. Mango peel extract mediated novel route for synthesis of silver nanoparticles and antibacterial application of silver nanoparticles loaded onto non-woven fabrics. Ind. Crops Prod. 2013, 48, 81–88. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, X.; Chen, Z. The formation of iron nanoparticles by Eucalyptus leaf extract and used to remove Cr(VI). Sci. Total Environ. 2018, 627, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Niraimathi, K.L.; Sudha, V.; Lavanya, R.; Brindha, P. Biosynthesis of silver nanoparticles using Alternanthera sessilis (Linn.) extract and their antimicrobial, antioxidant activities. Colloids Surf. B Biointerfaces 2013, 102, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Kora, A.J.; Sashidhar, R.B.; Arunachalam, J. Aqueous extract of gum olibanum (Boswellia serrata): A reductant and stabilizer for the biosynthesis of antibacterial silver nanoparticles. Process Biochem. 2012, 47, 1516–1520. [Google Scholar] [CrossRef]
- Lagashetty, A.N. Green synthesis and characterization of silver nanoparticles using Piper betel leaf extract. Bull. Adv. Sci. Res. 2015, 1, 136–138. [Google Scholar]
- Patil, C.D.; Patil, S.V.; Borase, H.P.; Salunke, B.K.; Salunkhe, R.B. Larvicidal activity of silver nanoparticles synthesized using Plumeria rubra plant latex against Aedes aegypti and Anopheles stephensi. Parasitol. Res. 2012, 110, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Bar, H.; Bhui, D.K.; Sahoo, G.P.; Sarkar, P.; De, S.P.; Misra, A. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf. A Physicochem. Eng. Asp. 2009, 339, 134–139. [Google Scholar] [CrossRef]
- Yasmin, A.; Ramesh, K.; Rajeshkumar, S. Optimization and stabilization of gold nanoparticles by using herbal plant extract with microwave heating. Nano Converg. 2014, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M. Biosynthesis of gold nanoparticles using extract of grape (Vitis vinifera) leaves and seeds. Prog. Nanotechnol. Nanomater. 2014, 3, 1–12. [Google Scholar]
- Philip, D. Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2010, 77, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Mata, R.; Nakkala, J.R.; Sadras, S.R. Polyphenol stabilized colloidal gold nanoparticles from Abutilon indicum leaf extract induce apoptosis in HT-29 colon cancer cells. Colloids Surf. B Biointerfaces 2016, 143, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Rajathi, F.A.A.; Arumugam, R.; Saravanan, S.; Anantharaman, P. Phytofabrication of gold nanoparticles assisted by leaves of Suaeda monoica and its free radical scavenging property. J. Photochem. Photobiol. B Biol. 2014, 135, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Das, J.; Velusamy, P. Catalytic reduction of methylene blue using biogenic gold nanoparticles from Sesbania grandiflora L. J. Taiwan Inst. Chem. Eng. 2014, 45, 2280–2285. [Google Scholar] [CrossRef]
- Yu, J.; Xu, D.; Guan, H.N.; Wang, C.; Huang, L.K.; Chi, D.F. Facile one-step green synthesis of gold nanoparticles using Citrus maxima aqueous extracts and its catalytic activity. Mater. Lett. 2016, 166, 110–112. [Google Scholar] [CrossRef]
- Elbagory, A.M.; Meyer, M.; Cupido, C.N.; Hussein, A.A. Inhibition of bacteria associated with wound infection by biocompatible green synthesized gold nanoparticles from South African plant extracts. Nanomaterials 2017, 7, 417. [Google Scholar] [CrossRef] [PubMed]
- Chahardoli, A.; Karimi, N.; Sadeghi, F.; Fattahi, A. Green approach for synthesis of gold nanoparticles from Nigella arvensis leaf extract and evaluation of their antibacterial, antioxidant, cytotoxicity and catalytic activities. Artif. Cells Nanomed. Biotechnol. 2018, 46, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.; Mukherjee, S.; Barui, A.K.; Ganguly, A.; Sreedhar, B.; Patra, C.R. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater. Sci. Eng. C 2015, 53, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Bogireddy, N.K.R.; Hoskote Anand, K.K.; Mandal, B.K. Gold nanoparticles—Synthesis by Sterculia acuminata extract and its catalytic efficiency in alleviating different organic dyes. J. Mol. Liq. 2015, 211, 868–875. [Google Scholar] [CrossRef]
- Mohan Kumar, K.; Mandal, B.K.; Kiran Kumar, H.A.; Maddinedi, S.B. Green synthesis of size controllable gold nanoparticles. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Ankamwar, B. Biosynthesis of gold nanoparticles (Green-gold) using leaf extract of Terminalia catappa. E J. Chem. 2010, 7, 1334–1339. [Google Scholar] [CrossRef]
- Koperuncholan, M. Bioreduction of chloroauric acid (HAuCl4) for the synthesis of gold nanoparticles (GNPs): A special empathies of pharmacological activity. Int. J. Phytopharm. 2015, 5, 72–80. [Google Scholar]
- Venkatachalam, M.; Govindaraju, K.; Mohamed Sadiq, A.; Tamilselvan, S.; Ganesh Kumar, V.; Singaravelu, G. Functionalization of gold nanoparticles as antidiabetic nanomaterial. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 116, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Kang, S.; Cha, S.H.; Kim, H.S.; Song, K.; Lee, Y.J.; Kim, K.; Kim, Y.S.; Cho, S.; Park, Y. Platycodon saponins from Platycodi Radix (Platycodon grandiflorum) for the green synthesis of gold and silver nanoparticles. Nanoscale Res. Lett. 2018, 13, 018–2436. [Google Scholar] [CrossRef] [PubMed]
- Zayed, M.F.; Eisa, W.H. Phoenix dactylifera L. leaf extract phytosynthesized gold nanoparticles; controlled synthesis and catalytic activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2014, 121, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Shankar, S.; Jaiswal, L.; Aparna, R.S.L.; Prasad, R.G.S.V. Synthesis, characterization, in vitro biocompatibility, and antimicrobial activity of gold, silver and gold silver alloy nanoparticles prepared from Lansium domesticum fruit peel extract. Mater. Lett. 2014, 137, 75–78. [Google Scholar] [CrossRef]
- Islam, N.U.; Jalil, K.; Shahid, M.; Rauf, A.; Muhammad, N.; Khan, A.; Shah, M.R.; Khan, M.A. Green synthesis and biological activities of gold nanoparticles functionalized with Salix alba. Arab. J. Chem. 2015. [Google Scholar] [CrossRef]
- Francis, G.; Thombre, R.; Parekh, F.; Leksminarayan, P. Bioinspired Synthesis of Gold Nanoparticles Using Ficus benghalensis (Indian Banyan) Leaf Extract. Chem. Sci. Trans. 2014, 2014, 470–474. [Google Scholar]
- Arunachalam, K.D.; Arun, L.B.; Annamalai, S.K.; Arunachalam, A.M. Biofunctionalized gold nanoparticles synthesis from gymnema sylvestre and its preliminary anticancer activity. Int. J. Pharm. Pharm. Sci. 2014, 6, 423–430. [Google Scholar]
- Gopinath, K.; Venkatesh, K.S.; Ilangovan, R.; Sankaranarayanan, K.; Arumugam, A. Green synthesis of gold nanoparticles from leaf extract of Terminalia arjuna, for the enhanced mitotic cell division and pollen germination activity. Ind. Crops Prod. 2013, 50, 737–742. [Google Scholar] [CrossRef]
- Ahmad, T.; Bustam, M.A.; Irfan, M.; Moniruzzaman, M.; Anwaar Asghar, H.M.; Bhattacharjee, S. Green synthesis of stabilized spherical shaped gold nanoparticles using novel aqueous Elaeis guineensis (oil palm) leaves extract. J. Mol. Struct. 2018, 1159, 167–173. [Google Scholar] [CrossRef]
- Varun, S.; Sivakumar, S.; Rafiqkhan, M.; Vijayakumar, S. Green synthesis of gold nanoparticles using Argemone mexicana L. Leaf extract and its characterization. Int. J. Pharm. Sci. Rev. Res. 2015, 32, 42–44. [Google Scholar]
- Correa, S.N.; Naranjo, A.M.; Herrera, A.P. Biosynthesis and characterization of gold nanoparticles using extracts of Tamarindus indica L. Leaves. J. Phys. Conf. Ser. 2016, 687, 012082. [Google Scholar] [CrossRef]
- Isaac, R.S.R.; Sakthivel, G.; Murthy, C. Green synthesis of gold and silver nanoparticles using Averrhoa bilimbi fruit extract. J. Nanotechnol. 2013, 2013, 6. [Google Scholar] [CrossRef]
- Sathishkumar, G.; Jha, P.K.; Vignesh, V.; Rajkuberan, C.; Jeyaraj, M.; Selvakumar, M.; Jha, R.; Sivaramakrishnan, S. Cannonball fruit (Couroupita guianensis, Aubl.) extract mediated synthesis of gold nanoparticles and evaluation of its antioxidant activity. J. Mol. Liq. 2016, 215, 229–236. [Google Scholar]
- Dutta, P.P.; Bordoloi, M.; Gogoi, K.; Roy, S.; Narzary, B.; Bhattacharyya, D.R.; Mohapatra, P.K.; Mazumder, B. Antimalarial silver and gold nanoparticles: Green synthesis, characterization and in vitro study. Biomed. Pharmacother. 2017, 91, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Zarzuela, R.; Luna, M.J.; Gil, M.L.A.; Ortega, M.J.; Palacios-Santander, J.M.; Naranjo-Rodríguez, I.; Delgado, J.J.; Cubillana-Aguilera, L.M. Analytical determination of the reducing and stabilization agents present in different Zostera noltii extracts used for the biosynthesis of gold nanoparticles. J. Photochem. Photobiol. B Biol. 2018, 179, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, T.; Anuradha, J.; Ganaie, S.U.; Abbasi, S.A. Gainful utilization of the highly intransigent weed ipomoea in the synthesis of gold nanoparticles. J. King Saud Univ. Sci. 2015, 27, 15–22. [Google Scholar] [CrossRef]
- Vankar, P.S.; Bajpai, D. Preparation of gold nanoparticles from Mirabilis jalapa flowers. Indian J. Biochem. Biophys. 2010, 47, 157–160. [Google Scholar] [PubMed]
- Jimenez Perez, Z.E.; Mathiyalagan, R.; Markus, J.; Kim, Y.J.; Kang, H.M.; Abbai, R.; Seo, K.H.; Wang, D.; Soshnikova, V.; Yang, D.C. Ginseng-berry-mediated gold and silver nanoparticle synthesis and evaluation of their in vitro antioxidant, antimicrobial, and cytotoxicity effects on human dermal fibroblast and murine melanoma skin cell lines. Int. J. Nanomed. 2017, 12, 709–723. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M. Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 2017, 10, S3029–S3039. [Google Scholar] [CrossRef]
- Hajra, A.; Dutta, S.; Mondal, N.K. Mosquito larvicidal activity of cadmium nanoparticles synthesized from petal extracts of marigold (Tagetes sp.) and rose (Rosa sp.) flower. J. Parasit. Dis. Off. Organ Indian Soc. Parasitol. 2016, 40, 1519–1527. [Google Scholar] [CrossRef] [PubMed]
- Nazar, N.; Bibi, I.; Kamal, S.; Iqbal, M.; Nouren, S.; Jilani, K.; Umair, M.; Ata, S. Cu nanoparticles synthesis using biological molecule of P. granatum seeds extract as reducing and capping agent: Growth mechanism and photo-catalytic activity. Int. J. Biol. Macromol. 2018, 106, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, A.D.; Cohen, K.A.; St. Angelo, S.K. Ultrasmall copper nanoparticles synthesized with a plant eea reducing agent. ACS Sustain. Chem. Eng. 2014, 2, 1933–1939. [Google Scholar] [CrossRef]
- Cheirmadurai, K.; Biswas, S.; Murali, R.; Thanikaivelan, P. Green synthesis of copper nanoparticles and conducting nanobiocomposites using plant and animal sources. RSC Adv. 2014, 4, 19507–19511. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Mohammad Sajadi, S. Pd nanoparticles synthesized in situ with the use of Euphorbia granulate leaf extract: Catalytic properties of the resulting particles. J. Colloid Interface Sci. 2016, 462, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Sajadi, S.M.; Maham, M. Green synthesis of palladium nanoparticles using Hippophae rhamnoides Linn leaf extract and their catalytic activity for the Suzuki–Miyaura coupling in water. J. Mol. Catal. A Chem. 2015, 396, 297–303. [Google Scholar] [CrossRef]
- Dauthal, P.; Mukhopadhyay, M. Biosynthesis of palladium nanoparticles using Delonix regia leaf extract and its catalytic activity for nitro-aromatics hydrogenation. Ind. Eng. Chem. Res. 2013, 52, 18131–18139. [Google Scholar] [CrossRef]
- Zheng, B.; Kong, T.; Jing, X.; Odoom-Wubah, T.; Li, X.; Sun, D.; Lu, F.; Zheng, Y.; Huang, J.; Li, Q. Plant-mediated synthesis of platinum nanoparticles and its bioreductive mechanism. J. Colloid Interface Sci. 2013, 396, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Song, J.Y.; Kwon, E.Y.; Kim, B.S. Biological synthesis of platinum nanoparticles using Diopyros kaki leaf extract. Bioprocess Biosyst. Eng. 2010, 33, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Nitnavare, R.; Dewle, A.; Tomar, G.B.; Chippalkatti, R.; More, P.; Kitture, R.; Kale, S.; Bellare, J.; Chopade, B.A. Novel platinum-palladium bimetallic nanoparticles synthesized by Dioscorea bulbifera: Anticancer and antioxidant activities. Int. J. Nanomed. 2015, 10, 7477–7790. [Google Scholar]
- Suresh, D.; Nethravathi, P.C.; Udayabhanu; Rajanaika, H.; Nagabhushana, H; Sharma, S.C. Green synthesis of multifunctional zinc oxide (ZnO) nanoparticles using Cassia fistula plant extract and their photodegradative, antioxidant and antibacterial activities. Mater. Sci. Semicond. Process. 2015, 31, 446–454. [Google Scholar] [CrossRef]
- Elumalai, K.; Velmurugan, S. Green synthesis, characterization and antimicrobial activities of zinc oxide nanoparticles from the leaf extract of Azadirachta indica (L.). Appl. Surf. Sci. 2015, 345, 329–336. [Google Scholar] [CrossRef]
- Jafarirad, S.; Mehrabi, M.; Divband, B.; Kosari-Nasab, M. Biofabrication of zinc oxide nanoparticles using fruit extract of Rosa canina and their toxic potential against bacteria: A mechanistic approach. Mater. Sci. Eng. C 2016, 59, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Dwivedi, S.; Azam, A.; Saquib, Q.; Al-Said, M.S.; Alkhedhairy, A.A.; Musarrat, J. Aloe vera extract functionalized zinc oxide nanoparticles as nanoantibiotics against multi-drug resistant clinical bacterial isolates. J. Colloid Interface Sci. 2016, 472, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Thema, F.T.; Manikandan, E.; Dhlamini, M.S.; Maaza, M. Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett. 2015, 161, 124–127. [Google Scholar] [CrossRef]
- Dobrucka, R.; Dlugaszewska, J. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J. Biol. Sci. 2016, 23, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Rajiv, P.; Rajeshwari, S.; Venckatesh, R. Bio-fabrication of zinc oxide nanoparticles using leaf extract of Parthenium hysterophorus L. and its size-dependent antifungal activity against plant fungal pathogens. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 112, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Anbuvannan, M.; Ramesh, M.; Viruthagiri, G.; Shanmugam, N.; Kannadasan, N. Anisochilus carnosus leaf extract mediated synthesis of zinc oxide nanoparticles for antibacterial and photocatalytic activities. Mater. Sci. Semicond. Process. 2015, 39, 621–628. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Sreekanth, T.V.M.; Tettey, C.O.; Jun, Y.I.; Mook, S.H. Characterization, antibacterial, antioxidant, and cytotoxic activities of ZnO nanoparticles using Coptidis Rhizoma. Bioorgan. Med. Chem. Lett. 2014, 24, 4298–4303. [Google Scholar] [CrossRef] [PubMed]
- Gawade, V.V.; Gavade, N.L.; Shinde, H.M.; Babar, S.B.; Kadam, A.N.; Garadkar, K.M. Green synthesis of ZnO nanoparticles by using Calotropis procera leaves for the photodegradation of methyl orange. J. Mater. Sci. Mater. Electron. 2017, 28, 14033–14039. [Google Scholar] [CrossRef]
- Avoseh, O.N.; Oyedeji, O.O.; Aremu, O.; Nkeh-Chungag, B.N.; Songca, S.P.; Oyedeji, A.O.; Mohan, S.; Oluwafemi, O.S. Biosynthesis of silver nanoparticles from Acacia mearnsii De Wild stem bark and its antinociceptive properties. Green Chem. Lett. Rev. 2017, 10, 59–68. [Google Scholar] [CrossRef]
- Halder, A.; Das, S.; Bera, T.; Mukherjee, A. Rapid synthesis for monodispersed gold nanoparticles in kaempferol and anti-leishmanial efficacy against wild and drug resistant strains. RSC Adv. 2017, 7, 14159–14167. [Google Scholar] [CrossRef]
- Kavitha, S.; Dhamodaran, M.; Prasad, R.; Ganesan, M. Synthesis and characterisation of zinc oxide nanoparticles using terpenoid fractions of Andrographis paniculata leaves. Int. Nano Lett. 2017, 7, 141–147. [Google Scholar] [CrossRef]
- Hwang, S.J.; Jun, S.H.; Park, Y.; Cha, S.-H.; Yoon, M.; Cho, S.; Lee, H.-J.; Park, Y. Green synthesis of gold nanoparticles using chlorogenic acid and their enhanced performance for inflammation. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 1677–1688. [Google Scholar] [CrossRef] [PubMed]
- Sahu, N.; Soni, D.; Chandrashekhar, B.; Satpute, D.B.; Saravanadevi, S.; Sarangi, B.K.; Pandey, R.A. Synthesis of silver nanoparticles using flavonoids: Hesperidin, naringin and diosmin, and their antibacterial effects and cytotoxicity. Int. Nano Lett. 2016, 6, 173–181. [Google Scholar] [CrossRef]
- Kachlicki, P.; Piasecka, A.; Stobiecki, M.; Marczak, Ł. Structural Characterization of Flavonoid Glycoconjugates and Their Derivatives with Mass Spectrometric Techniques. Molecules 2016, 21, 1494. [Google Scholar] [CrossRef] [PubMed]
- Kostyuk, V.A.; Potapovich, A.I.; Kostyuk, T.V.; Cherian, M.G. Metal complexes of dietary flavonoids: Evaluation of radical scavenger properties and protective activity against oxidative stress in vivo. Cell Mol. Biol. 2007, 53, 62–69. [Google Scholar] [PubMed]
- Samsonowicz, M.; Regulska, E.; Kalinowska, M. Hydroxyflavone metal complexes—Molecular structure, antioxidant activity and biological effects. Chem. Biol. Interact. 2017, 273, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Pusz, J.; Nitka, B. Synthesis and physicochemical properties of the complexes of Co(II), Ni(II), and Cu(II) with chrysin. Microchem. J. 1997, 56, 373–381. [Google Scholar] [CrossRef]
- Dowling, S.; Regan, F.; Hughes, H. The characterisation of structural and antioxidant properties of isoflavone metal chelates. J. Inorg. Biochem. 2010, 104, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, G.; Karadag, R.; Eler, A. Aluminium(III), Fe(II) complexes and dyeing properties of apigenin(5,7,4′-trihydroxy flavone). Rev. Anal. Chem. 2010, 29, 211–232. [Google Scholar] [CrossRef]
- Tan, M.; Zhu, J.; Pan, Y.; Chen, Z.; Liang, H.; Liu, H.; Wang, H. Synthesis, cytotoxic activity, and DNA binding properties of Copper (II) complexes with hesperetin, naringenin, and apigenin. Bioinorg. Chem. Appl. 2009, 2009, 347872. [Google Scholar] [CrossRef] [PubMed]
- Perez, C.A.; Wei, Y.; Guo, M. Iron-binding and anti-Fenton properties of baicalein and baicalin. J. Inorg. Biochem. 2009, 103, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Dimitrić Marković, J.M.; Marković, Z.S.; Brdarić, T.P.; Pavelkić, V.M.; Jadranin, M.B. Iron complexes of dietary flavonoids: Combined spectroscopic and mechanistic study of their free radical scavenging activity. Food Chem. 2011, 129, 1567–1577. [Google Scholar] [CrossRef]
- Rygula, A.; Wrobel, T.P.; Szklarzewicz, J.; Baranska, M. Raman and UV–vis spectroscopy studies on luteolin–Al(III) complexes. Vib. Spectrosc. 2013, 64, 21–26. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Toscano, M. The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem. 2011, 125, 288–306. [Google Scholar] [CrossRef]
- Raza, A.; Xu, X.; Xia, L.; Xia, C.; Tang, J.; Ouyang, Z. Quercetin-Iron complex: Synthesis, characterization, antioxidant, DNA binding, DNA cleavage, and antibacterial activity studies. J. Fluoresc. 2016, 26, 2023–2031. [Google Scholar] [CrossRef] [PubMed]
- Sanna, V.; Pala, N.; Dessi, G.; Manconi, P.; Mariani, A.; Dedola, S.; Rassu, M.; Crosio, C.; Iaccarino, C.; Sechi, M. Single-step green synthesis and characterization of gold-conjugated polyphenol nanoparticles with antioxidant and biological activities. Int. J. Nanomed. 2014, 9, 4935–4951. [Google Scholar]
- Gudikandula, K.; Charya Maringanti, S. Synthesis of silver nanoparticles by chemical and biological methods and their antimicrobial properties. J. Exp. Nanosci. 2016, 11, 714–721. [Google Scholar] [CrossRef]
- Kunjiappan, S.; Chowdhury, R.; Bhattacharjee, C. A green chemistry approach for the synthesis and characterization of bioactive gold nanoparticles using Azolla microphylla methanol extract. Front. Mater. Sci. 2014, 8, 123–135. [Google Scholar] [CrossRef]
- Nazeruddin, G.M.; Prasad, N.R.; Waghmare, S.R.; Garadkar, K.M.; Mulla, I.S. Extracellular biosynthesis of silver nanoparticle using Azadirachta indica leaf extract and its anti-microbial activity. J. Alloys Compd. 2014, 583, 272–277. [Google Scholar] [CrossRef]
- Bagherzade, G.; Tavakoli, M.M.; Namaei, M.H. Green synthesis of silver nanoparticles using aqueous extract of saffron (Crocus sativus L.) wastages and its antibacterial activity against six bacteria. Asian Pac. J. Trop. Biomed. 2017, 7, 227–233. [Google Scholar] [CrossRef]
- Gomathi, M.; Rajkumar, P.V.; Prakasam, A.; Ravichandran, K. Green synthesis of silver nanoparticles using Datura stramonium leaf extract and assessment of their antibacterial activity. Resour. Eff. Technol. 2017, 3, 280–284. [Google Scholar] [CrossRef]
- Abdelmoteleb, A.; Valdez-Salas, B.; Ceceña-Duran, C.; Tzintzun-Camacho, O.; Gutiérrez-Miceli, F.; Grimaldo-Juarez, O.; González-Mendoza, D. Silver nanoparticles from Prosopis glandulosa and their potential application as biocontrol of Acinetobacter calcoaceticus and Bacillus cereus. Chem. Speciat. Bioavailab. 2017, 29, 1–5. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Rajini, N.; Nellaiah, H.; Kathiresan, T.; Jawaid, M.; Rajulu, A.V. Preparation and properties of cellulose nanocomposite films with in situ generated copper nanoparticles using Terminalia catappa leaf extract. Int. J. Biol. Macromol. 2017, 95, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Tareq, F.K.; Fayzunnesa, M.; Kabir, M.S.; Nuzat, M. Mechanism of bio molecule stabilized selenium nanoparticles against oxidation process and Clostridium Botulinum. Microb. Pathog. 2018, 115, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Tahir, K.; Nazir, S.; Ahmad, A.; Li, B.; Khan, A.U.; Khan, Z.U.; Khan, F.U.; Khan, Q.U.; Khan, A.; Rahman, A.U. Facile and green synthesis of phytochemicals capped platinum nanoparticles and in vitro their superior antibacterial activity. J. Photochem. Photobiol. B 2017, 166, 246–251. [Google Scholar] [CrossRef] [PubMed]
- Subhapriya, S.; Gomathipriya, P. Green synthesis of titanium dioxide (TiO2) nanoparticles by Trigonella foenum-graecum extract and its antimicrobial properties. Microb. Pathog. 2018, 116, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.; Velmurugan, P.; Park, J.-H.; Chang, W.-S.; Park, Y.-J.; Jayanthi, P.; Cho, M.; Oh, B.-T. Green synthesis of silver oxide nanoparticles and its antibacterial activity against dental pathogens. 3 Biotech 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, X.; Geo Vigila, A.V.; Parimelazhagan, T.; Muralidhara-Rao, D.; Zhang, S. Biosynthesis, characterization, and evaluation of bioactivities of leaf extract-mediated biocompatible silver nanoparticles from an early tracheophyte, Pteris tripartita Sw. Int. J. Nanomed. 2016, 11, 5789–5806. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.; Panghal, M.; Kadyan, S.; Chaudhary, U.; Yadav, J.P. Green silver nanoparticles of Phyllanthus amarus: As an antibacterial agent against multi drug resistant clinical isolates of Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.S.D.; Houreld, N.N.; Kroukamp, E.M.; Abrahamse, H. Cellular imaging and bactericidal mechanism of green-synthesized silver nanoparticles against human pathogenic bacteria. J. Photochem. Photobiol. B Biol. 2018, 178, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Yasir, M.; Singh, J.; Tripathi, M.K.; Singh, P.; Shrivastava, R. Green synthesis of silver nanoparticles using leaf extract of common arrowhead houseplant and its anticandidal activity. Pharmacogn. Mag. 2018, 13 (Suppl. 4), S840–S844. [Google Scholar] [PubMed]
- Zahir, A.A.; Rahuman, A.A. Evaluation of different extracts and synthesised silver nanoparticles from leaves of Euphorbia prostrata against Haemaphysalis bispinosa and Hippobosca maculata. Vet. Parasitol. 2012, 187, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, G.; Abdul Rahuman, A. Larvicidal activity of synthesized silver nanoparticles using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Tropica 2011, 118, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Kumar, G.; Das, R.; Agrawal, V. Strong larvicidal potential of silver nanoparticles (AgNPs) synthesized using Holarrhena antidysenterica (L.) Wall. bark extract against malarial vector, Anopheles stephensi Liston. Process Saf. Environ. Prot. 2018, 116, 137–148. [Google Scholar] [CrossRef]
- Jayaseelan, C.; Rahuman, A.A.; Rajakumar, G.; Vishnu Kirthi, A.; Santhoshkumar, T.; Marimuthu, S.; Bagavan, A.; Kamaraj, C.; Zahir, A.A.; Elango, G. Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heartleaf moonseed plant, Tinospora cordifolia Miers. Parasitol. Res. 2011, 109, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ghramh, H.A.; Al-Ghamdi, K.M.; Mahyoub, J.A.; Ibrahim, E.H. Chrysanthemum extract and extract prepared silver nanoparticles as biocides to control Aedes aegypti (L.), the vector of dengue fever. J. Asia Pac. Entomol. 2018, 21, 205–210. [Google Scholar] [CrossRef]
- Chai, S.H.; Wang, Y.; Qiao, Y.; Wang, P.; Li, Q.; Xia, C.; Ju, M. Bio fabrication of silver nanoparticles as an effective wound healing agent in the wound care after anorectal surgery. J. Photochem. Photobiol. B 2018, 178, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Kanjikar, A.P.; Hugar, A.L.; Londonkar, R.L. Characterization of phyto-nanoparticles from Ficus krishnae for their antibacterial and anticancer activities. Drug Dev. Ind. Pharm. 2018, 44, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Suriyakalaa, U.; Antony, J.J.; Suganya, S.; Siva, D.; Sukirtha, R.; Kamalakkannan, S.; Pichiah, P.B.; Achiraman, S. Hepatocurative activity of biosynthesized silver nanoparticles fabricated using Andrographis paniculata. Colloids Surf. B Biointerfaces 2013, 102, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sudha, A.; Jeyakanthan, J.; Srinivasan, P. Green synthesis of silver nanoparticles using Lippia nodiflora aerial extract and evaluation of their antioxidant, antibacterial and cytotoxic effects. Resour. Eff. Technol. 2017, 3, 506–515. [Google Scholar] [CrossRef]
- Velu, V.; Das, M.; Dua, K.; Malipeddi, H. Evaluation of in vitro and in vivo anti-urolithiatic activity of silver nanoparticles containing aqueous leaf extract of Tragia involucrata. Drug Deliv. Transl. Res. 2017, 7, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Sukhwal, A.; Jain, D.; Joshi, A.; Rawal, P.; Kushwaha, H.S. Biosynthesised silver nanoparticles using aqueous leaf extract of Tagetes patula L. and evaluation of their antifungal activity against phytopathogenic fungi. IET Nanobiotechnol. 2017, 11, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.; Barik, S.K.; Behera, T.; Nayak, S.K.; Sahoo, S.K.; Mishra, S.S.; Swain, P. Green synthesis of gold nanoparticles using root and leaf extracts of Vetiveria zizanioides and Cannabis sativa and its antifungal activities. BioNanoScience 2016, 6, 205–213. [Google Scholar] [CrossRef]
- Panja, S.; Chaudhuri, I.; Khanra, K.; Bhattacharyya, N. Biological application of green silver nanoparticle synthesized from leaf extract of Rauvolfia serpentina Benth. Asian Pac. J. Trop. Dis. 2016, 6, 549–556. [Google Scholar] [CrossRef]
- Daisy, P.; Saipriya, K. Biochemical analysis of Cassia fistula aqueous extract and phytochemically synthesized gold nanoparticles as hypoglycemic treatment for diabetes mellitus. Int. J. Nanomed. 2012, 7, 1189–1202. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Saha, S.K.; Gayen, P.; Chowdhury, P.; Sinha Babu, S.P. Exploration of antifilarial activity of gold nanoparticle against human and bovine filarial parasites: A nanomedicinal mechanistic approach. Colloids Surf. B Biointerfaces 2018, 161, 236–243. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.U.; Khan, I.; Rauf, A.; Muhammad, N.; Shahid, M.; Shah, M.R. Antinociceptive, muscle relaxant and sedative activities of gold nanoparticles generated by methanolic extract of Euphorbia milii. BMC Complement Altern. Med. 2015, 15, 160. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Smita, K.; Cumbal, L.; Debut, A. Green synthesis of silver nanoparticles using Andean blackberry fruit extract. Saudi J. Biol. Sci. 2017, 24, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Gubitosa, J.; Rizzi, V.; Lopedota, A.; Fini, P.; Laurenzana, A.; Fibbi, G.; Fanelli, F.; Petrella, A.; Laquintana, V.; Denora, N.; et al. One pot environmental friendly synthesis of gold nanoparticles using Punica Granatum Juice: A novel antioxidant agent for future dermatological and cosmetic applications. J. Colloid Interface Sci. 2018, 521, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, N.B.; Hussain, I.; Singh, H. Effect of biologically synthesized copper oxide nanoparticles on metabolism and antioxidant activity to the crop plants Solanum lycopersicum and Brassica oleracea var. botrytis. J. Biotechnol. 2017, 262, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Sulaiman, G.M.; Tawfeeq, A.T.; Jaaffer, M.D. Biogenic synthesis of copper oxide nanoparticles using olea europaea leaf extract and evaluation of their toxicity activities: An in vivo and in vitro study. Biotechnol. Prog. 2018, 34, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.; Singh, P.; Jang, M.; Kim, Y.-J.; Castro-Aceituno, V.; Simu, S.Y.; Kim, Y.J.; Yang, D.-C. Gold nanoflowers synthesized using Acanthopanacis cortex extract inhibit inflammatory mediators in LPS-induced RAW264.7 macrophages via NF-κB and AP-1 pathways. Colloids Surf. B Biointerfaces 2018, 162, 398–404. [Google Scholar] [CrossRef] [PubMed]
- Ateeq, M.; Shah, M.R.; Ali, H.; Kabir, N.; Khan, A.; Nadeem, S. Hepatoprotective and urease inhibitory activities of garlic conjugated gold nanoparticles. New J. Chem. 2015, 39, 5003–5007. [Google Scholar] [CrossRef]
- Aswathy Aromal, S.; Philip, D. Green synthesis of gold nanoparticles using Trigonella foenum-graecum and its size-dependent catalytic activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 97, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Devipriya, D.; Roopan, S.M. Cissus quadrangularis mediated ecofriendly synthesis of copper oxide nanoparticles and its antifungal studies against Aspergillus niger, Aspergillus flavus. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 80, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Gnanavel, V.; Palanichamy, V.; Roopan, S.M. Biosynthesis and characterization of copper oxide nanoparticles and its anticancer activity on human colon cancer cell lines (HCT-116). J. Photochem. Photobiol. B 2017, 171, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Şahin, B.; Aygün, A.; Gündüz, H.; Şahin, K.; Demir, E.; Akocak, S.; Şen, F. Cytotoxic effects of platinum nanoparticles obtained from pomegranate extract by the green synthesis method on the MCF-7 cell line. Colloids Surf. B Biointerfaces 2018, 163, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Jayaseelan, C.; Gandhi, P.R.; Rajasree, S.R.R.; Suman, T.Y.; Mary, R.R. Toxicity studies of nanofabricated palladium against filariasis and malaria vectors. Environ. Sci. Pollut. Res. Int. 2018, 25, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, H.; Zhang, R.; Zhang, G.; Yang, Y.; Liu, Z. Biofabrication of polyphenols coated Nano palladium and its in-vitro cytotoxicity against human leukemia cell lines (K562). J. Photochem. Photobiol. B 2017, 175, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, C.; Murali, M.; Manasa, G.; Ponnamma, P.; Abhilash, M.R.; Lakshmeesha, T.R.; Satish, A.; Amruthesh, K.N.; Sudarshana, M.S. Antibacterial and antimitotic potential of bio-fabricated zinc oxide nanoparticles of Cochlospermum religiosum (L.). Microb. Pathog. 2017, 110, 620–629. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, P.R.; Jayaseelan, C.; Mary, R.R.; Mathivanan, D.; Suseem, S.R. Acaricidal, pediculicidal and larvicidal activity of synthesized ZnO nanoparticles using Momordica charantia leaf extract against blood feeding parasites. Exp. Parasitol. 2017, 181, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Ishwarya, R.; Vaseeharan, B.; Kalyani, S.; Banumathi, B.; Govindarajan, M.; Alharbi, N.S.; Kadaikunnan, S.; Al-anbr, M.N.; Khaled, J.M.; Benelli, G. Facile green synthesis of zinc oxide nanoparticles using Ulva lactuca seaweed extract and evaluation of their photocatalytic, antibiofilm and insecticidal activity. J. Photochem. Photobiol. B Biol. 2018, 178, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Angel Ezhilarasi, A.; Judith Vijaya, J.; Kaviyarasu, K.; John Kennedy, L.; Ramalingam, R.J.; Al-Lohedan, H.A. Green synthesis of NiO nanoparticles using Aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J. Photochem. Photobiol. B 2018, 180, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, Y. Biosynthesis of cerium oxide nanoparticles and their effect on lipopolysaccharide (LPS) induced sepsis mortality and associated hepatic dysfunction in male Sprague Dawley rats. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 83, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Thandapani, K.; Kathiravan, M.; Namasivayam, E.; Padiksan, I.A.; Natesan, G.; Tiwari, M.; Giovanni, B.; Perumal, V. Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ. Sci. Pollut. Res. Int. 2017, 23, 10328–10339. [Google Scholar] [CrossRef] [PubMed]
- Khatami, M.; Alijani, H.; Sharifi, I.; Sharifi, F.; Pourseyedi, S.; Kharazi, S.; Lima Nobre, M.A.; Khatami, M. Leishmanicidal activity of biogenic Fe3O4 nanoparticles. Sci. Pharm. 2017, 85, 36. [Google Scholar] [CrossRef] [PubMed]
- Sisubalan, N.; Ramkumar, V.S.; Pugazhendhi, A.; Karthikeyan, C.; Indira, K.; Gopinath, K.; Hameed, A.S.H.; Basha, M.H.G. ROS-mediated cytotoxic activity of ZnO and CeO2 nanoparticles synthesized using the Rubia cordifolia L. leaf extract on MG-63 human osteosarcoma cell lines. Environ. Sci. Pollut. Res. Int. 2017, 29, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sowndarya, P.; Ramkumar, G.; Shivakumar, M.S. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1490–1495. [Google Scholar] [CrossRef] [PubMed]
- Defaei, M.; Taheri-Kafrani, A.; Miroliaei, M.; Yaghmaei, P. Improvement of stability and reusability of α-amylase immobilized on naringin functionalized magnetic nanoparticles: A robust nanobiocatalyst. Int. J. Biol. Macromol. 2018, 113, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Barua, S.; Sarkar, S.; Karak, N.; Bhattacharyya, P.; Raza, N.; Kim, K.-H.; Bhattacharya, S.S. Plant extract–mediated green silver nanoparticles: Efficacy as soil conditioner and plant growth promoter. J. Hazard. Mater. 2018, 346, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Dayakar, T.; Rao, K.V.; Bikshalu, K.; Rajendar, V.; Park, S.H. Novel synthesis and characterization of pristine Cu nanoparticles for the non-enzymatic glucose biosensor. J. Mater. Sci. Mater. Med. 2017, 28, 109. [Google Scholar] [CrossRef] [PubMed]
- Kurepa, J.; Nakabayashi, R.; Paunesku, T.; Suzuki, M.; Saito, K.; Woloschak, G.E.; Smalle, J.A. Direct isolation of flavonoids from plants using ultra-small anatase TiO2 nanoparticles. Plant J. 2014, 77, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Cao, X.; De La Torre-Roche, R.; Tan, C.; Yang, T.; White, J.C.; Xiao, H.; Xing, B.; He, L. A green, facile, and rapid method for microextraction and Raman detection of titanium dioxide nanoparticles from milk powder. RSC Adv. 2017, 7, 21380–21388. [Google Scholar] [CrossRef]
- Xiao, D.; Zhang, C.; Yuan, D.; He, J.; Wu, J.; Zhang, K.; Lin, R.; He, H. Magnetic solid-phase extraction based on Fe3O4 nanoparticle retrieval of chitosan for the determination of flavonoids in biological samples coupled with high performance liquid chromatography. RSC Adv. 2014, 4, 64843–64854. [Google Scholar] [CrossRef]
- Cao, X.; Ma, C.; Gao, Z.; Zheng, J.; He, L.; McClements, D.J.; Xiao, H. Characterization of the interactions between titanium dioxide nanoparticles and polymethoxyflavones using surface-enhanced Raman spectroscopy. J. Agric. Food Chem. 2016, 64, 9436–9441. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Wallace, W.T.; Islam, S.Z.; Nagpure, S.; Strzalka, J.; Littleton, J.M.; Rankin, S.E.; Knutson, B.L. Adsorption and recovery of polyphenolic flavonoids using TiO2-functionalized mesoporous silica nanoparticles. ACS Appl. Mater. Interfaces 2017, 9, 32114–32125. [Google Scholar] [CrossRef] [PubMed]
- Schlipf, D.M.; Jones, C.A.; Armbruster, M.E.; Rushing, E.S.; Wooten, K.C.; Rankin, S.E.; Knutson, B.L. Flavonoid adsorption and stability on titania-functionalized silica nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2015, 478, 15–21. [Google Scholar] [CrossRef]
- Lee, D.; Ko, W.-K.; Hwang, D.-S.; Heo, D.N.; Lee, S.J.; Heo, M.; Lee, K.-S.; Ahn, J.-Y.; Jo, J.; Kwon, I.K. Use of baicalin-conjugated gold nanoparticles for apoptotic induction of breast cancer cells. Nanoscale Res. Lett. 2016, 11, 381. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Rani, M.; Singh, J.; Moudgil, L.; Sharma, P.; Kumar, S.; Saini, G.S.S.; Tripathi, S.K.; Singh, G.; Kaura, A. Identifying the preferred interaction mode of naringin with gold nanoparticles through experimental, DFT and TDDFT techniques: Insights into their sensing and biological applications. RSC Adv. 2016, 6, 79470–79484. [Google Scholar] [CrossRef]
- Pal, R.; Panigrahi, S.; Bhattacharyya, D.; Chakraborti, A.S. Characterization of citrate capped gold nanoparticle-quercetin complex: Experimental and quantum chemical approach. J. Mol. Struct. 2013, 1046, 153–163. [Google Scholar] [CrossRef]
- Krishnan, G.; Subramaniyan, J.; Chengalvarayan Subramani, P.; Muralidharan, B.; Thiruvengadam, D. Hesperetin conjugated PEGylated gold nanoparticles exploring the potential role in anti-inflammation and anti-proliferation during diethylnitrosamine-induced hepatocarcinogenesis in rats. Asian J. Pharm. Sci. 2017, 12, 442–455. [Google Scholar] [CrossRef]
- Pak, P.J.; Go, E.B.; Hwang, M.H.; Lee, D.G.; Cho, M.J.; Joo, Y.H.; Chung, N. Evaluation of the cytotoxicity of gold nanoparticle-quercetin complex and its potential as a drug delivery vesicle. J. Appl. Biol. Chem. 2016, 59, 145–147. [Google Scholar] [CrossRef]
- Rajesh Kumar, S.; Priyatharshni, S.; Babu, V.N.; Mangalaraj, D.; Viswanathan, C.; Kannan, S.; Ponpandian, N. Quercetin conjugated superparamagnetic magnetite nanoparticles for in-vitro analysis of breast cancer cell lines for chemotherapy applications. J. Colloid Interface Sci. 2014, 436, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.; Fawcett, D.; Sharma, S.; Tripathy, S.K.; Poinern, G.E.J. Green synthesis of metallic nanoparticles via biological entities. Materials 2015, 8, 7278–7308. [Google Scholar] [CrossRef]
- Shaik, M.; Ali, Z.; Khan, M.; Kuniyil, M.; Assal, M.; Alkhathlan, H.; Al-Warthan, A.; Siddiqui, M.; Khan, M.; Adil, S. Green synthesis and characterization of palladium nanoparticles using Origanum vulgare L. rxtract and their catalytic activity. Molecules 2017, 22, 165. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.P.; Sengodan, K. Synthesis and characterization of zinc oxide and iron oxide nanoparticles using Sesbania grandiflora leaf extract as reducing agent. J. Nanosci. 2017, 2017, 7. [Google Scholar] [CrossRef]



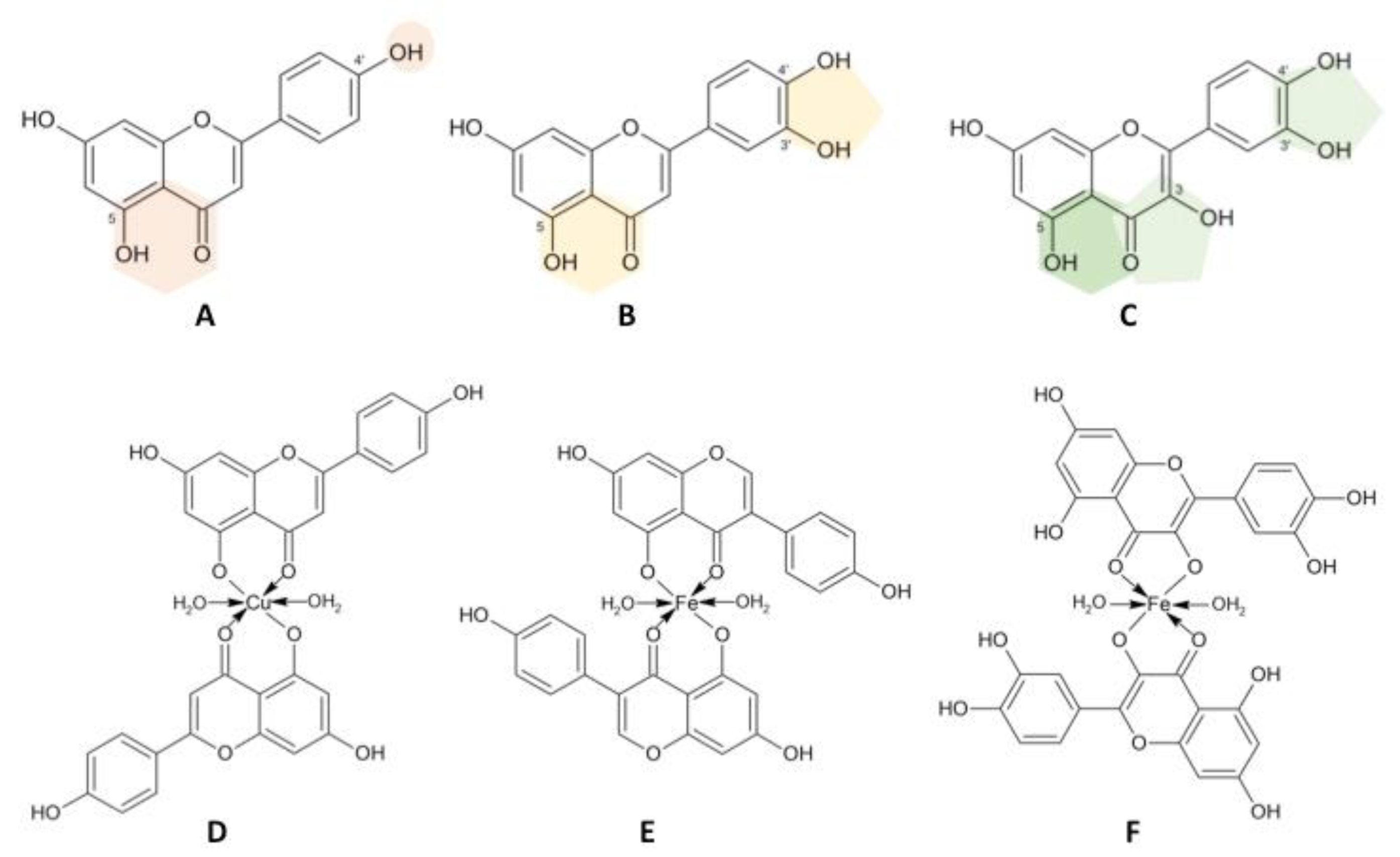
| Plant Species | NPs | Metabolites Identified in the Extract/NPs | Reference |
|---|---|---|---|
| Coleus aromaticus | Ag | Flavonoids | [44] |
| Syzygium cumini | Ag | Flavonoids | [45] |
| Azadirachta indica | Ag | Flavanoids, terpenoids | [46] |
| Citrus sinensis | Ag | Flavonoids, ascorbic acid, volatile oils | [47] |
| Zingiber officinale | Ag | Flavonoid, alkaloids | [48] |
| Ocimum sanctum | Ag | Eugenols, linalool, terpenes | [49] |
| Glycyrrhiza glabra | Ag | Flavonoids, thiamine and terpenoids | [50] |
| Nigella arvensis | Ag | Flavonoids, alkaloids | [51] |
| Dioscorea bulbifera | Ag | Flavonoids, polyphenols | [52] |
| Lantana camara | Ag | Flavonoids, glycosides and carbohydrates | [53] |
| Helianthus annuus | Ag | Fatty acids, triglycerides, phenolics, tocopherols | [54] |
| Rosmarinus officinalis | Ag | Polyphenols | [55] |
| Mimusops elengi | Ag | Polyphenols | [56] |
| Syzygium cumini | Ag | Polyphenols | [57] |
| Ocimum sanctum | Ag | Quercetin | [58] |
| Acalypha indica | Ag | Quercetin, plant pigment | [59] |
| Gardenia jasminoides | Ag | Rutin, gallic acid, chlorogenic acid | [60] |
| Withania somnifera | Ag | Catechin p-coumaric acid, luteolin-7-glucoside, withanolides | [14] |
| Lippia citriodora | Ag | Verbascoside, isoverbascoside, chrysoeriol-7-O-diglucoronide, luteonin-7-O-diglucoronide | [61] |
| Decalepis hamiltonii | Ag | Polyols, phenols | [62] |
| Achyranthes aspera | Ag | Polyols | [63] |
| Hybanthus enneaspermus | Ag | Several bioactive phytochemical compounds | [64] |
| Desmodium triflorum | Ag | Ascorbic acid | [65] |
| Sesuvium portulacastrum | Ag | Flavones, proteins, terpenoids | [66] |
| Solanum xanthocarpum | Ag | Alcohols, phenols, carboxylic anions | [67] |
| Mentha piperita | Ag | Alkaloids, flavones, steroids, polysaccharides, amino acids, oximes, proteins, menthol | [41] |
| Anacardium occidentale | Ag | Proteins, polyols | [68] |
| Dioscorea bulbifera | Ag | Diosgenin, ascorbic acid | [52] |
| Iresine herbstii | Ag | Phenolic compound | [69] |
| Trianthema decandra | Ag | Catechins, hydroxyflavones | [70] |
| Morinda pubescens | Ag | Flavonoids, triterpenoids, polyphenols | [71] |
| Carica papaya | Ag | Proteins, alcohols, phenolics | [72] |
| Annona squamosa | Ag | Alkaloids, glycoside, saponins, tannins, phenolic, carbohydrates | [73] |
| Trianthema decandra | Ag | Saponin | [74] |
| Aegle marmelos | Ag | Tannin | [75] |
| Rosa rugosa | Ag | Carboxylate, amine groups | [76] |
| Hibiscus rosa- sinensis | Ag | Carboxylate ion groups | [77] |
| Leonuri herba | Ag | Hydroxyl, polyphenols groups | [78] |
| Lonicera japonica | Ag | Phenolic and hydroxyl groups of chlorogenic acid | [79] |
| Mangifera indica | Ag | Ketone, aldehydes, hydroxyl, carboxyl groups | [80] |
| Eucalyptus | Fe | Alcohol, phenols, alkylaldehyde | [81] |
| Alternanthera sessilis | Ag | Tannins, carbohydrates, proteins, ascorbic acid | [82] |
| Boswellia serrata | Ag | Proteins | [83] |
| Piper betle | Ag | Amide, aromatic amine | [84] |
| Plumeria rubra | Ag | Proteins | [85] |
| Jatropha curcas | Ag | Cyclic peptides (curcacycline A and curcacycline B) | [86] |
| Hibiscus rosa- sinensis | Au | Flavonoids | [87] |
| Vitis vinifera | Au | Flavonoids | [88] |
| Mangifera indica | Au | Favonoids, terpenoids, thiamine | [89] |
| Abutilon indicum | Au | Flavonoids, phenolic compounds | [90] |
| Suaeda monoica | Au | Flavonoids, terpenoids, soluble proteins | [91] |
| Sesbania grandiflora | Au | Flavonoids, polyphenols | [92] |
| Citrus maxima | Au | Flavonoids, terpenes, vitamins | [93] |
| Hypoxis hemerocallidea | Au | Flavonoids, terpenoids, phenolic compounds and/or carbohydrates | [94] |
| Galenia africana | Au | Flavonoids, terpenoids, phenolic compounds and/or carbohydrates | [94] |
| Nigella arvensis | Au | Flavonoids, phenolic compounds | [95] |
| Butea monosperma | Au | Polyphenols | [96] |
| Sterculia acuminata | Au | Polyphenols | [97] |
| Terminalia arjuna | Au | Polyphenols | [98] |
| Terminalia catappa | Au | Hydroxyl group of phenols | [99] |
| Hygrophila spinosa | Au | Hydroxyl group | [100] |
| Cassia auriculata | Au | Hydroxyl group | [101] |
| Platycodon grandiflorum | Au | Hydroxyl group | [102] |
| Phoenix dactylifera | Au | Hydroxyl group | [103] |
| Lansium domesticum | Au | Carboxylic acid | [104] |
| Salix alba | Au | Proteins, metabolites having functional groups of amines, alcohols, ketones, aldehydes, carboxylic acids (salicin) | [105] |
| Cinnamomum zeylanicum | Au | Proteins | [39] |
| Ficus benghalensis | Au | Proteins | [106] |
| Jatropha | Au | Proteins | [16] |
| Morinda citrifolia | Au | Proteins | [42] |
| Gymnema sylvestre | Au | Proteins, polypeptides | [107] |
| Olea europaea | Au | Proteins | [40] |
| Trianthema decandra | Au | Saponin | [74] |
| Terminalia arjuna | Au | Hydrolyzable tannins | [108] |
| Elaeis guineensis | Au | Phenolic, carboxylic, amines | [109] |
| Mentha piperita | Au | Menthol | [41] |
| Argemone mexicana | Au | Phosphorous compounds | [110] |
| Tamarindus indica | Au | Phenolic compounds | [111] |
| Averrhoa bilimbi | Au | Phenols, tertiary amides | [112] |
| Couroupita guianensis | Au | Phenol group | [113] |
| Syzygium jambos | Au | Saccharides, phenolics | [114] |
| Zostera noltii | Au | Flavone sulfates | [115] |
| Ipomoea carnea | Au | Polysaccharides, protein | [116] |
| Mirabilis jalapa | Au | Polysaccharides | [117] |
| Panax ginseng | Au | Polysaccharides, phenolic compounds | [118] |
| Galaxaura elongata | Au | Glutamic acid, hexadecanoic acid, oleic acid, 11-eicosenoic acid, stearic acid, gallic acid, epigallocatechin, catechin, epicatechin gallate | [119] |
| Tagetes sp. and Rosa sp. | Cd | Alcoholic, amide, C–C, –OCH3 groups (tannins, flavonoids, alkaloids and carotenoids) | [120] |
| Punica granatum | Cu | Flavonoids, alkaloids, polyphenols | [121] |
| Cymbopogon citratus | Cu | Polyphenols, proteins | [122] |
| Lawsonia inermis | Cu | Phenolic compounds | [123] |
| Euphorbia granulate | Pd | Hydroxyflavones, phenolics | [124] |
| Hippophae rhamnoides | Pd | Flavonoids | [125] |
| Delonix regia | Pd | Polyphenols | [126] |
| Cacumen platycladi | Pt | Flavonoids, proteins | [127] |
| Diospyros kaki | Pt | Terpenoids | [128] |
| Dioscorea bulbifera | Pt-Pd | Hydroxyl group of polyphenolic compounds | [129] |
| Cassia fistula | ZnO | Flavonoids, polyphenols | [130] |
| Azadirachta indica | ZnO | Flavonoids, phenolic acid, terpenoids, protein | [131] |
| Rosa canina | ZnO | Phenolic and carboxylic acids | [132] |
| Aloe barbadensis | ZnO | Phenol, amines, alcohol groups | [133] |
| Agathosma betulina | ZnO | Hydroxyl group | [134] |
| Trifolium pratense | ZnO | Hydroxyl group | [135] |
| Parthenium hysterophorus | ZnO | Phosphorus compound, secondary sulfonamide, monosubstituted alkyne | [136] |
| Anisochilus carnosus | ZnO | Phenol, carboxylic acid | [137] |
| Coptis chinensis | ZnO | Alcohol, carboxylic acid, alkyl halide, alkynes | [138] |
| Calotropis procera | ZnO | Hydroxyl groups, aldehydes, amines, ketones, carboxylic acids | [139] |
| NPs | Plant Species Used | Bioactivity Reported | Reference |
|---|---|---|---|
| Ag | Withania somnifera | Antibacterial, anticandidal | [14] |
| Ag | Lansium domesticum | Antibacterial | [104] |
| Ag | Crocus sativus | Antibacterial | [161] |
| Ag | Datura stramonium | Antibacterial | [162] |
| Ag | Prosopis glandulosa | Antibacterial | [163] |
| Ag | Azadirachta indica | Antibacterial | [46] |
| Au | Hypoxis hemerocallidea | Antibacterial | [94] |
| Au | Galenia africana | Antibacterial | [94] |
| Cu | Terminalia catappa | Antibacterial | [164] |
| Se | Azadirachta indica | Antibacterial | [165] |
| Pt | Taraxacum laevigatum | Antibacterial | [166] |
| TiO2 | Trigonella foenum-graecum | Antibacterial | [167] |
| Ag2O | Ficus benghalensis | Antibacterial | [168] |
| Ag | Pteris tripartita | Antibacterial, antifungal, antioxidant, antiinflammatory | [169] |
| Ag | Phyllanthus amarus | Antibacterial | [170] |
| Ag | Aloe arborescens | Antibacterial | [171] |
| Ag | Syngonium podophyllum | Anticandidal | [172] |
| Ag | Euphorbia prostrata | Antiplasmodial | [173] |
| Ag | Ocimum sanctum | Antibacterial | [58] |
| Ag | Hybanthus enneaspermus | Larvicidal | [64] |
| Ag | Eclipta prostrata | Larvicidal | [174] |
| Cd | Tagetes sp. and Rosa sp. | Larvicidal | [120] |
| Ag | Holarrhena antidysenterica | Larvicidal | [175] |
| Ag | Tinospora cordifolia | Larvicidal | [176] |
| Ag | Chrysanthemum | Larvicidal | [177] |
| Ag | Delonix elata | Wound healing | [178] |
| Ag | Ficus krishnae | Antibacterial, anticancer | [179] |
| Ag | Andrographis paniculata | Hepatocurative | [180] |
| Ag | Lippia nodiflora | Antioxidant, antibacterial, cytotoxic | [181] |
| Ag | Tragia involucrata | Antiurolithic | [182] |
| Ag | Tagetes patula | Antifungal | [183] |
| Au | Vetiveria zizanioides | Antifungal | [184] |
| Au | Cannabis sativa | Antifungal | [184] |
| Ag | Rauvolfia serpentina | Antimicrobial, larvicidal and cytotoxic | [185] |
| Au | Cassia fistula | Antihypoglycemic | [186] |
| Au | Terminalia chebula | Antifilarial | [187] |
| Au | Euphorbia milii | Antinociceptive, muscle relaxant, sedative | [188] |
| Ag | Rubus glaucus | Antioxidant | [189] |
| Au | Punica Granatum | Antioxidant | [190] |
| Au | Azolla microphylla | Antioxidant | [159] |
| CuO | Morus alba | Antioxidant | [191] |
| CuO | Olea europaea | Antioxidant | [192] |
| Au | Acanthopanacis cortex | Anti-inflammatory | [193] |
| Au | Allium sativum | Hepatoprotective | [194] |
| Au | Trigonella foenum-graecum | Catalytic | [195] |
| CuO | Cissus quadrangularis | Antifungal | [196] |
| CuO | Ormocarpum cochinchinense | Anticancer | [197] |
| Pt | Punica granatum | Cytotoxic | [198] |
| Pd | Tinospora cordifolia | Antifilarial, antimalarial | [199] |
| Pd | Pelargonium graveolens | Cytotoxic | [200] |
| Zn | Cochlospermum religiosum | Antibacterial, antimitotic | [201] |
| Zn | Momordica charantia | Acaricidal, pediculicidal, larvicidal | [202] |
| ZnO | Ulva lactuca | Insecticidal | [203] |
| ZnO | Hibiscus sabdariffa | Antibacterial, antidiabetic | [20] |
| ZnO | Calotropis procera | Photocatalytic | [139] |
| Ni | Desmodium gangeticum | Antioxidant, antibacterial | [19] |
| NiO | Aegle marmelos | Cytotoxic, antibacterial | [204] |
| CeO2 | Camellia sinensis | Healing of liver sepsis | [205] |
| TiO2 | Parthenium hysterophorus | Larvicidal, antibacterial, photocatalytic | [206] |
| Fe3O4 | Rosmarinus officinalis | Leishmanicidal | [207] |
| CeO2 | Rubia cordifolia | Anticancer | [208] |
| Se | Clausena dentata | Larvicidal | [209] |
| Au | Salix alba | Antifungal, antinociceptive, muscle relaxant | [105] |
| NP | Source | Compound(s) Trapped/Conjugated | Reference |
|---|---|---|---|
| Ag | Withania somnifera leaf extract | Catechin, p-coumaric acid, and luteolin-7-glucoside and withanolides | [14] |
| TiO2 | Arabidopsis thaliana seedlings | Flavonoids | [213] |
| TiO2 | Food samples | Myricetin | [214] |
| Fe3O4 | Urine and blood | Luteolin, quercetin, kaempferol | [215] |
| TiO2 | Flavonoids | Flavonoids | [216] |
| TiO2-SiO2 | Quercetin, rutin | Quercetin, rutin | [217] |
| TiO2-SiO2 | Quercetin | Quercetin | [218] |
| Au | Baicalin | Baicalin | [219] |
| Au | Naringin | Naringin | [220] |
| Au | Quercetin | Quercetin | [221] |
| Au | Hesperetin | Hesperetin | [222] |
| Au | Quercetin | Quercetin | [223] |
| Fe3O4 | Quercetin | Quercetin | [224] |
| Fe3O4 | Naringin | Naringin | [210] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marslin, G.; Siram, K.; Maqbool, Q.; Selvakesavan, R.K.; Kruszka, D.; Kachlicki, P.; Franklin, G. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials 2018, 11, 940. https://doi.org/10.3390/ma11060940
Marslin G, Siram K, Maqbool Q, Selvakesavan RK, Kruszka D, Kachlicki P, Franklin G. Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials. 2018; 11(6):940. https://doi.org/10.3390/ma11060940
Chicago/Turabian StyleMarslin, Gregory, Karthik Siram, Qaisar Maqbool, Rajendran Kamalabai Selvakesavan, Dariusz Kruszka, Piotr Kachlicki, and Gregory Franklin. 2018. "Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles" Materials 11, no. 6: 940. https://doi.org/10.3390/ma11060940
APA StyleMarslin, G., Siram, K., Maqbool, Q., Selvakesavan, R. K., Kruszka, D., Kachlicki, P., & Franklin, G. (2018). Secondary Metabolites in the Green Synthesis of Metallic Nanoparticles. Materials, 11(6), 940. https://doi.org/10.3390/ma11060940





