Effect of Sigma Phase Morphology on the Degradation of Properties in a Super Duplex Stainless Steel
Abstract
1. Introduction
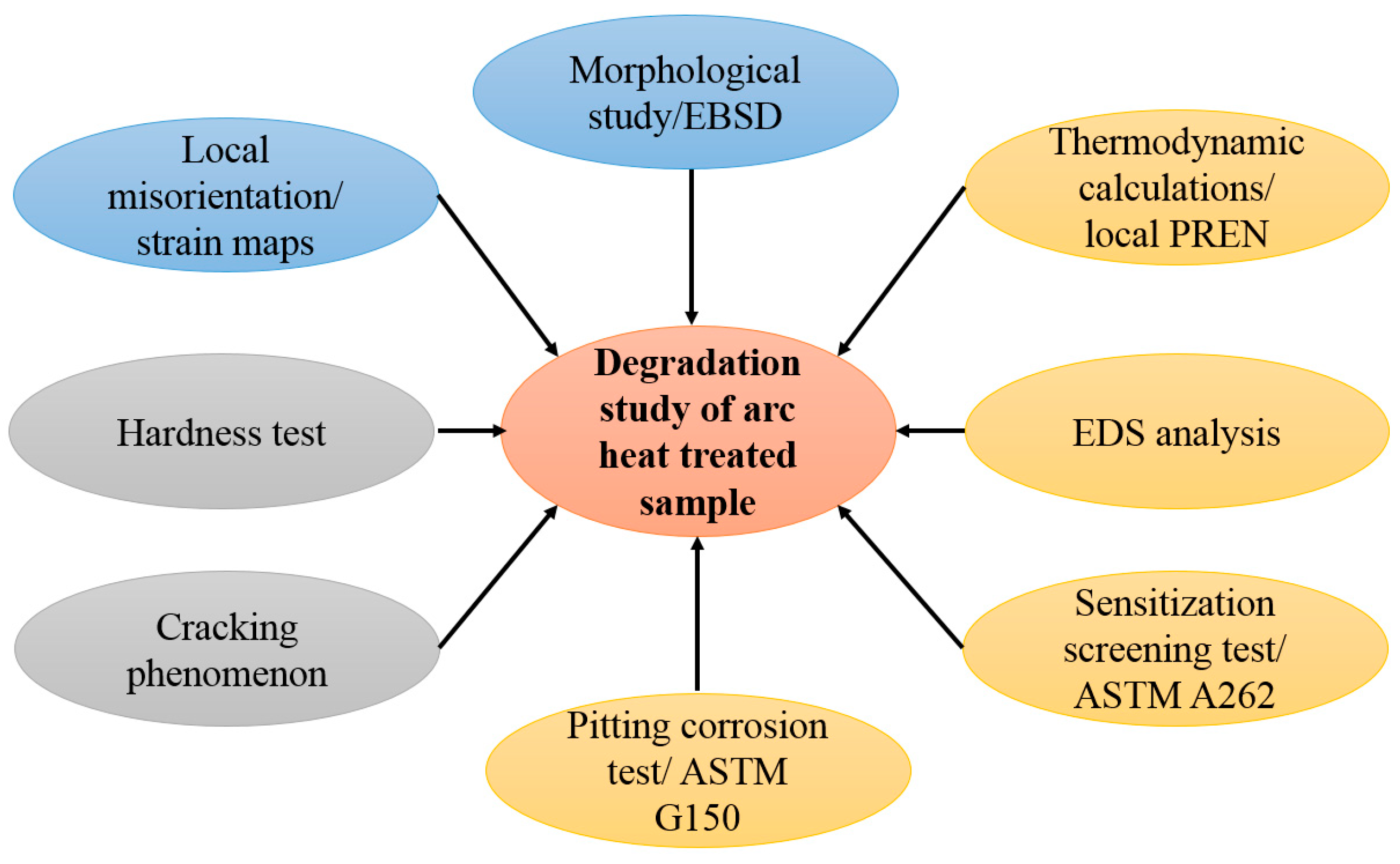
2. Materials and Methods
2.1. Material and Heat Treatment
2.2. Testing and Evaluation
2.3. Modeling and Calculations
3. Results
3.1. Microstructure before Arc Heat Treatment
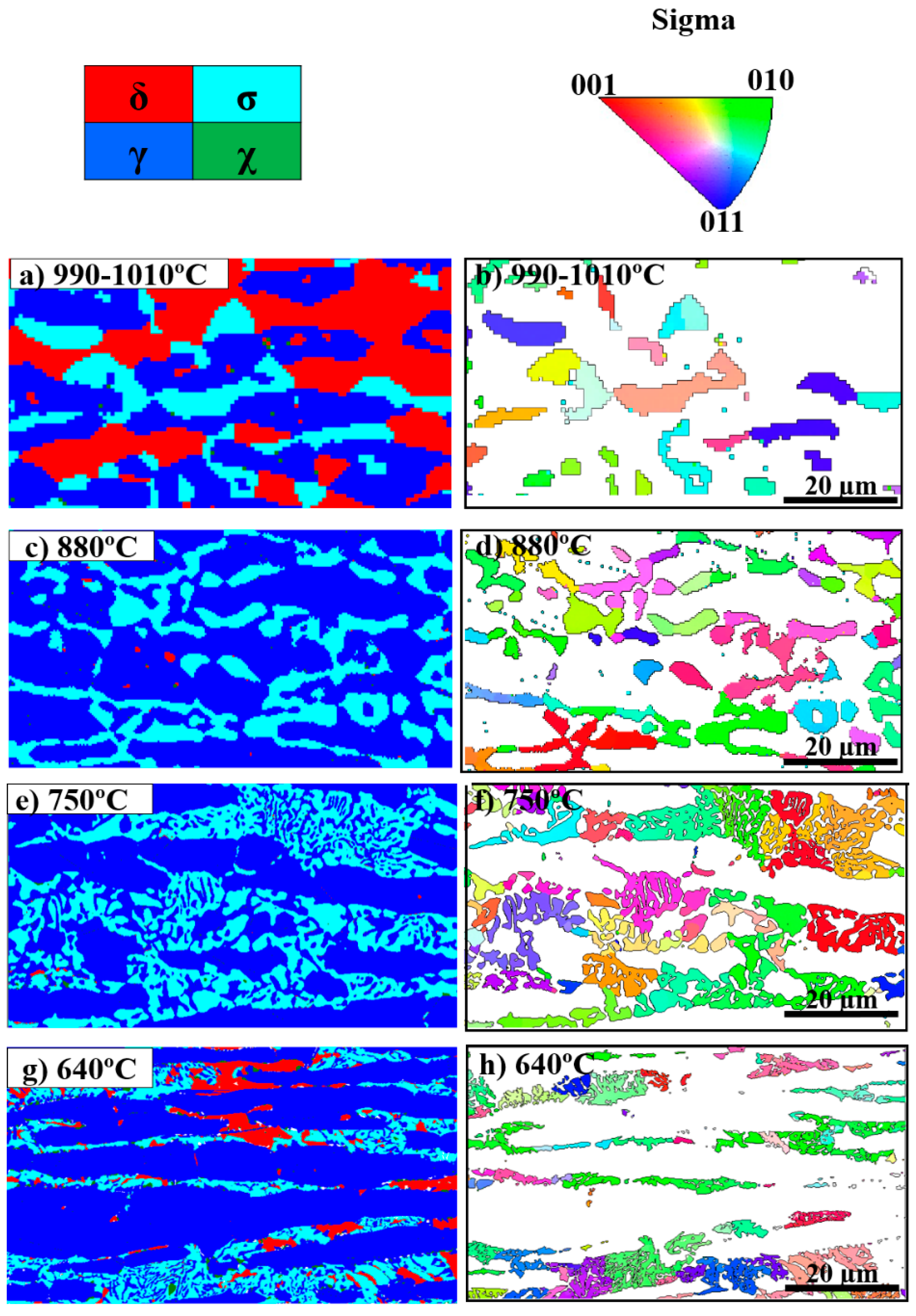
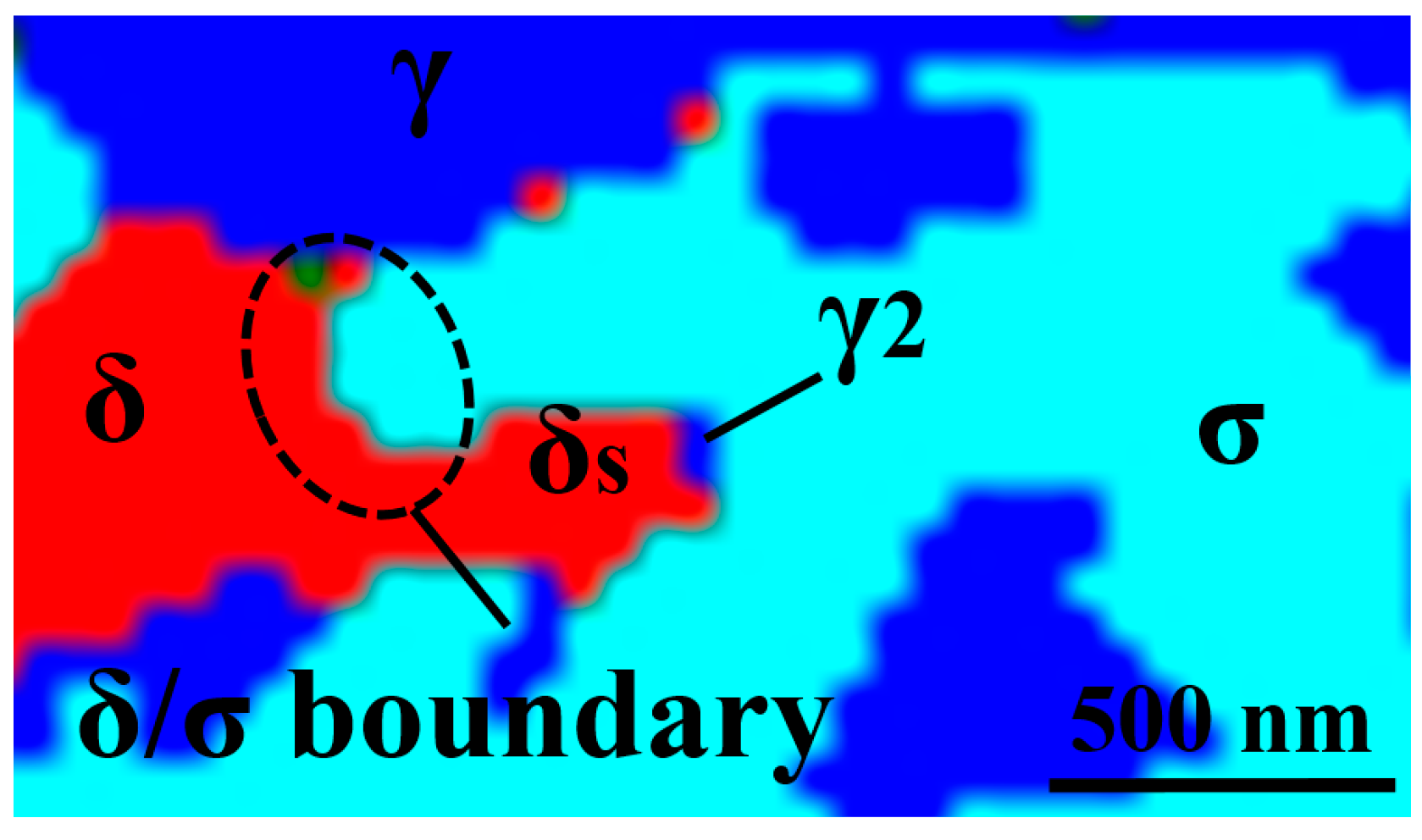
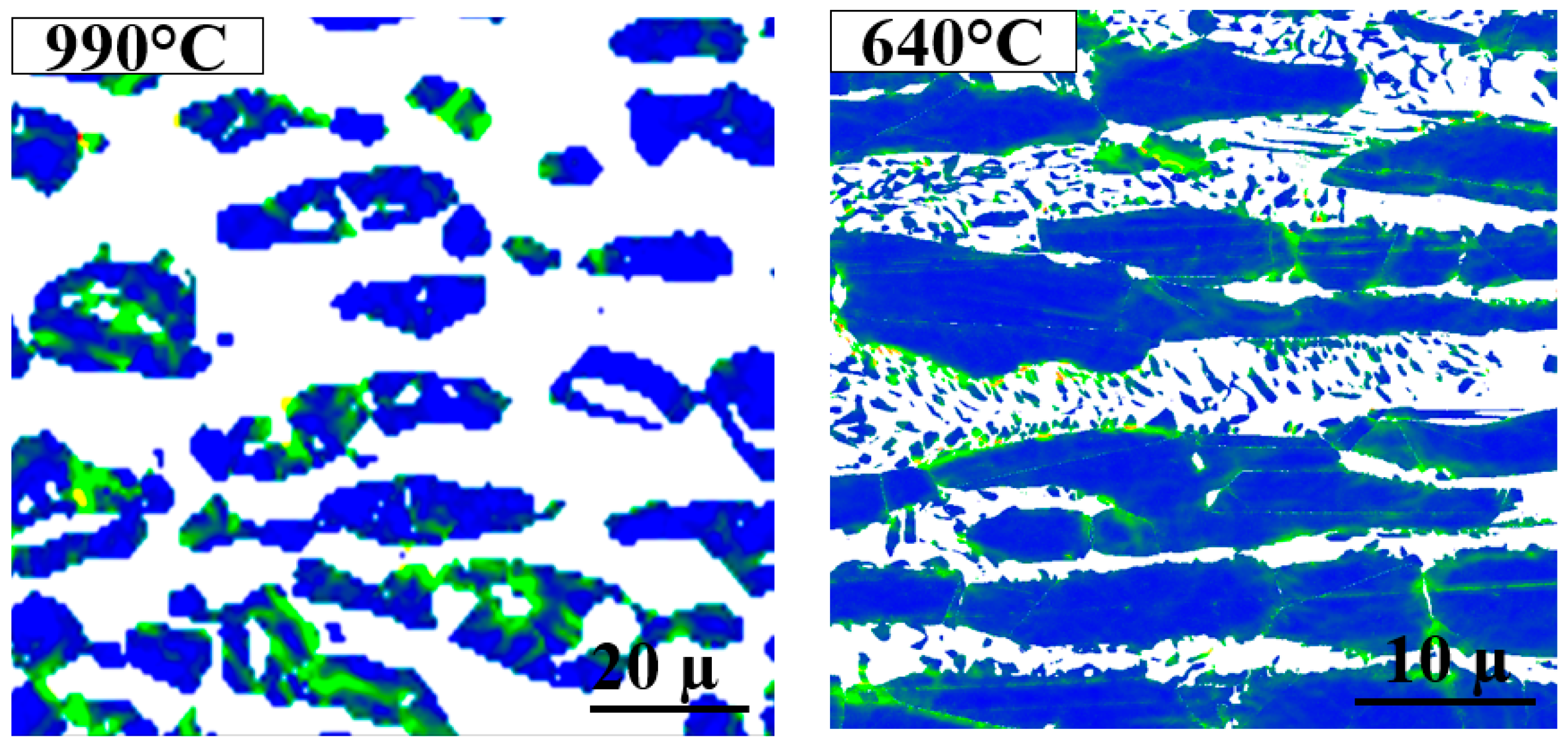
3.2. Sigma Phase Characteristics in Functionally Graded Microstructure
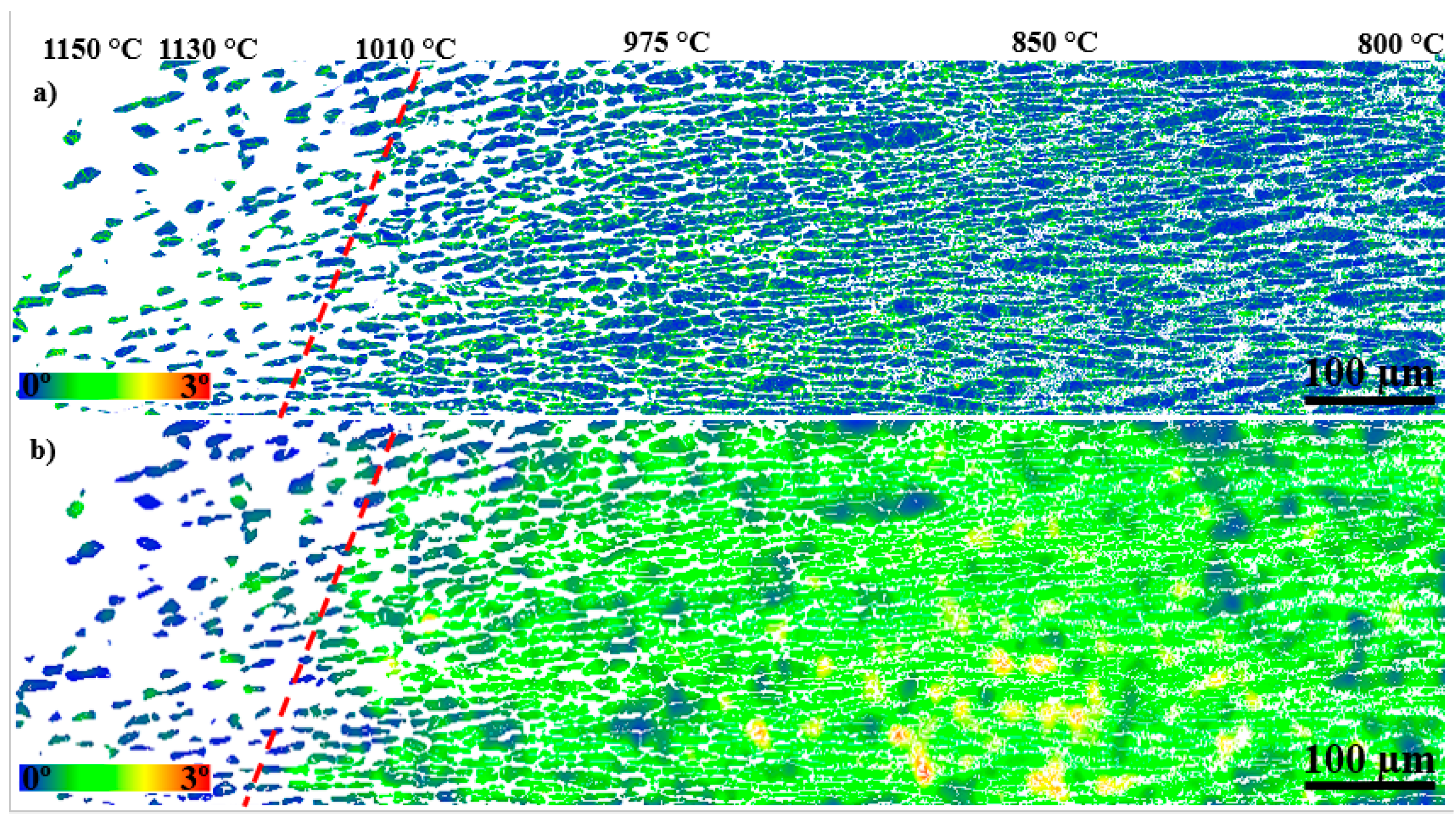
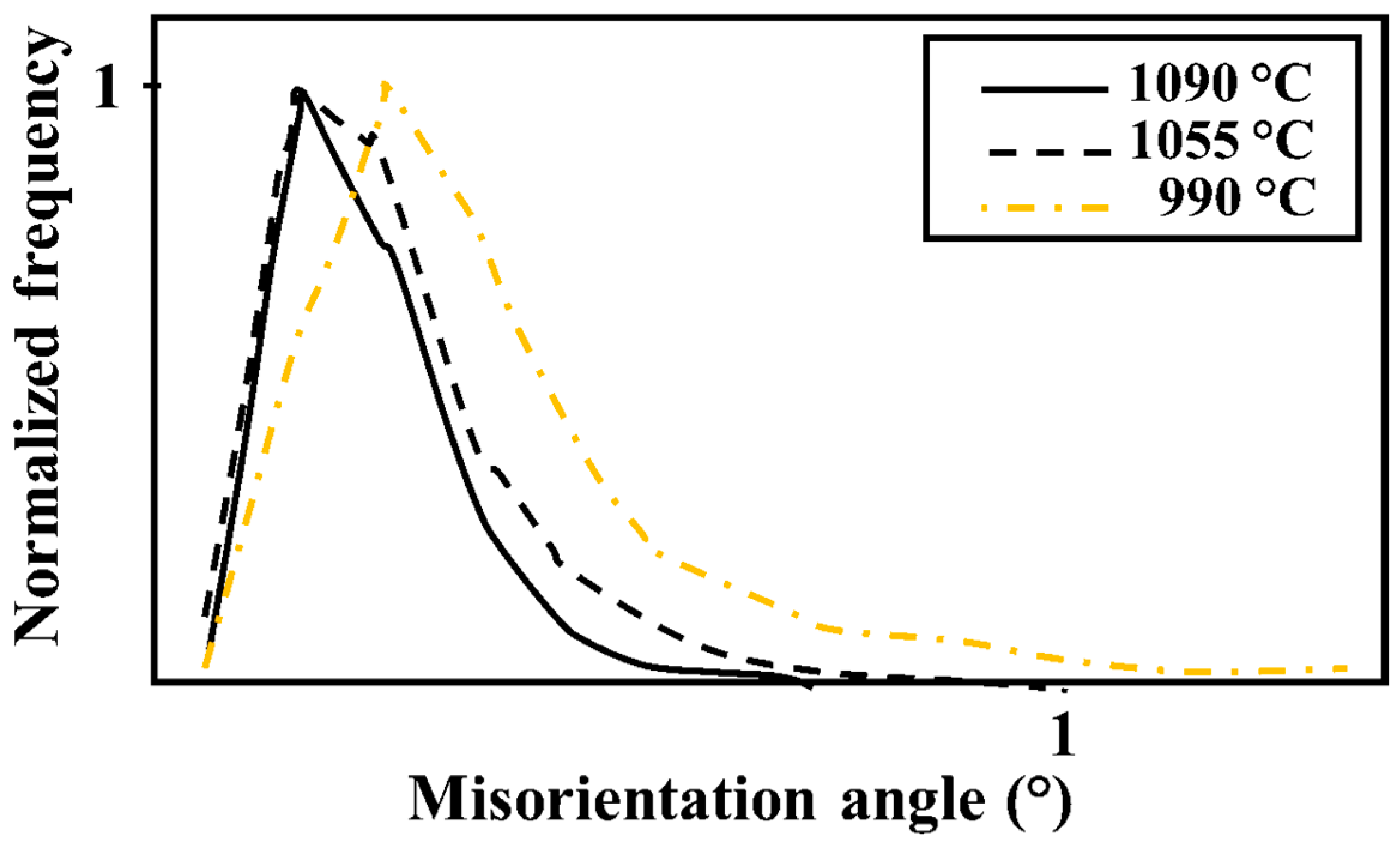
3.3. Local Misorientation and Strain Distribution Analyses of SDSS FGM
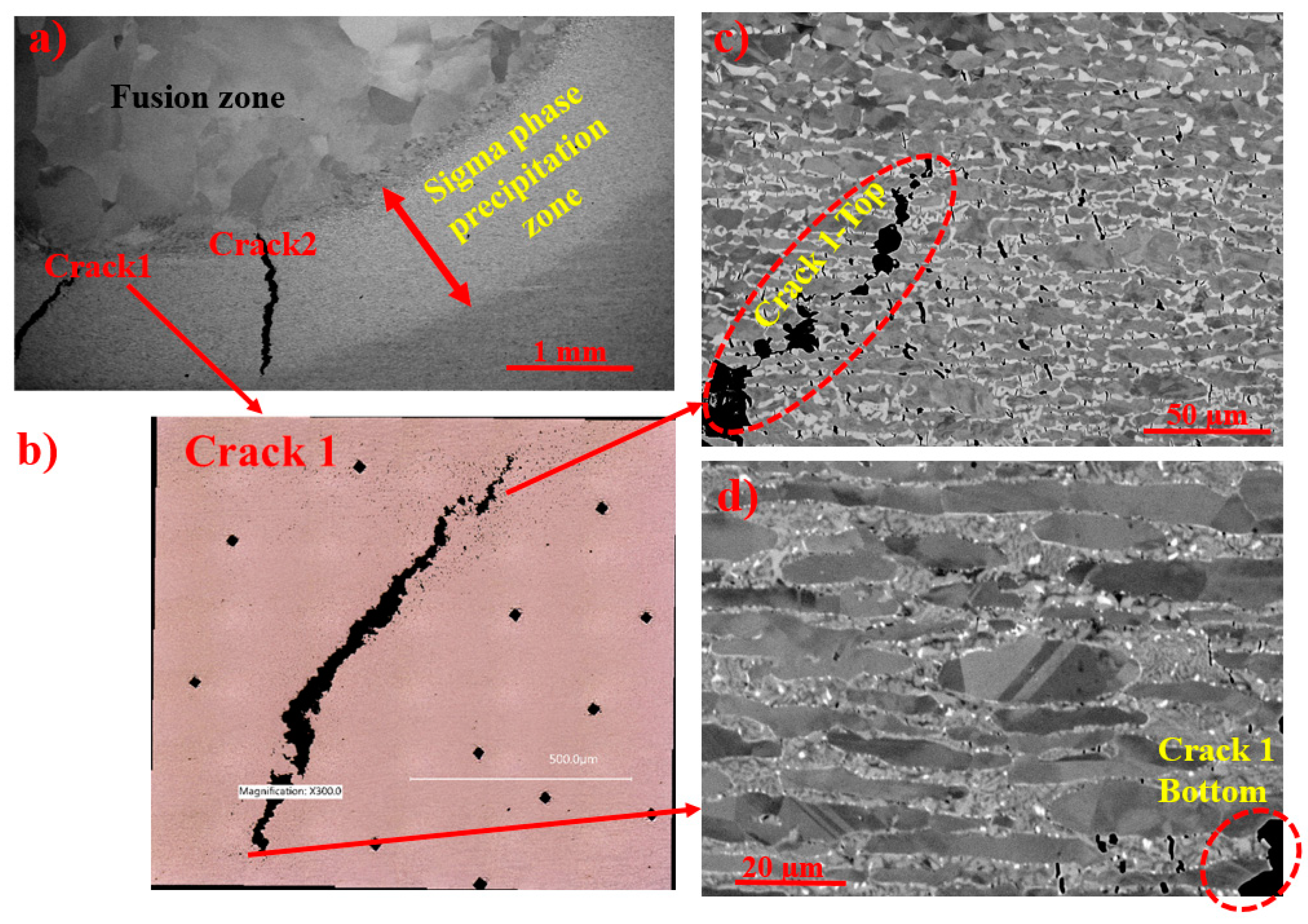
3.4. Cracking in Sigma Phase Formation Zone
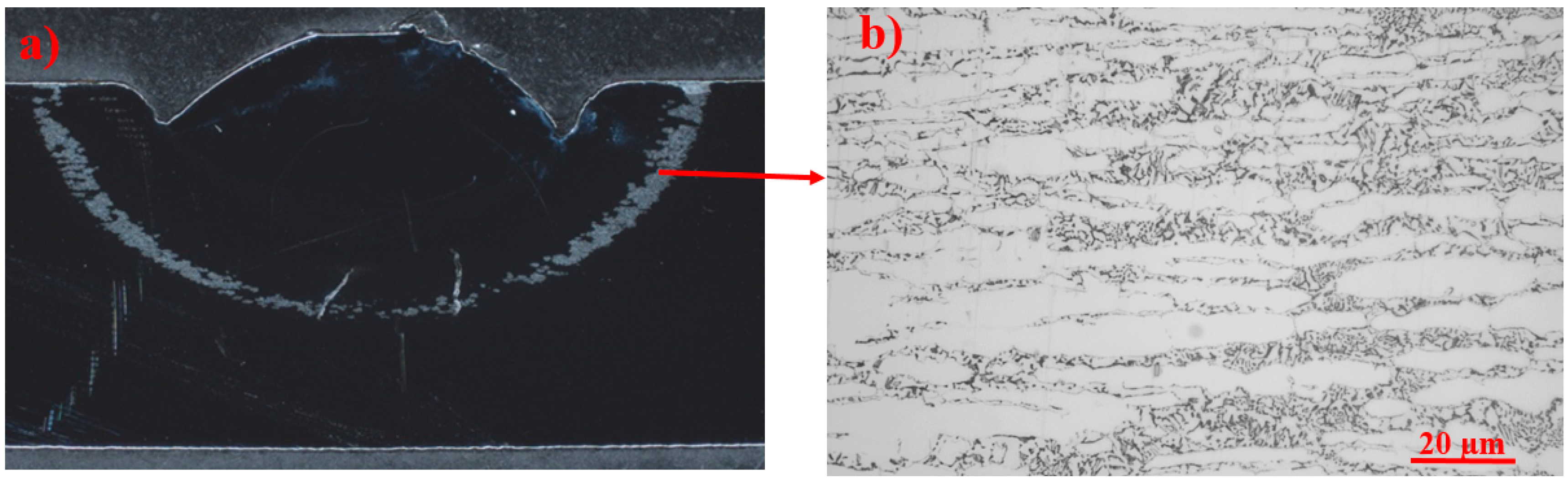
3.5. Sensitization
3.6. Electrochemical Critical Pitting Temperature Test
4. Discussion
4.1. Use of FGMs for Material Characterization
4.2. Sigma Phase Characteristics
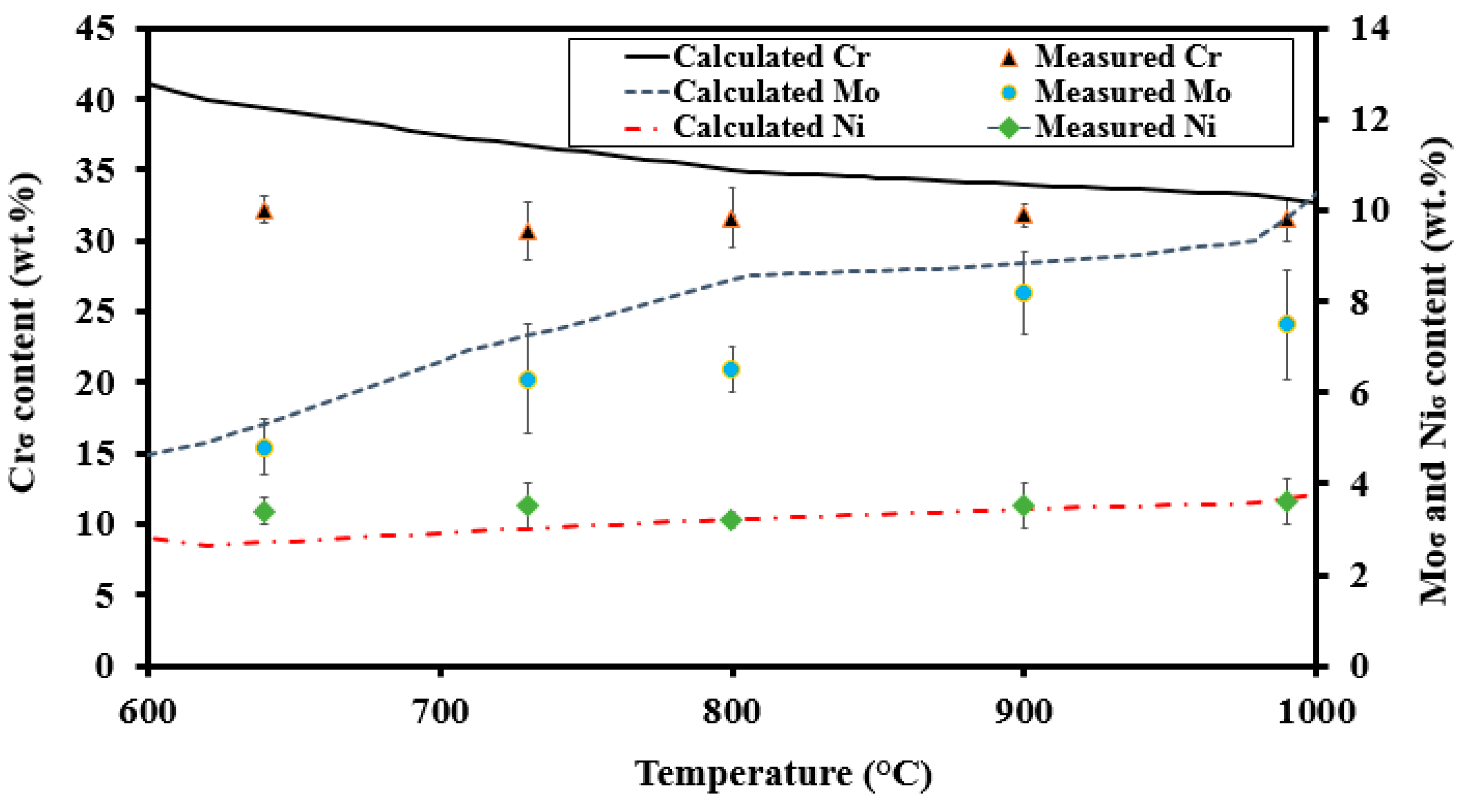
- Above 950 °C, the measured Mo and Cr contents were somewhat lower than predicted. Lower concentrations of Mo and Cr could be a possible consequence of higher σ fractions compared to the Thermo-Calc prediction [14].
- The calculated and measured Mo contents were quite similar around 900 °C. The predicted equilibrium σ content was met and the time was sufficient to approach the equilibrium content.
- Between 750 °C and 850 °C, the Mo content was significantly lower than predicted. Although the calculated equilibrium σ fraction was met at 800 °C [14], the time was not sufficient for the Mo content to approach its equilibrium level.
- Below 750 °C, the Mo content again was close to its predicted equilibrium value, as Mo contents of the parent δ and the final σ were quite similar.
4.3. Corrosion Behavior of σ-Containing Microstructures
4.4. Hardness, Local Misorientation, and Crack Propagation
4.5. Effects of σ Morphology on Degradation
5. Conclusions
- At temperatures between 630 and 1010 °C, σ precipitated with a maximum fraction of 34% at 750 °C;
- A maximum hardness of about 480 HV0.2 was obtained for aging at about 750 °C;
- The σ morphology changed from blocky to fine coral-shaped with decreasing aging temperature and its thickness decreased by about 80% from 1010 °C to 640 °C. No specific crystallography texturing was found in σ at different aging temperatures;
- The measured chemical composition of σ was similar to that predicted by thermodynamic calculations for 800–900 °C, but deviated at higher and lower temperatures;
- The microstructure containing blocky σ showed a higher local misorientation in the bulk of primary γ grains at 990 °C; however, local misorientation was distributed more locally in the γ2 grains next to coral-shaped σ at 640 °C;
- Macroscale cracks were propagated where σ precipitated. Blocky σ particles were more susceptible to microscale cracking;
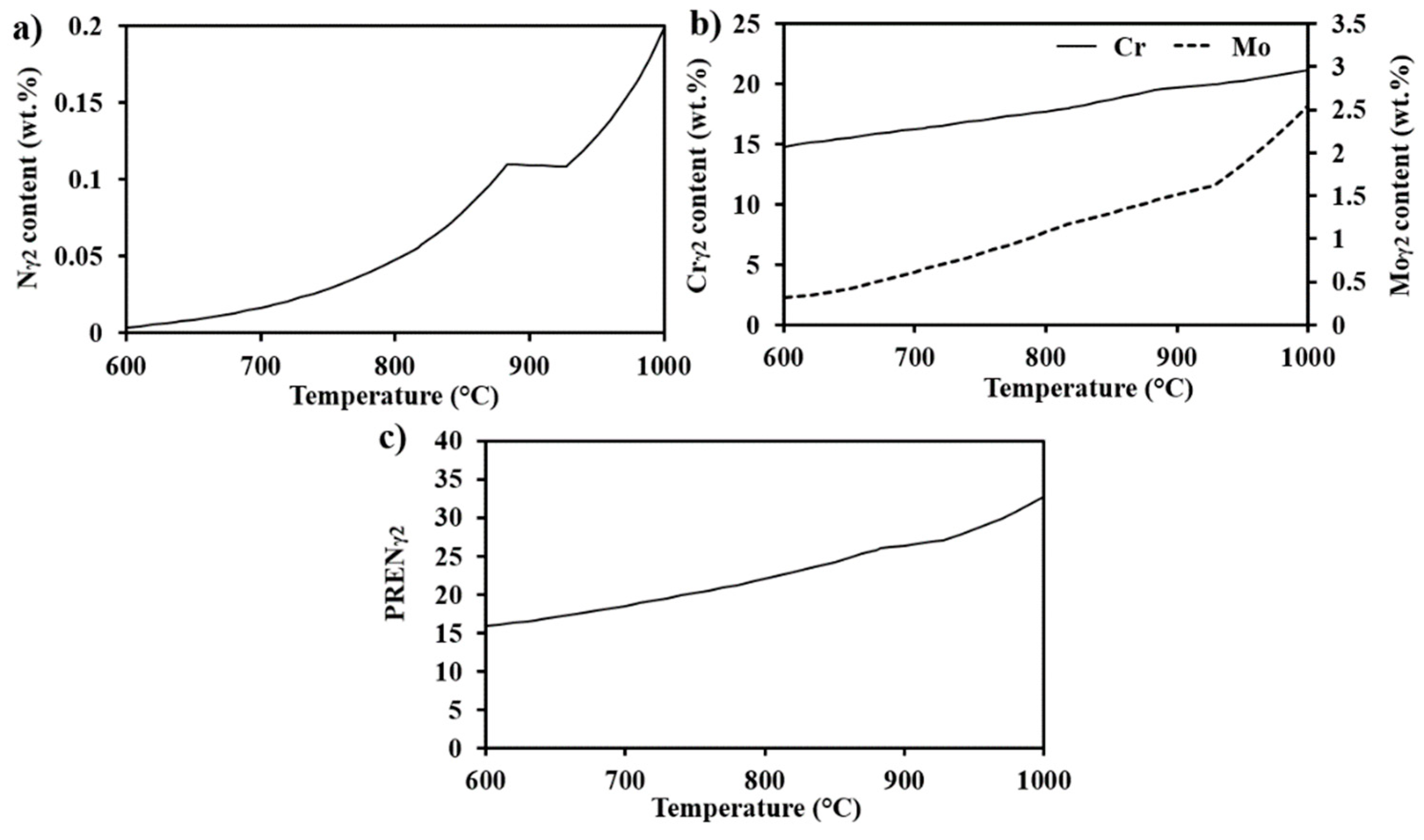
- Pitting corrosion testing showed that selective corrosion occurred in the regions aged between 630 °C and 750 °C in the microstructure containing fine coral-shaped σ. Sensitization screening testing showed more sensitization at the temperature range of 630–750 °C. Low PREN γ2, predicted by thermodynamic calculations, and δ were the location of local corrosion attacks.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nilsson, J.-O.; Chai, G. The physical metallurgy of duplex stainless steels. In Proceedings of the Duplex Stainless Steel Conference, Beaune, France, 13–15 October 2010. [Google Scholar]
- Tan, H.; Jiang, Y.; Deng, B.; Sun, T.; Xu, J.; Li, J. Effect of annealing temperature on the pitting corrosion resistance of super duplex stainless steel UNS S32750. Mater. Charact. 2009, 60, 1049–1054. [Google Scholar] [CrossRef]
- Deng, B.; Jiang, Y.; Gao, J.; Li, J. Effect of annealing treatment on microstructure evolution and the associated corrosion behavior of a super-duplex stainless steel. J. Alloys Compd. 2010, 493, 461–464. [Google Scholar] [CrossRef]
- Nilsson, J.-O. Super duplex stainless steels. Mater. Sci. Technol. 1992, 8, 685–700. [Google Scholar] [CrossRef]
- Handbook of Stainless Steel; Outokumpu Oyj: Helsinki, Finland, 2013.
- Pohl, M.; Storz, O.; Glogowski, T. Effect of intermetallic precipitations on the properties of duplex stainless steel. Mater. Charact. 2007, 58, 65–71. [Google Scholar] [CrossRef]
- Redjaimia, A.; Metauer, G.; Gantois, M. Decomposition of Delta Ferrite in an Fe–22 Cr–5 Ni–3 Mo–0. 03 C Duplex Stainless Steel. A Morphological and Structural Study. In Proceedings of the Duplex Stainless Steels (’91), Beaune, France, 28–30 October 1991; Volume 1, pp. 119–126. [Google Scholar]
- Hosseini, V.A.; Bermejo, M.A.V.; Gårdstam, J.; Hurtig, K.; Karlsson, L. Influence of multiple thermal cycles on microstructure of heat-affected zone in TIG-welded super duplex stainless steel. Weld. World 2016, 60, 233–245. [Google Scholar] [CrossRef]
- Zhang, Z.; Jing, H.; Xu, L.; Han, Y.; Zhao, L. The influence of microstructural evolution on selective corrosion in duplex stainless steel flux-cored arc welded joints. Corros. Sci. 2017, 120 (Suppl. C), 194–210. [Google Scholar] [CrossRef]
- Kim, H.-J.; Jeon, S.-H.; Kim, S.-T.; Park, Y.-S. Influence of the shielding gas composition on the passive film and erosion corrosion of tube-to-tube sheet welds of hyper duplex stainless steel. Corros. Sci. 2015, 91 (Suppl. C), 140–150. [Google Scholar] [CrossRef]
- Deng, B.; Wang, Z.; Jiang, Y.; Wang, H.; Gao, J.; Li, J. Evaluation of localized corrosion in duplex stainless steel aged at 850 °C with critical pitting temperature measurement. Electrochim. Acta 2009, 54, 2790–2794. [Google Scholar] [CrossRef]
- Pohl, M.; Storz, O. Sigma-phase in duplex-stainless steels. Zeitschrift für Metallkunde 2004, 95, 631–638. [Google Scholar] [CrossRef]
- Hosseini, V.A.; Karlsson, L.; Hurtig, K.; Choquet, I.; Engelberg, D.; Roy, M.J.; Kumara, C. A novel arc heat treatment technique for producing graded microstructures through controlled temperature gradients. Mater. Des. 2017, 121, 11–23. [Google Scholar] [CrossRef]
- Hosseini, V.A.; Karlsson, L.; Örnek, C.; Reccagni, P.; Wessman, S.; Engelberg, D. Functionally Graded Microstructure of Super Duplex Stainless Steel. Mater. Charact. 2018, 139, 390–400. [Google Scholar] [CrossRef]
- Hosseini, V.A.; Karlsson, L.; Engelberg, D.; Wessman, S. Time-temperature-precipitation and property diagrams for super duplex stainless steel weld metals. Weld. World 2018, 62, 317–533. [Google Scholar] [CrossRef]
- Hosseini, V.A.; Thuvander, M.; Wessman, S.; Karlsson, L. Spinodal decomposition in functionally graded super duplex stainless steel and weld metal. Metall. Mater. Trans. A 2018, 1–14. [Google Scholar] [CrossRef]
- Gregori, A.; Nilsson, J.-O. Decomposition of ferrite in commercial superduplex stainless steel weld metals; microstructural transformations above 700 °C. Metall. Mater. Trans. A 2002, 33, 1009–1018. [Google Scholar] [CrossRef]
- Nishimoto, K.; Saida, K.; Katsuyama, O. Prediction of Sigma Phase Precipitation in Super Duplex Stainless Steel Weldments. Weld. World 2006, 50, 13–28. [Google Scholar] [CrossRef]
- Jepson, M.A.E.; Rowlett, M.; Higginson, R.L. The Effect of Surface Preparation on the Precipitation of Sigma During High Temperature Exposure of S32205 Duplex Stainless Steel. Metall. Mater. Trans. A 2017, 48, 1491–1500. [Google Scholar] [CrossRef]
- Malik, A.; Odqvist, J.; Höglund, L.; Hertzman, S.; Ågren, J. Phase-Field Modeling of Sigma-Phase Precipitation in 25Cr7Ni4Mo Duplex Stainless Steel. Metall. Mater. Trans. A 2017, 48, 4914–4928. [Google Scholar] [CrossRef]
- Wan, J.; Ruan, H.; Wang, J.; Shi, S. The Kinetic diagram of sigma phase and its precipitation hardening effect on 15Cr-2Ni duplex stainless steel. Mater. Sci. Eng. A 2018, 711, 571–578. [Google Scholar] [CrossRef]
- Fukuoka, C.; Morishima, K.; Yoshizawa, H.; Mino, K. Misorientation development in grains of tensile strained and crept 2.25%Cr–1%Mo steel. Scr. Mater. 2002, 46, 61–66. [Google Scholar] [CrossRef]
- ASTM International. ASTM E384-17: Standard Test Method for Microindentation Hardness of Materials; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- ASTM International. ASTM A262-15: Standard Practices for Detecting Susceptibility to Intergranular Attack in Austenitic Stainless Steels; ASTM International: West Conshohocken, PA, USA, 2015. [Google Scholar]
- Hosseini, V.; Hurtig, K.; Karlsson, L. Effect of multipass TIG welding on the corrosion resistance and microstructure of a super duplex stainless steel. Mater. Corros. 2017, 68, 405–415. [Google Scholar] [CrossRef]
- ASTM International. ASTM G150-13 Standard Test Method for Electrochemical Critical Pitting Temperature Testing of Stainless Steels; ASTM International: West Conshohocken, PA, USA, 2013. [Google Scholar]
- Kumara, C. Modelling of the Temperature Field in TIG arc Heat Treated Super Duplex Stainless Steel Samples; University West: Trollhättan, Sweden, 2016. [Google Scholar]
- Martins, M.; Casteletti, L.C. Sigma phase morphologies in cast and aged super duplex stainless steel. Mater. Charact. 2009, 60, 792–795. [Google Scholar] [CrossRef]
- Magnabosco, R.; Alonso-Falleiros, N. Pit morphology and its relation to microstructure of 850 °C aged duplex stainless steel. Corrosion 2005, 61, 130–136. [Google Scholar] [CrossRef]
- Adhe, K.; Kain, V.; Madangopal, K.; Gadiyar, H. Influence of sigma-phase formation on the localized corrosion behavior of a duplex stainless steel. J. Mater. Eng. Perform. 1996, 5, 500–506. [Google Scholar] [CrossRef]
- Ridley, N. A review of the data on the interlamellar spacing of pearlite. Metall. Mater. Trans. A 1984, 15, 1019–1036. [Google Scholar] [CrossRef]
- Hayes, F.; Hetherington, M.; Longbottom, R. Thermodynamics of duplex stainless steels. Mater. Sci. Technol. 1990, 6, 263–272. [Google Scholar] [CrossRef]
- Garin, J.L.; Mannheim, R.L. Sigma-phase precipitation upon industrial-like heating of cast heat-resistant steels. J. Mater. Process. Technol. 2009, 209, 3143–3148. [Google Scholar] [CrossRef]
- Sathirachinda, N.; Pettersson, R.; Pan, J. Depletion effects at phase boundaries in 2205 duplex stainless steel characterized with SKPFM and TEM/EDS. Corros. Sci. 2009, 51, 1850–1860. [Google Scholar] [CrossRef]
- Warren, A.; Harniman, R.; Guo, Z.; Younes, C.; Flewitt, P.; Scott, T. Quantification of sigma-phase evolution in thermally aged 2205 duplex stainless steel. J. Mater. Sci. 2016, 51, 694–707. [Google Scholar] [CrossRef]
- Nilsson, J.-O.; Kangas, P.; Wilson, A.; Karlsson, T. Mechanical properties, microstructural stability and kinetics of σ-phase formation in 29Cr-6Ni-2Mo-0.38 N superduplex stainless steel. Metall. Mater. Trans. A 2000, 31, 35–45. [Google Scholar] [CrossRef]
- Llorca-Isern, N.; López-Luque, H.; López-Jiménez, I.; Biezma, M.V. Identification of sigma and chi phases in duplex stainless steels. Mater. Charact. 2016, 112, 20–29. [Google Scholar] [CrossRef]
- Lo, K.H.; Kwok, C.T.; Chan, W.K. Characterisation of duplex stainless steel subjected to long-term annealing in the sigma phase formation temperature range by the dlepr test. Corros. Sci. 2011, 53, 3697–3703. [Google Scholar] [CrossRef]
- Bassiouni, M.; Ward, L.; Singh Raman, R.; O’Mullane, A.; Gideon, B. (Eds.) Study on the Microstructural Changes and Impact on the Pitting Corrosion Behaviour of 2507 Duplex Stainless Steel Weld Regions. In Proceedings of the Australian Corrosion and Prevention Conference, Adelaide, Australia, 14–17 November 2010. [Google Scholar]
- Yousefieh, M.; Shamanian, M.; Saatchi, A. Influence of step annealing temperature on the microstructure and pitting corrosion resistance of SDSS UNS S32760 welds. J. Mater. Eng. Perform. 2011, 20, 1678–1683. [Google Scholar] [CrossRef]
- Kobayashi, D.Y.; Wolynec, S. Evaluation of the low corrosion resistant phase formed during the sigma phase precipitation in duplex stainless steels. Mater. Res. 1999, 2, 239–247. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, B.; Wang, J.; Yin, J. The influence of solution treatment temperature on microstructure and corrosion behavior of high temperature ageing in 25% Cr duplex stainless steel. J. Alloys Compd. 2011, 509, 8870–8879. [Google Scholar] [CrossRef]
- Jinlong, L.; Tongxiang, L.; Limin, D.; Chen, W. Influence of sensitization on microstructure and passive property of AISI 2205 duplex stainless steel. Corros. Sci. 2016, 104, 144–151. [Google Scholar] [CrossRef]
- Kamaya, M. Measurement of local plastic strain distribution of stainless steel by electron backscatter diffraction. Mater. Charact. 2009, 60, 125–132. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, Q.; Lu, Z.; Ru, X.; Han, G.; Tian, Y.; Shoji, T. The Effects of Prior-Deformation on Anodic Dissolution Kinetics and Pitting Behavior of 316L Stainless Steel. Int. J. Electrochem. Sci. 2016, 11, 1395–1415. [Google Scholar]
- Ubhi, H.S.; Saithala, J.; Jiang, H. An EBSD study of the micro structural development during annealing of a folded super duplex stainless steel sheet sample. Baosteel Tech. Res. 2010. [Google Scholar]
- Elmer, J.; Olson, D.; Matlock, D. Thermal expansion characteristics of stainless steel weld metal. Weld. J. 1982, 61, 293–301. [Google Scholar]
- Babakr, A.; Al-Ahmari, A.; Al-Jumayiah, K.; Habiby, F. Sigma phase formation and embrittlement of cast iron-chromium-nickel (Fe-Cr-Ni) alloys. J. Miner. Mater. Charact. Eng. 2008, 7, 127. [Google Scholar] [CrossRef]





| Element | Content (wt %) |
|---|---|
| C | 0.016 |
| Si | 0.44 |
| Mn | 0.76 |
| P | 0.028 |
| S | 0.001 |
| Cr | 25.04 |
| Ni | 6.93 |
| Mo | 3.78 |
| N | 0.265 |
| Cu | 0.40 |
| Fe | Bal. |
| T (°C) | δ (%) | γ (%) | σ (%) | Average σ Thickness (µm) | Hardness (HV0.2) |
|---|---|---|---|---|---|
| As-received material | 49.7 ± 2 | 50.3 ± 2 | 0 | - | 280 |
| 1020 | 82 ± 2 | 18 ± 2 | 0 | - | 277 |
| 980–990 | 36.0–64.3 | 42.7–28.2 | 22.3–14.1 | 2.9 ± 1.7 | 359 |
| 950 | - | - | 27.0 ± 2.9 | - | 438 |
| 880 | 0.5 ± 0.2 | 71.3 ± 1.3 | 27.9 ± 1.2 | 1.5 ± 1.1 | |
| 750 | 0.3 ± 0.1 | 65.0 ± 0.8 | 34.4 ± 0.7 | 0.9 ± 0.7 | 480 |
| 640 | 7.8 ± 3.6 | 68.3 ± 6.2 | 21.4 ± 2.7 | 0.5 ± 0.4 | 363 |
| 630 * | - | - | 5.4 | - | |
| 620 * | - | - | 0 | - |
| Temperature (°C) | Cr (wt %) | Ni (wt %) | Mo (wt %) | Fe (wt %) |
|---|---|---|---|---|
| 990 | 31.5 ± 1.5 | 3.6 ± 0.9 | 7.5 ± 1.2 | Balance |
| 900 | 31.8 ± 0.8 | 3.5 ± 0.5 | 8.2 ± 0.9 | Balance |
| 800 | 31.6 ± 2.1 | 3.2 ± 0.2 | 6.5 ± 0.5 | Balance |
| 730 | 30.7 ± 2.0 | 3.5 ± 0.5 | 6.3 ± 1.2 | Balance |
| 640 | 32.2 ± 1.0 | 3.4 ± 0.3 | 4.8 ± 0.6 | Balance |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hosseini, V.A.; Karlsson, L.; Wessman, S.; Fuertes, N. Effect of Sigma Phase Morphology on the Degradation of Properties in a Super Duplex Stainless Steel. Materials 2018, 11, 933. https://doi.org/10.3390/ma11060933
Hosseini VA, Karlsson L, Wessman S, Fuertes N. Effect of Sigma Phase Morphology on the Degradation of Properties in a Super Duplex Stainless Steel. Materials. 2018; 11(6):933. https://doi.org/10.3390/ma11060933
Chicago/Turabian StyleHosseini, Vahid A., Leif Karlsson, Sten Wessman, and Nuria Fuertes. 2018. "Effect of Sigma Phase Morphology on the Degradation of Properties in a Super Duplex Stainless Steel" Materials 11, no. 6: 933. https://doi.org/10.3390/ma11060933
APA StyleHosseini, V. A., Karlsson, L., Wessman, S., & Fuertes, N. (2018). Effect of Sigma Phase Morphology on the Degradation of Properties in a Super Duplex Stainless Steel. Materials, 11(6), 933. https://doi.org/10.3390/ma11060933





