1. Introduction
With the rapid development of technology, the demand for various mobile electronic products such as cell phones, tablets, and smart healthcare devices has increased explosively [
1,
2,
3], and studies with the aim of developing lithium-ion batteries (LIB) of a high efficiency, stability, and low cost have been actively conducted. Graphite (372 mAh g
−1) [
4] has been used as the anode material in the fabrication of Li-ion batteries with a low theoretical capacity, because the anode material directly affects the capacity of Li-ion batteries. Although silicon-based material is emerging as an ideal replacement for the commercial carbonaceous material owing to its highest theoretical capacity (4200 mAh g
−1), it would be difficult to replace that because of the volume expansion of the silicon-based anode during the charge/discharge process. Innovative silicon nanostructures, therefore, have been used in numerous studies in order to prevent volume expansion of Si-based anodes (i.e., nanoparticles [
5,
6], nanowires [
7], and nanotubes [
8]). Despite the important achievements mentioned, poisonous and dangerous reagents are not appropriate in a synthesis process. When sophisticated equipment is necessary or expensive and multi-stage synthesis is adopted, it is not easy to increase the scale. Accordingly, there still remain significant challenges to overcome the commercialization of the Si anode.
In the case of transition metal oxides, the capacity is much higher than the capacity of the graphitic carbon, and the volume change is not as great as the silicon material. Therefore, the use of a transition metal oxide as the anode material appears to be an appropriate way to overcome the drawbacks of the alloy anode material [
9]. Copper (Cu)-based oxides such as copper (Cu
2O) and copper (CuO) oxides, as well as transition metal oxides, are under the spotlight for promising candidates for use in LIB, solar cells, supercapacitors, gas sensors, biosensors and catalysts due to their inherent properties as p-type semiconductors with a low band gap energy, high optical absorption and high catalytic activity.
Cupric oxide (CuO) [
10] and cuprous oxide (Cu
2O) [
11], with the respective capacities of 674 mAh g
−1 and 375 mAh g
−1, have received enormous attention owing to high theoretical capacities, low costs, non-toxicity, and abundant natural reserves. Alternatively, regarding the LIB for which CuO and Cu
2O serve as the anode materials, the generation of very large volume expansions occurs during the battery lithiation and delithiation processes, resulting in not only a separation from the copper (Cu) current collector, but a poor cyclability, low capacity, and low electronic conductivity [
12,
13]. To solve these problems, numerous studies have been conducted to mitigate the volume-expansion stress, where the anode material nanostructure has been fabricated into nanoparticles, nanowires, nanotubes, nanorods and nanoflake structures [
14,
15,
16,
17]. Notwithstanding the significant improvements in the electrochemical performance of the Li-ion batteries that are enabled by these studies, the corresponding methods require exorbitant devices, complicated preparations, and time-consuming tasks, and difficulties of low-cost large-area processing are encountered as well.
Meanwhile, fabrication of nanostructured metal oxides generated on the metal substrates by fast pulsed laser ablation has been reported. A series of nanostructured metal oxides have been synthesized by pulsed laser ablation of metal substrates.
Here, graphite, the anode material of the conventional battery, was replaced with CuO through the formation of CuO on the Cu surface that was achieved by a simple, fast, innocuous, and inexpensive laser irradiation of the Cu current collector; nanostructures, which were made through laser ablation, prevented the degradation of the battery capacity and cyclability that are results of the volume expansion. The laser ablation spontaneously allowed the formation of the nanostructure on the Cu current collector. Then, the microstructure was transferred onto the Cu current collector through the imprinting of the microstructural metal mold on the Cu current collector, wherein the nanostructure was formed. This process was carried out considering the use of a hierarchical structure [
18] consisting of both the nano- and microstructures, which accommodate volume expansion of Si more than solely the nanostructure. The lithium-ion battery that is based on the hierarchically nanostructured CuO–Cu current collector with the micro- and nanostructures not only maintained a cyclability that is similar to those of the conventional types, but the capacity was improved twofold. The proposed method of this paper can be directly applied to the existing battery-fabrication process, as laser ablation enables an increase in the battery capacity via a chemical-free process in a fast and simple manner.
3. Results and Discussion
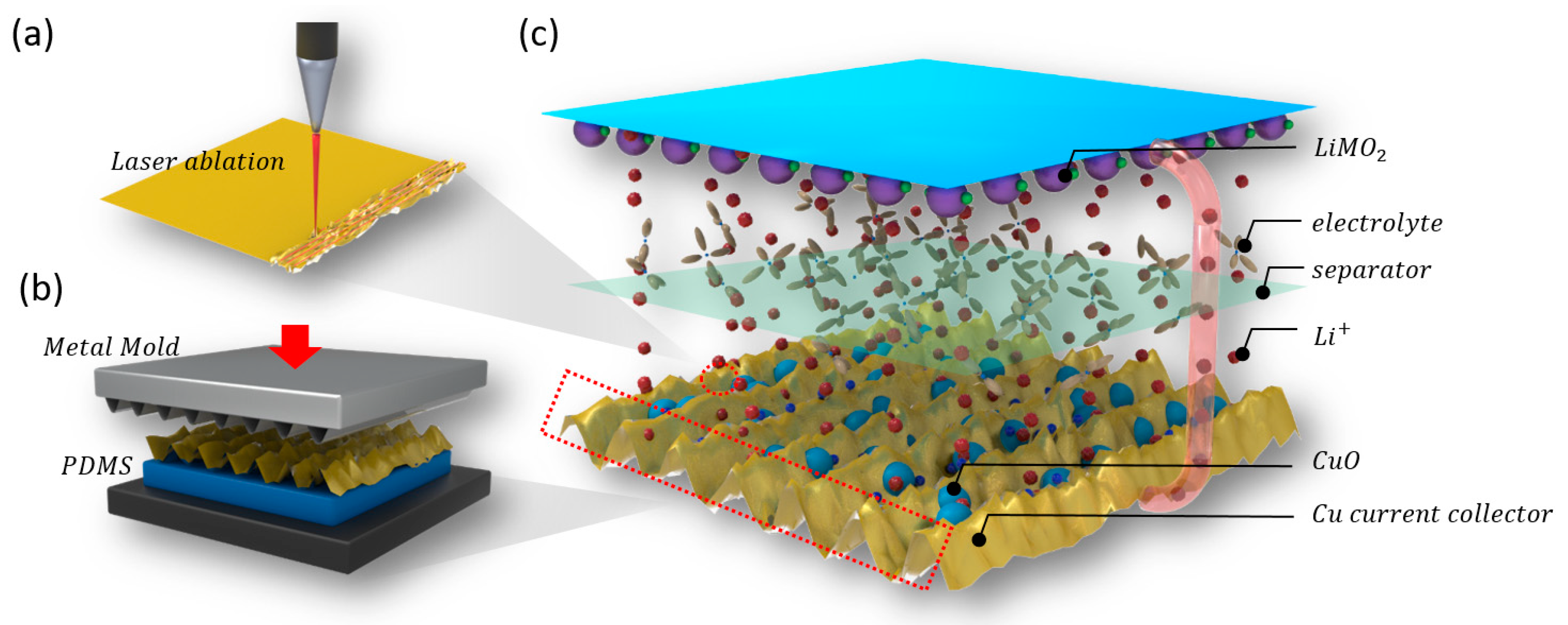
Figure 1 illustrates the overall fabrication process of the hierarchically nanostructured CuO–Cu current collector and its application in the design of the lithium-ion battery. The first step, as shown in
Figure 1a, represents the nanosecond laser ablation on the Cu current collector; The surface of the Cu collector in the laser-cutting process was immediately vaporized by high energy and thermal laser pulses. The avalanche ionization induced by the intense laser field excited the copper vapor to the plasma. Plasma and/or superfine Cu particles were then oxidized in air and deposited on the surface of the Cu current collector substrate and cooled to room temperature within a very short time. Finally, due to the very high pressure of the atmospheric atmosphere in which the superfine particles were generated, a uniformly distributed faulty CuO hierarchical nanostructure which was firmly adhered to the surface of the Cu current collector after the CuO particles retracted onto the substrate. The Cu was evaporated by the heat generated and converted into CuO through the two-step oxidation of 4Cu + O
2 → 2Cu
2O, 2Cu
2O + O
2 → 4CuO in air [
21].
The second step of
Figure 1b shows the imprinting of the line-patterned metal mold on the Cu current collector with the nanostructure constructed by the laser ablation. The sinusoidal pathway of the proposed line-patterned metal mold obtained the 500-μm wavelength. Then, the Cu current collector in which the nanostructure was formed was placed on polydimethylsiloxane (PDMS), and the line pattern was transferred onto the Cu current collector using imprinting by the generated line-patterned metal mold (under the pressure of 10 MPa for 5 min). The PDMS served as the buffer layer and allowed the microsize line pattern to be properly transferred to the Cu current collector while the ablation-produced nanostructure was entirely maintained. The CuO–Cu current collector was consequently fabricated with a hierarchical structure that is frequently used to broaden the surface area in nature.
The last step, shown in
Figure 1c, is the demonstration of the application of the hierarchically nanostructured CuO–Cu current collector, which replaced the conventional Cu current collector and the graphite anode material, in the design of the lithium-ion battery. The cycling-performance test was subsequently carried out.
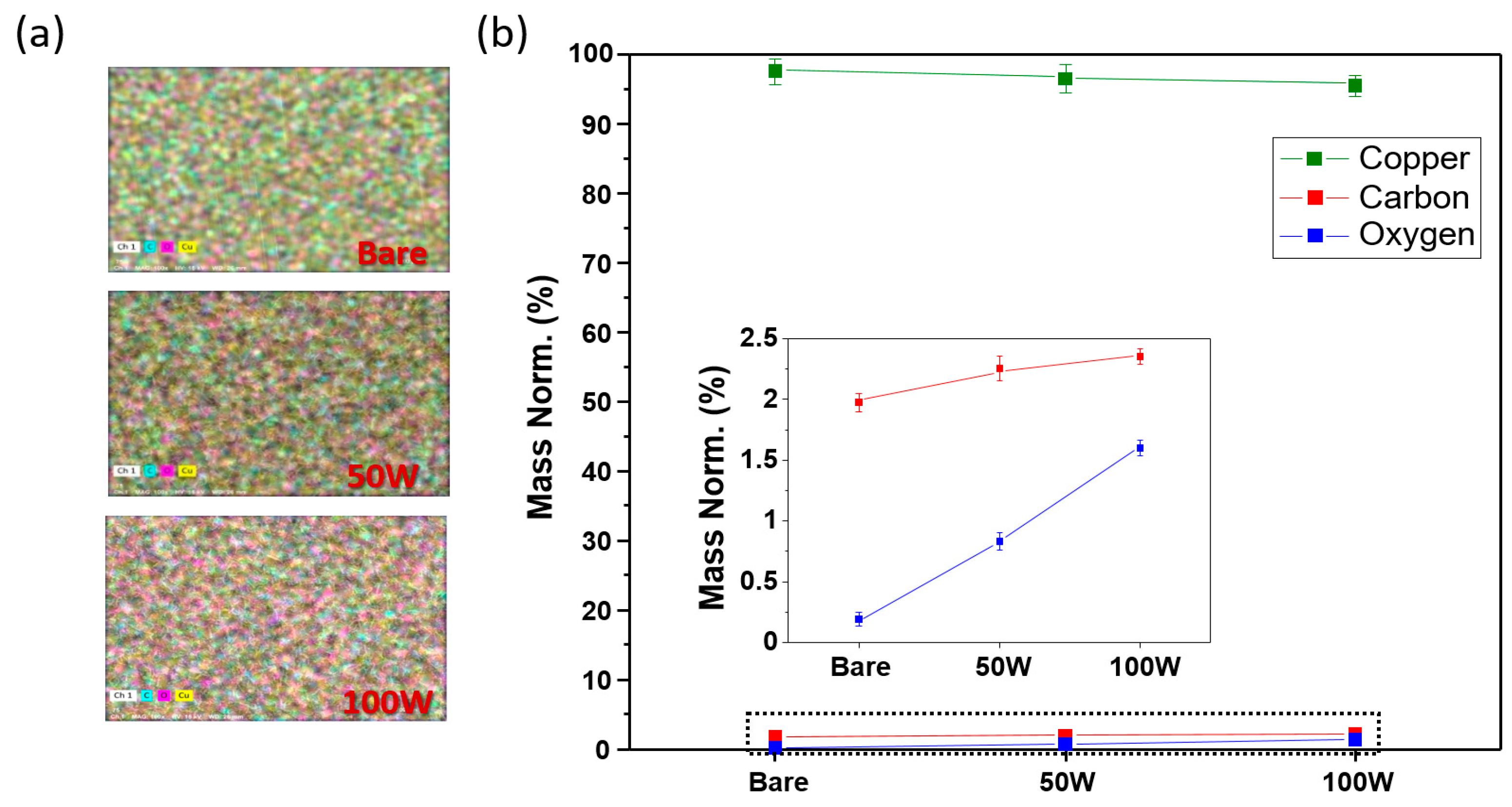
Figure 2 shows the EPMA and EDS chemical analyses regarding the CuO–Cu current collector, which was formed as a result of the laser ablation on the bare Cu current collector. In
Figure 2a, the top sections of each of the EPMA images indicate the regions on the bare Cu current collector under the 50-W and 100-W ablation powers. As the analysis data of the bare Cu current collector show, at the top of the image the oxygen component of the pink region appears to be low, and then it gradually increases further down the image; that is, as the Cu current collector was oxidized by the laser’s high energy and heat, the CuO became evenly generated on the surface. The EDS numerical-analysis data are shown in
Figure 2b, where the graph indicates the relative contents of Cu, C, and O
2 as quantitative materials; moreover, the inset graph, an enlargement of the C and O
2 results, demonstrates that the bare Cu current collector consisting of 97.80% of Cu and 0.22% of O
2 represents significantly lower oxygen content compared with the Cu content. Alternatively, the Cu current collector at 100 W comprising 96.94% of Cu and 0.81% of O
2 is the result of the state at 50 W consisting of 96.09% of Cu and 1.55% of O
2, respectively, and this presents the rapid O increase. This result shows the combining of the Cu and O
2 in the air and the subsequent deposition of this combination on the surface that increased the oxygen floating on the surface. The Cu current collector that was ablated with the 100-W laser was used in the subsequent experiments, where the efficiency was increased as the CuO content grew. Furthermore,
Figure 2 shows that laser ablation can quickly and simply produce CuO with a high theoretical capacity without any chemical treatment, and a higher capacity that outperforms the conventional lithium-ion battery is anticipated.
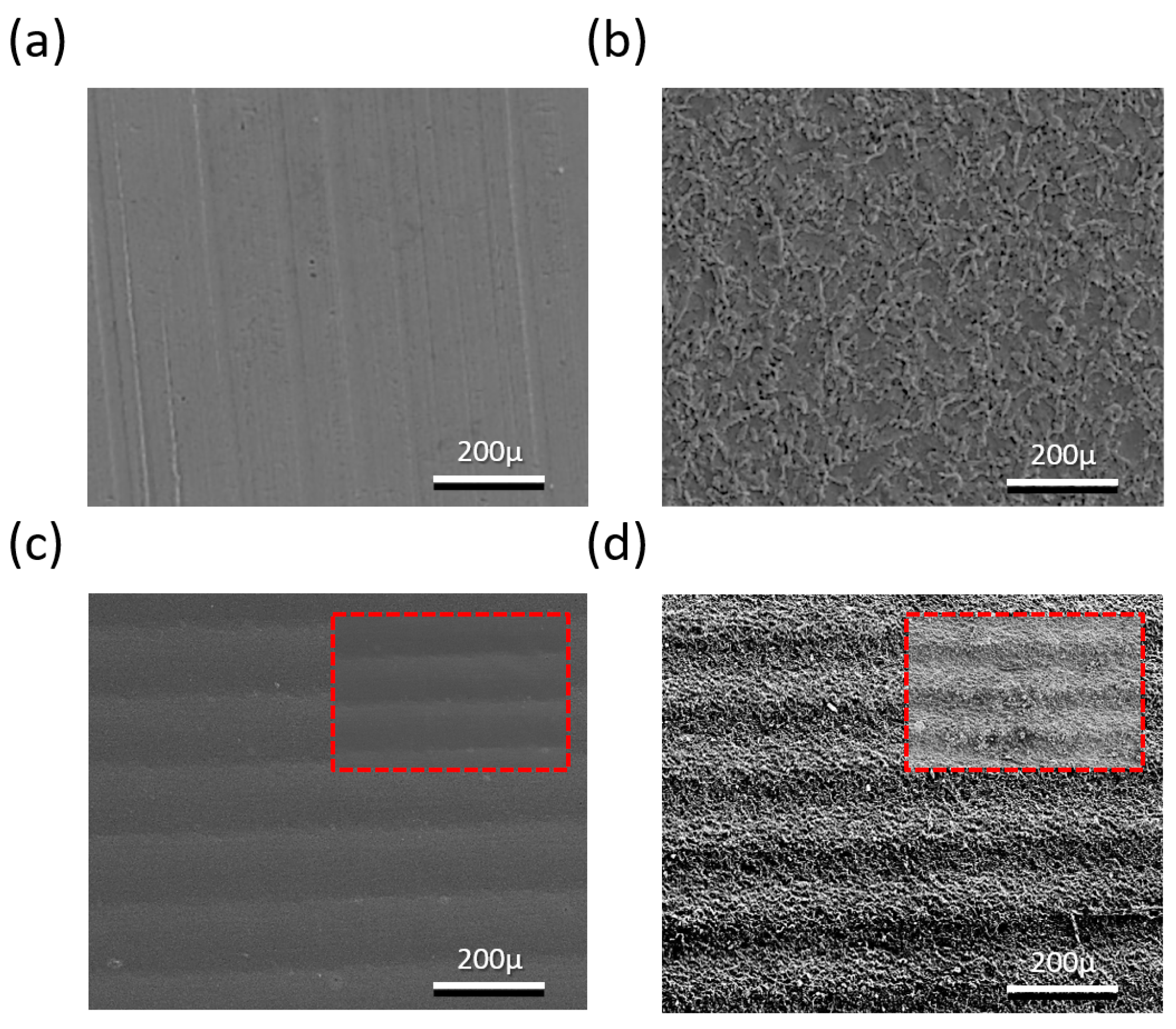
SEM (500×) images of the bare Cu current collector, laser-ablated Cu current collector, imprinted Cu current collector, and laser + imprinted Cu current collector are represented in
Figure 3a–d, respectively. A flat surface is seen in
Figure 3a, while in
Figure 3b, the surface becomes very rough due to the explosion of the laser energy.
Figure 3c shows the illustration of the sinusoidal pathway of the Cu current collector of the 500-μm wavelength that was constructed using the imprinting process.
Figure 3d shows the laser-ablated nanostructure and the combined-hierarchy Cu current collector with the microstructure that was formed using metal-mold imprinting.
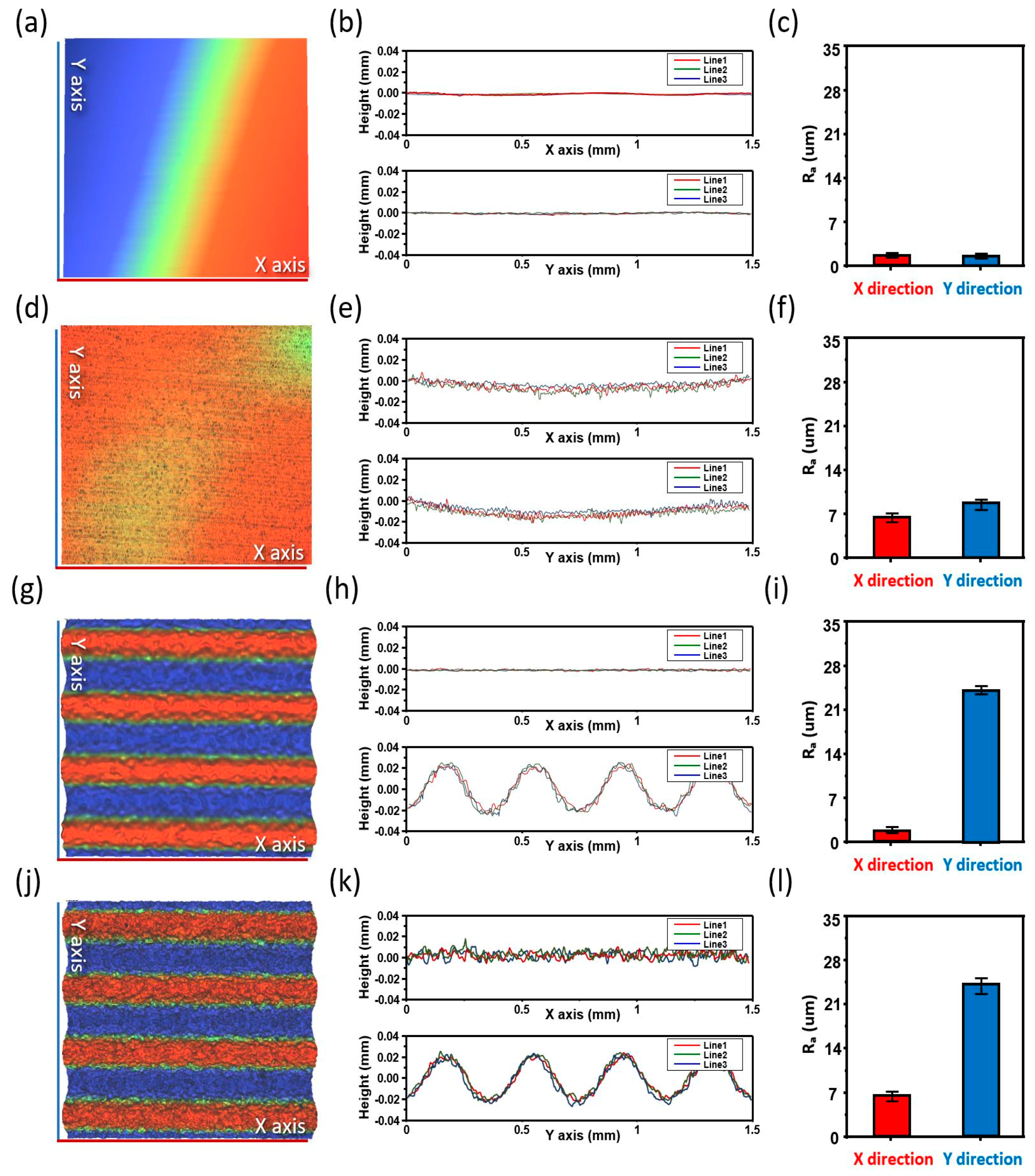
Figure 4a,d,g,j respectively show the three-dimensional (3D) surface-analysis images of the four types of the fabricated Cu current collector, as follows: bare Cu current collector, laser-ablated Cu current collector, imprinted Cu current collector, and laser + imprinted Cu current collector. The figure shows that 3D images provide results that are the same as those of
Figure 3. The line-scan profiles of the bare Cu current collector, laser-ablated Cu current collector, imprinted Cu current collector, and laser + imprinted Cu current collector, measured using the non-contact 3D microtiter, are shown in
Figure 4b,e,h,k, respectively. The graphs above and below show the line-scan profiles of the Cu current collectors that were extracted at three different spots along the
x- and
y-axes, respectively. The line-scan profile indicates the elevation of the surface relative to zero. Also, the greater the amount of ripples, the rougher the surface and the wider the surface area; these results also indicate that the surface is flat, which is shown by the
x- and
y-axes on the line-scan profiles where only straight lines are apparent.
Moreover, as illustrated in
Figure 4e, the line-scan profile comprises many ripples on the
x- and
y-axes relative to zero. Accordingly, the surface area per unit area of the proposed Cu current collector became larger than that of the bare Cu current collector due to the spontaneously generated laser-ablation ripple. From the line-scan profile of
Figure 4h, the 500-μm wavelength occurred periodically, thereby resulting in an effectively performed transfer of the metal-mold pattern and the incremental transfer of the surface area per unit area compared with the bare Cu current collector.
Figure 4k, in contrast, shows the line-scan profile with numerous ripples and periodic appearances of the μm wavelengths that are owing to the simultaneously presented laser-ablation nanostructure and the metal-mold microstructure. This result represents a nanostructure that is not affected by the imprinting, a well-transferred microstructure, and a hierarchical structure that became the widest surface area per unit area.
Quantitative evaluation was performed to compare the increase of the surface area accurately.
Figure 4c,f,i,l show the surface-roughness averages (R
a) of the bare Cu current collector, laser-ablated Cu current collector, imprinted Cu current collector, and laser + imprinted Cu current collector, respectively. The R
a values indicate the degree of the surface roughness, where the value increases with the increasing roughness of the surface; that is, the incremental increase of the R
a value compared with the previous value means that the surface area per unit area has increased [
22]. Furthermore, the bar values along the
x- and
y-axes and the standard error demonstrate the mean and the standard deviation of the red, green, and blue along the
x- and
y-axes of the line-scan profiles shown in
Figure 4b,e,h,k. The R
a values of the
x- and
y-axes in
Figure 4c are 1.73 and 1.72, respectively. Compared with
Figure 4c,f shows increases of the R
a values of the
x- and
y-axes (6.63 and 8.72) by 383% and 506%, respectively, thereby quantitatively confirming an enormous increase of the surface area. In
Figure 4i, the R
a values of the
x- and
y-axes are 1.76 and 21.7, respectively, while the R
a value of the
x-axis is similar to that of
Figure 4c and the
y-axis was increased by 1261%. As shown in
Figure 4i, the R
a values of the
x- and
y-axes are 6.52 and 24.4, respectively. Here, the figures suggest that the surface area per unit area among the samples increased the most due to the increase of the R
a values of the
x- and
y-axes by 376% and 1418%, respectively, compared with
Figure 4c. The results of
Figure 3 and
Figure 4 provide the optimal method for maximizing the surface area for which the hierarchically nanostructured structures were used.
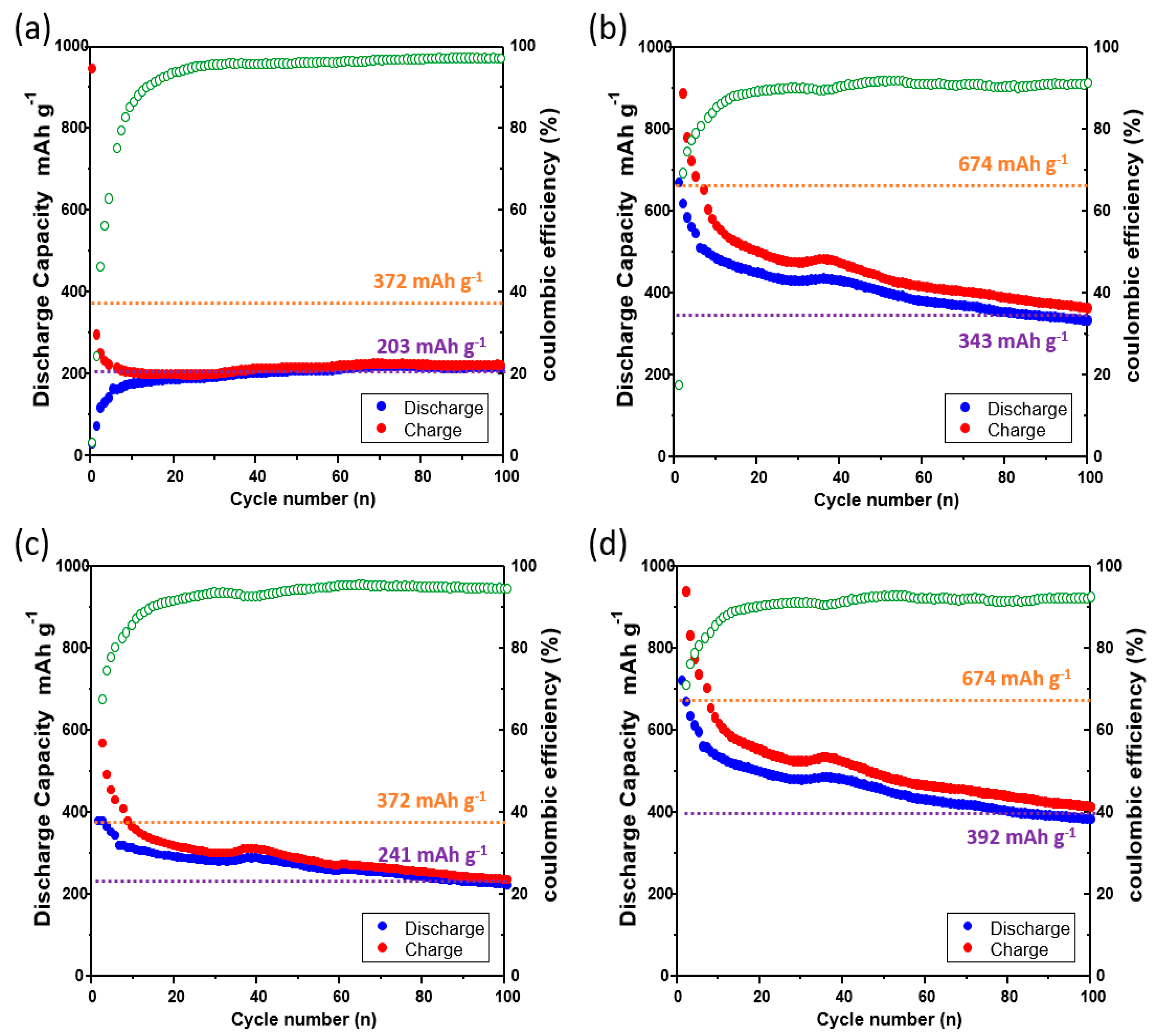
Figure 5 shows the charge–discharge curves of the lithium-ion batteries that were prepared using the bare Cu current collector, laser-ablated Cu current collector, imprinted Cu current collector, and laser + imprinted Cu current collector. Graphite was used as the anode material of the bare Cu current collector and the imprinted Cu current collector. Alternatively, CuO, deposited on the surfaces of the laser-ablated Cu current collector and the laser + imprinted Cu current collector as the product of the laser oxidation of the Cu current collector, was used as the anode material. The charging and discharging were carried out at the current density of 6 A g
−1 in the range of 0.01–1.50 V.
Figure 5a shows the charge–discharge curves of the lithium-ion battery of the bare Cu current collector. The capacity after 100 cycles is 203 mAh g
−1. The initial coulombic efficiency (CE) of
Figure 5a was maintained at 98% after 25 cycles, where the CE signifies the battery efficiency [
23].
Figure 5b illustrates the charge–discharge curves of the lithium-ion battery of the laser-ablated Cu current collector. The capacity after 100 cycles is 343 mAh g
−1. The CE was maintained at 92% after 25 cycles. The capacity after 100 cycles increased by 168%, and the CE was analogously maintained in comparison with
Figure 5a; this is because the capacity was increased as CuO, which was formed by the laser ablation, was used as the anode material. Moreover, the nanostructure, which was constructed by the laser ablation, created a void space. The void space not only provides space for CuO to accommodate the volumetric expansion during charging and discharging, but it also enlarges the surface area so that the increased interatomic attraction force enables the effective adherence between CuO and the Cu current collector; this leads to an improved conductivity and helps to improve the capacity as well.
Figure 5c shows the charge–discharge curves of the lithium-ion battery of the imprinted Cu current collector. The capacity after 100 cycles is 241 mAh g
−1. The CE was maintained at 95% after 25 cycles. The capacity after 100 cycles increased slightly by 119% compared with
Figure 5a. This finding is because of the unaltered theoretical capacity that is due to the use of the same anode material, graphite, but also since the microstructural sinusoidal pathway increases the surface area, which may provide the positive impacts as it expands the surface area where graphite and lithium react. Therefore, the imprinted Cu current collector has better conductivity than the bare Cu current collector.
Figure 5d demonstrates the charge–discharge curves of the lithium-ion battery of the laser + imprinted Cu current collector. The capacity after 100 cycles is 392 mAh g
−1. The CE was maintained at 94% after 25 cycles. The capacity after 100 cycles was increased by 193% compared with
Figure 5a, and the CE is analogous to
Figure 4c. In comparison with the other samples, the capacity after 100 cycles is the highest. This finding is because of the micro and nano mixed structures that are used to increase the surface area in nature; that is, the surface area became the widest among the samples, thereby leading to the reduced separation between CuO and the Cu current collector that is due to the highest increase of the interatomic attraction.