Polysulfobetaines in Aqueous Solution and in Thin Film Geometry
Abstract
:1. Introduction
2. Results
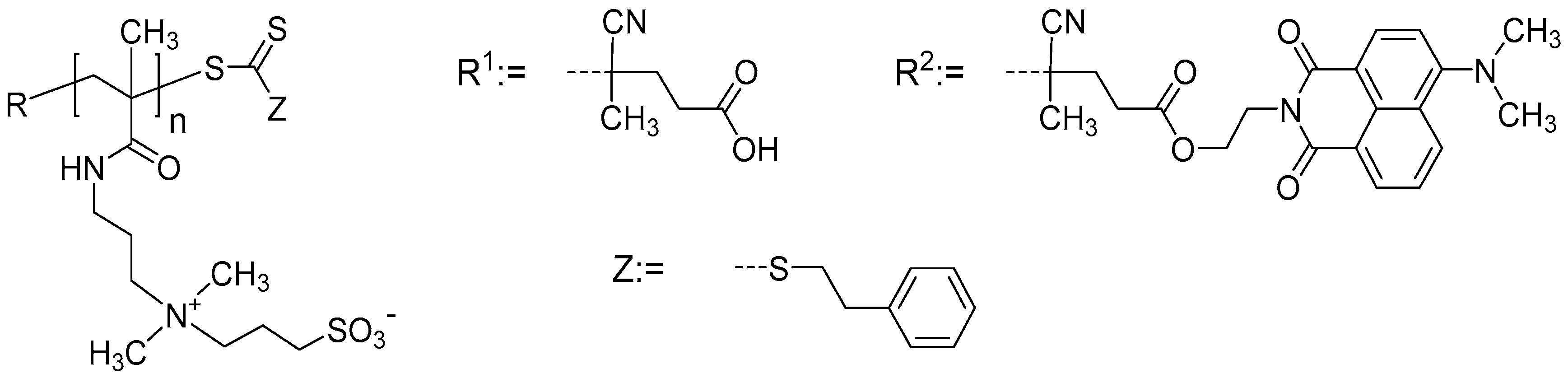
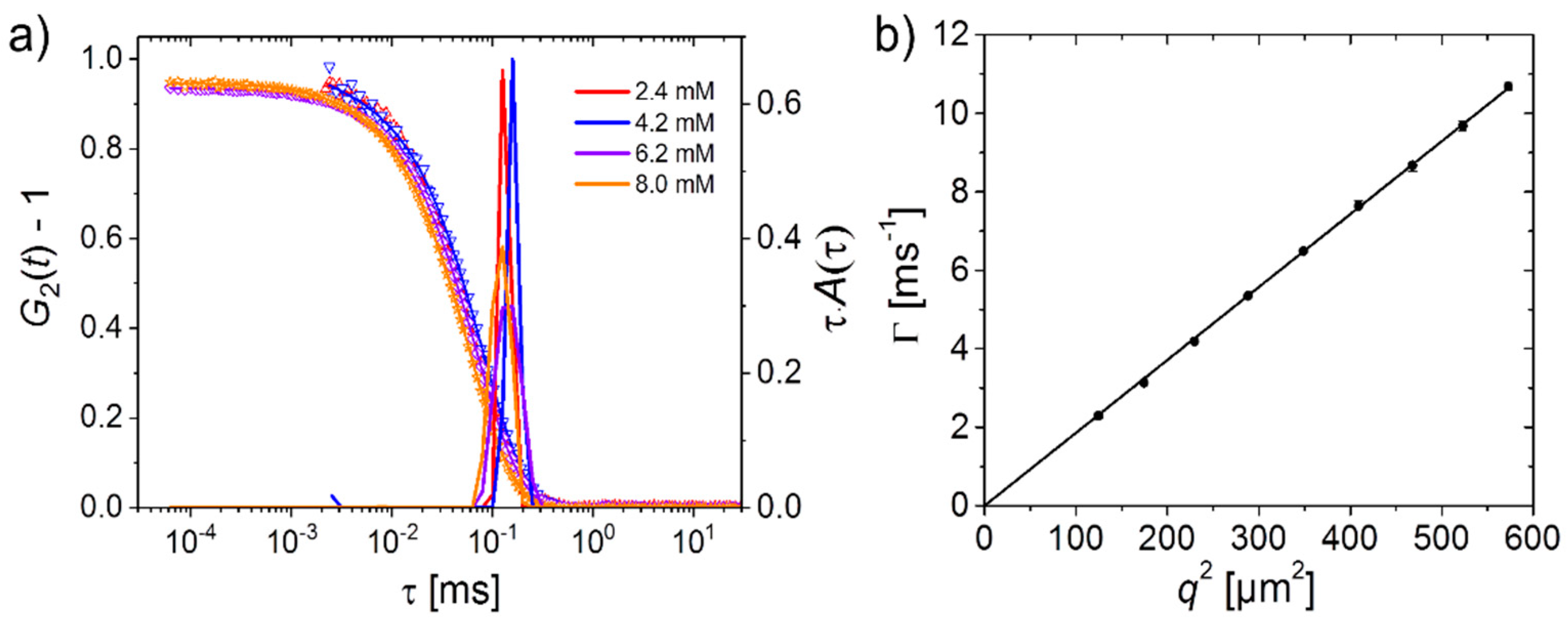
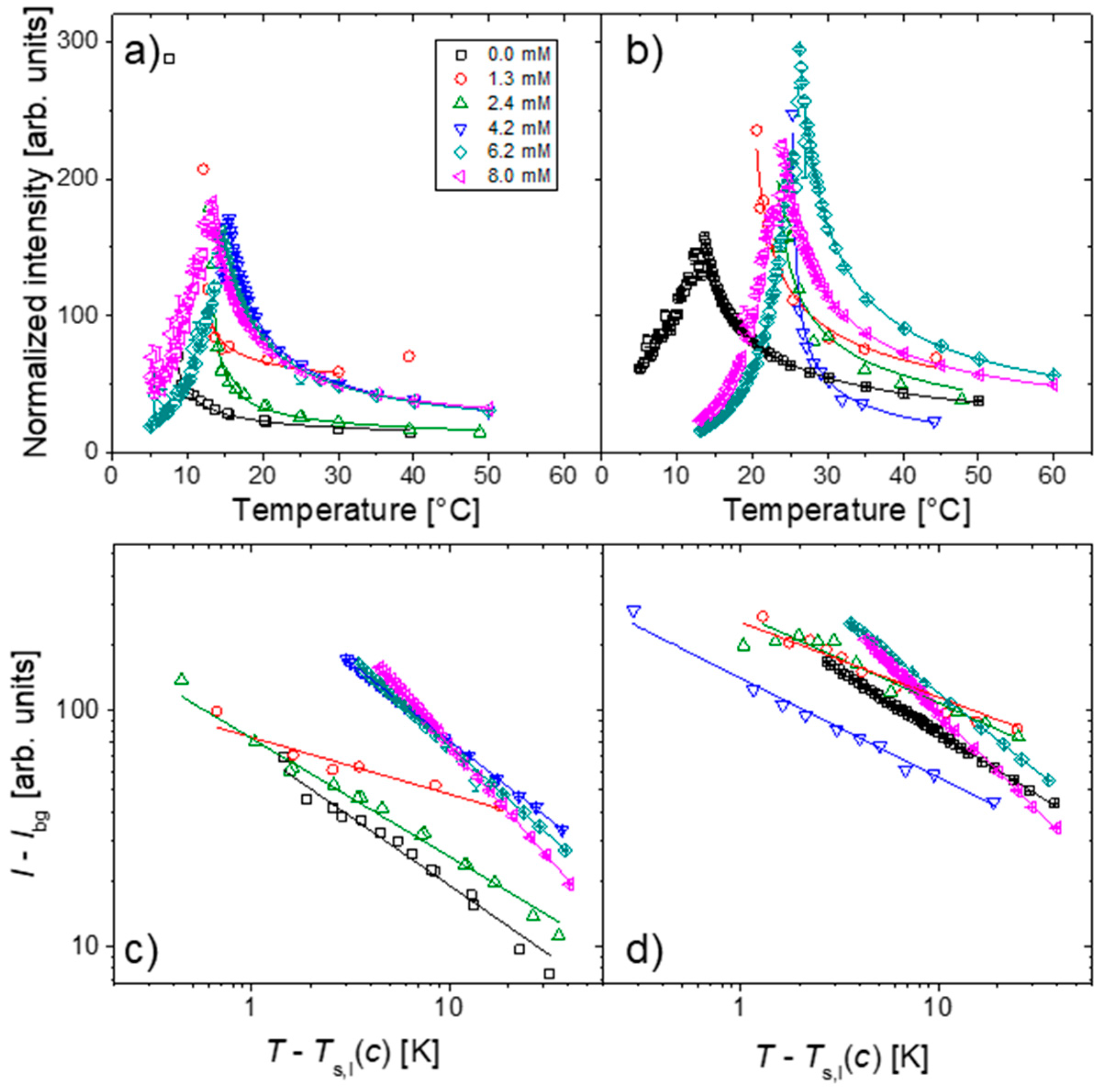
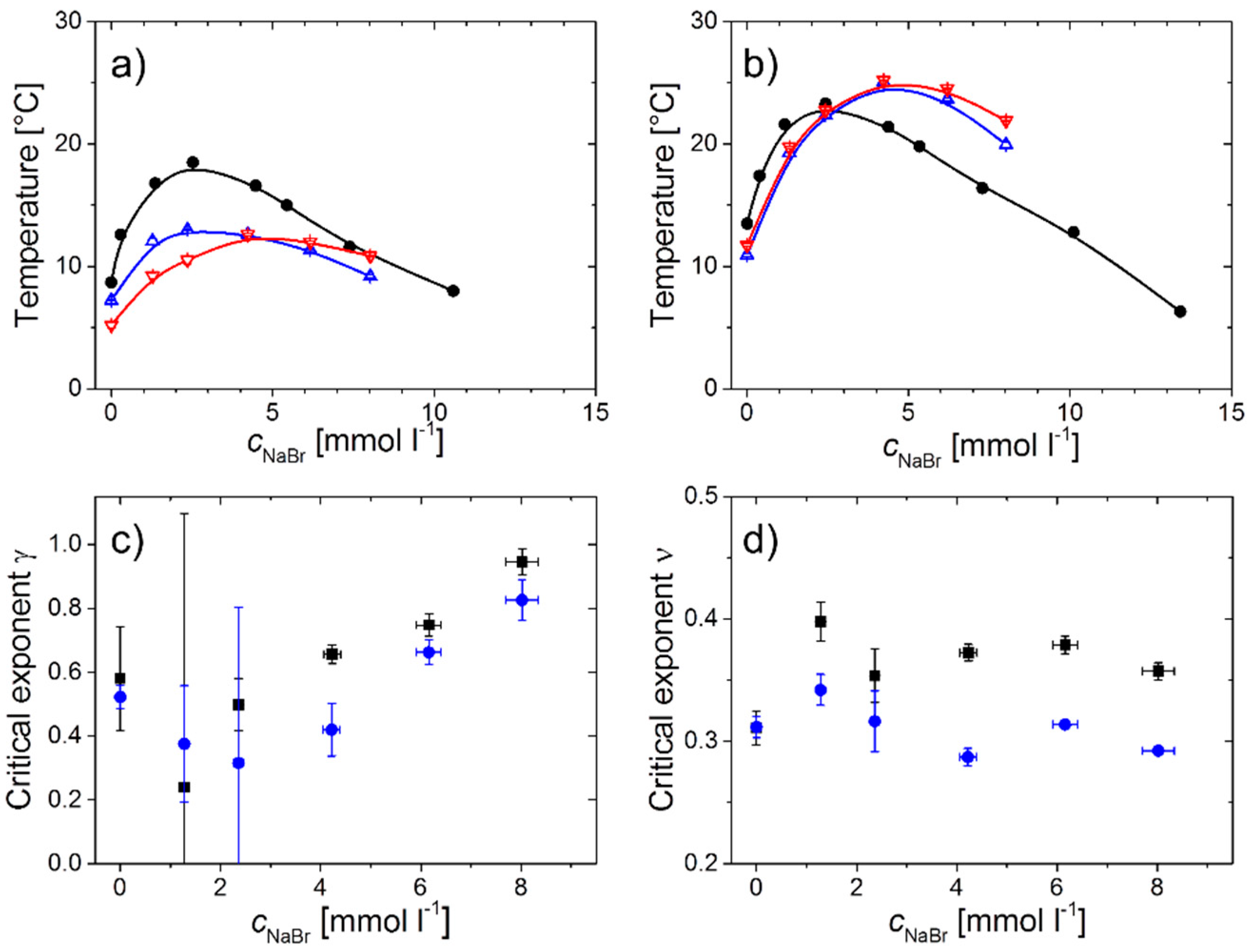
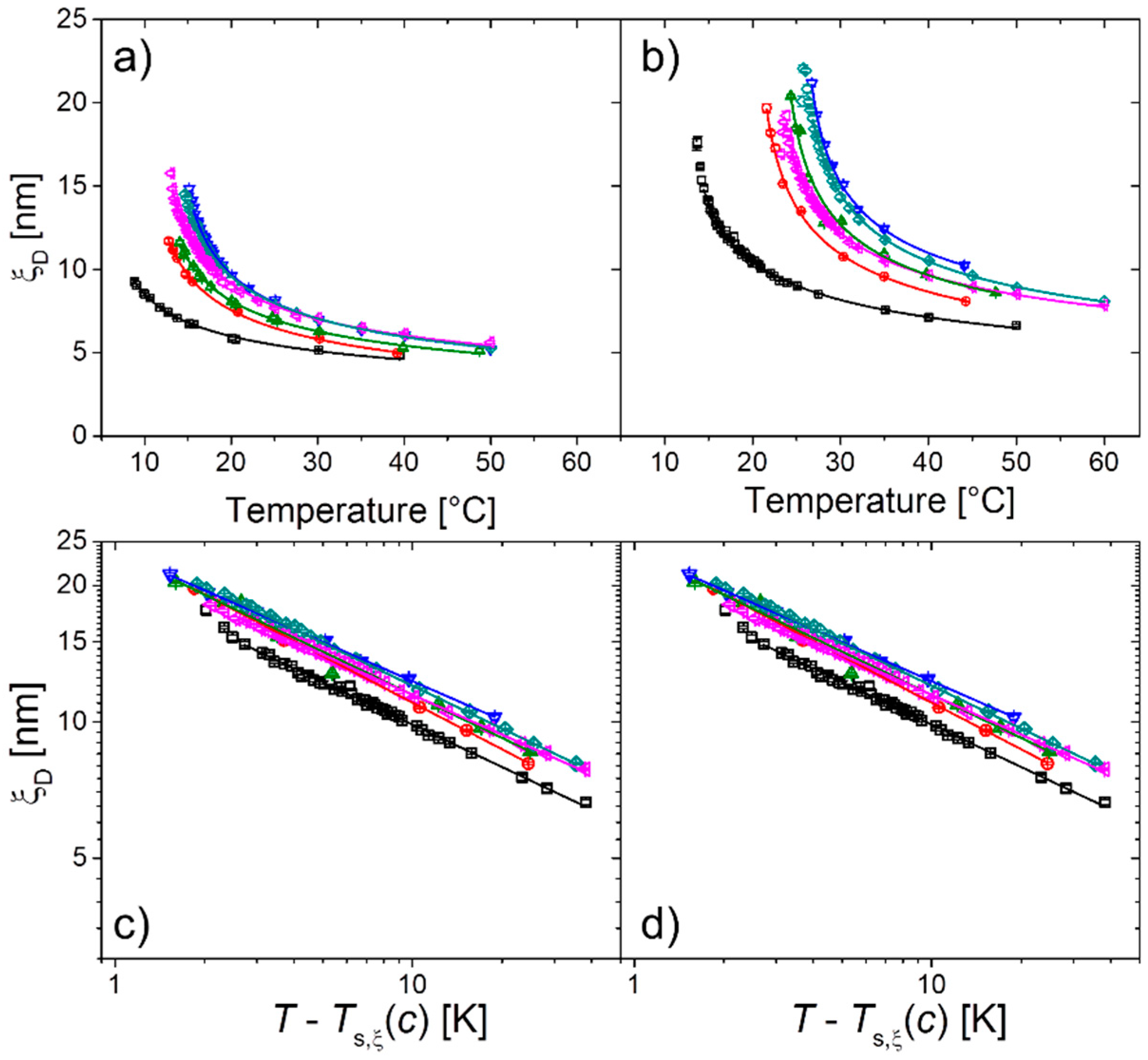
2.1. Polymer Solutions
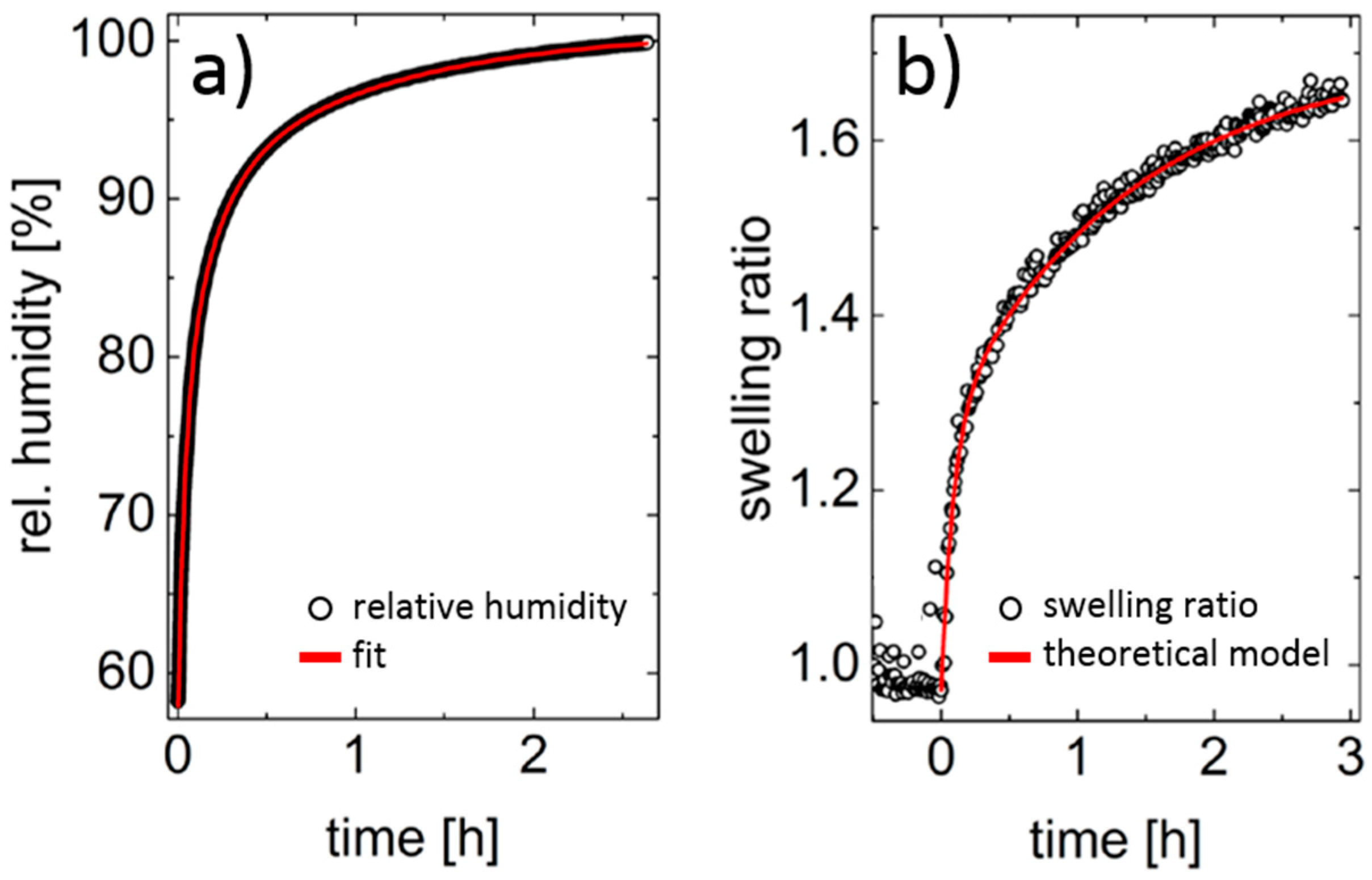
2.2. Thin Films
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoffman, A.S. Applications of thermally reversible polymers and hydrogels in therapeutics and diagnostics. J. Controlled Release 1987, 6, 297–305. [Google Scholar] [CrossRef]
- Gil, E.S.; Hudson, S. Stimuli-responsive polymers and their bioconjugates. Prog. Polym. Sci. 2004, 29, 1173–1222. [Google Scholar] [CrossRef]
- Roy, D.; Brooks, W.L.A.; Sumerlin, B.S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 47, 7214–7243. [Google Scholar] [CrossRef] [PubMed]
- Hocine, S.; Li, M.H. Thermoresponsive self-assembled polymer colloids in water. Soft Matter 2013, 9, 5839–5861. [Google Scholar] [CrossRef]
- Aseyev, V.; Tenhu, H.; Winnik, F.M. Non-ionic thermoresponsive polymers in water. Adv. Polym. Sci. 2011, 242, 29–89. [Google Scholar] [CrossRef]
- Halperin, A.; Kröger, M.; Winnik, F.M. Poly(N-isopropylacrylamide) phase diagrams: Fifty years of research. Angew. Chem. 2015, 54, 15342–15367. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Nakamura, T.; Ikeda, I. Changes in the hydration states of poly(N-alkylacrylamide)s during their phase transitions in water observed by FTIR spectroscopy. Macromolecules 2001, 34, 1391–1399. [Google Scholar] [CrossRef]
- Wu, C.; Wang, X. Globule-to-coil transition of a single homopolymer chain in solution. Phys. Rev. Lett. 1998, 80, 4092–4094. [Google Scholar] [CrossRef]
- Wang, W.; Troll, K.; Kaune, G.; Metwalli, E.; Ruderer, M.; Skrabania, K.; Laschewsky, A.; Roth, S.V.; Papadakis, C.M.; Müller-Buschbaum, P. Thin films of poly(N-isopropylacrylamide) end-capped with n-butyltrithiocarbonate. Macromolecules 2008, 41, 3209–3218. [Google Scholar] [CrossRef]
- Schulz, D.N.; Peiffer, D.G.; Agarwal, P.K.; Larabee, J.; Kaladas, J.J.; Soni, L.; Handwerker, B.; Garner, R.T. Phase behaviour and solution properties of sulphobetaine polymers. Polymer 1986, 27, 1734–1742. [Google Scholar] [CrossRef]
- Köberle, P.; Laschewsky, A.; Lomax, T.D. Interaction of a zwitterionic polysoap and its cationic analog with inorganic salts. Makromol. Chem. Rapid Commun. 1991, 12, 427–433. [Google Scholar] [CrossRef]
- Seuring, J.; Agarwal, S. Polymers with upper critical solution temperature in aqueous solution. Macromol. Rapid Commun. 2012, 33, 1898–1920. [Google Scholar] [CrossRef] [PubMed]
- Laschewsky, A. Structures and synthesis of zwitterionic polymers. Polymers 2014, 6, 1544–1601. [Google Scholar] [CrossRef]
- Ilčíková, M.; Tkáč, J.; Kasák, P. Switchable materials containing polyzwitterion moieties. Polymers 2015, 7, 2344–2370. [Google Scholar] [CrossRef]
- Grainger, D.W. All charged up about implanted biomaterials. Nature Biotechnol. 2013, 31, 507–509. [Google Scholar] [CrossRef] [PubMed]
- Mi, L.; Jiang, S. Integrated antimicrobial and nonfouling zwitterionic polymers. Angew. Chem. Int. Ed. 2014, 53, 1746–1754. [Google Scholar] [CrossRef] [PubMed]
- Mary, P.; Bendejacq, D.D.; Labeau, M.-P.; Dupuis, P. Reconciling low- and high-salt solution behavior of sulfobetaine polyzwitterions. J. Phys. Chem. B 2007, 111, 7767–7777. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, V.; Laschewsky, A.; Zehm, D. On the hydrophilicity of polyzwitterion poly (N,N-dimethyl-N-(3-(methacrylamido)propyl)ammoniopropane sulfonate) in water, deuterated water, and aqueous salt solutions. J. Biomater. Sci. Polym. Ed. 2014, 25, 1602–1618. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, V.; Laschewsky, A.; Wischerhoff, E. Modulating the solubility of zwitterionic poly((3-methacrylamidopropyl)ammonioalkane sulfonate)s in water and aqueous salt solutions via the spacer group separating the cationic and the anionic moieties. Polym. Chem. 2016, 7, 731–740. [Google Scholar] [CrossRef]
- Zhang, Y.; Furyk, S.; Bergbreiter, D.E.; Cremer, P.S. Specific ion effects on the water solubility of macromolecules: PNIPAM and the Hofmeister series. J. Am. Chem. Soc. 2005, 127, 14505–14510. [Google Scholar] [CrossRef] [PubMed]
- Lo Nostro, P.; Ninham, B.W. Hofmeister phenomena: An update on ion specificity in biology. Chem. Rev. 2012, 112, 2286–2322. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Paschew, G.; Klatt, S.; Lienig, J.; Arndt, K.-F.; Adler, H.-J.P. Review on hydrogel-based pH sensors and microsensors. Sensors 2008, 8, 561–581. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kaune, G.; Perlich, J.; Papadakis, C.M.; Bivigou Koumba, A.M.; Laschewsky, A.; Schlage, K.; Röhlsberger, R.; Roth, S.V.; Cubitt, R.; et al. Swelling and switching kinetics of gold coated end-capped poly(N-isopropylacrylamide) thin films. Macromolecules 2010, 43, 2444–2452. [Google Scholar] [CrossRef]
- Stuart, M.A.C.; Huck, W.T.S.; Genzer, J.; Müller, M.; Ober, C.; Stamm, M.; Sukhorukov, G.B.; Szleifer, I.; Tsukruk, V.V.; Urban, M.; et al. Emerging applications of stimuli-responsive polymer materials. Nat. Mater. 2010, 9, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Rotzetter, A.C.C.; Schumacher, C.M.; Bubenhofer, S.B.; Grass, R.N.; Gerber, L.C.; Zeltner, M.; Stark, W.J. Thermoresponsive polymer induced sweating surfaces as an efficient way to passively cool buildings. Adv. Mater. 2012, 24, 5352–5356. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tanaka, T. Kinetics of swelling and shrinking of gels. J. Chem. Phys. 1990, 92, 1365–1371. [Google Scholar] [CrossRef]
- Jaczewska, J.; Raptis, I.; Budkowski, A.; Goustouridis, D.; Raczkowska, J.; Sanopoulou, M.; Pamuła, E.; Bernasik, A.; Rysz, J. Swelling of poly(3-alkylthiophene) films exposed to solvent vapors and humidity: Evaluation of solubility parameters. Synth. Met. 2007, 157, 726–732. [Google Scholar] [CrossRef]
- Magerl, D.; Philipp, M.; Qiu, X.-P.; Winnik, F.M.; Müller-Buschbaum, P. Swelling and thermoresponsive behavior of linear versus cyclic poly(N-isopropylacrylamide) thin films. Macromolecules 2015, 48, 3104–3111. [Google Scholar] [CrossRef]
- Brown, W.; Nicolai, T. Dynamic properties of polymer solutions. In Dynamic Light Scattering. The Method and Some Applications; Brown, W., Ed.; Clarendon Press: Oxford, Great Britain, 1993; Chapter 6; pp. 272–318. ISBN 0-19-853942-8. [Google Scholar]
- Brown, W.; Nicolai, T. Static and dynamic behavior of semidilute polymer solutions. Colloid Polym. Sci. 1990, 268, 977–990. [Google Scholar] [CrossRef]
- Shibayama, M.; Isono, K.; Okabe, S.; Karino, T.; Nagao, M. SANS study on pressure-induced phase separation of poly(N-isopropylacrylamide) aqueous solutions and gels. Macromolecules 2004, 37, 2909–2918. [Google Scholar] [CrossRef]
- Hildebrand, V. Twofold Switchable Block Copolymers Based on New Polyzwitterions. Ph.D. Thesis, Universität Potsdam, Potsdam, Germany, 2016. [Google Scholar]
- Philipp, M.; Körstgens, V.; Magerl, D.; Heller, C.; Yao, Y.; Wang, W.; Santoro, G.; Roth, S.V.; Müller-Buschbaum, P. Sorption of water and initial stages of swelling of thin PNIPAM films. Langmuir 2015, 31, 9619–9627. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Rizzardo, E.; Thang, S.H. Toward living radical polymerization. Acc. Chem. Res. 2008, 41, 1133–1142. [Google Scholar] [CrossRef] [PubMed]
- Moad, G.; Rizzardo, E.; Thang, S.H. End-functional polymers, thiocarbonylthio group removal/transformation and reversible addition–fragmentation–chain transfer (RAFT) polymerization. Polym. Int. 2011, 60, 9–25. [Google Scholar] [CrossRef]
- Semsarilar, M.; Ladmiral, V.; Blanazs, A.; Armes, S.P. Anionice polyelectrolyte-stabilized nanoparticles via RAFT aqueous dispersion polymerization. Langmuir 2011, 28, 914–922. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, V.; Heydenreich, M.; Laschewsky, A.; Möller, H.M.; Müller-Buschbaum, P.; Papadakis, C.M.; Schanzenbach, D.; Wischerhoff, E. “Schizophrenic” self-assembly of dual thermoresponsive block copolymers bearing a zwitterionic and a non-ionic hydrophilic block. Polymer 2017, 122, 347–357. [Google Scholar] [CrossRef]
- Jakeš, J. Regularized positive exponential sum (REPES) program—A way of investigating Laplace transform data obtained by dynamic light scattering. Collect. Czech. Chem. Commun. 1995, 60, 1781–1797. [Google Scholar] [CrossRef]
| d0 [nm] | χeff | τswell [s] |
|---|---|---|
| 30 nm | 0.89 ± 0.01 | 242 ± 11 |
| 99 nm | 0.74 ± 0.01 | 37 ± 5 |
| 104 nm | 0.78 ± 0.01 | 76 ± 2 |
| 117 nm | 0.82 ± 0.01 | 81 ± 5 |
| Sample | Mn (kg mol−1) a | DPna | CP (°C) b |
|---|---|---|---|
| PSPP80 c | 24 | 80 | 12 |
| PSPP85 d | 26 | 85 | 8.7 |
| PSPP280 d | 82 | 280 | 13.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niebuur, B.-J.; Puchmayr, J.; Herold, C.; Kreuzer, L.P.; Hildebrand, V.; Müller-Buschbaum, P.; Laschewsky, A.; Papadakis, C.M. Polysulfobetaines in Aqueous Solution and in Thin Film Geometry. Materials 2018, 11, 850. https://doi.org/10.3390/ma11050850
Niebuur B-J, Puchmayr J, Herold C, Kreuzer LP, Hildebrand V, Müller-Buschbaum P, Laschewsky A, Papadakis CM. Polysulfobetaines in Aqueous Solution and in Thin Film Geometry. Materials. 2018; 11(5):850. https://doi.org/10.3390/ma11050850
Chicago/Turabian StyleNiebuur, Bart-Jan, Jonas Puchmayr, Christian Herold, Lucas P. Kreuzer, Viet Hildebrand, Peter Müller-Buschbaum, André Laschewsky, and Christine M. Papadakis. 2018. "Polysulfobetaines in Aqueous Solution and in Thin Film Geometry" Materials 11, no. 5: 850. https://doi.org/10.3390/ma11050850
APA StyleNiebuur, B.-J., Puchmayr, J., Herold, C., Kreuzer, L. P., Hildebrand, V., Müller-Buschbaum, P., Laschewsky, A., & Papadakis, C. M. (2018). Polysulfobetaines in Aqueous Solution and in Thin Film Geometry. Materials, 11(5), 850. https://doi.org/10.3390/ma11050850






