Enhanced Enzymatic Hydrolysis of Rice Straw Pretreated by Oxidants Assisted with Photocatalysis Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemical Reagents
2.2. Photocatalysis Pretreatment
2.3. Enzymatic Hydrolysis of Rice Straw
2.4. Addition of Oxidants in a TiO2/UV System
2.5. Characterization Analyses
3. Results
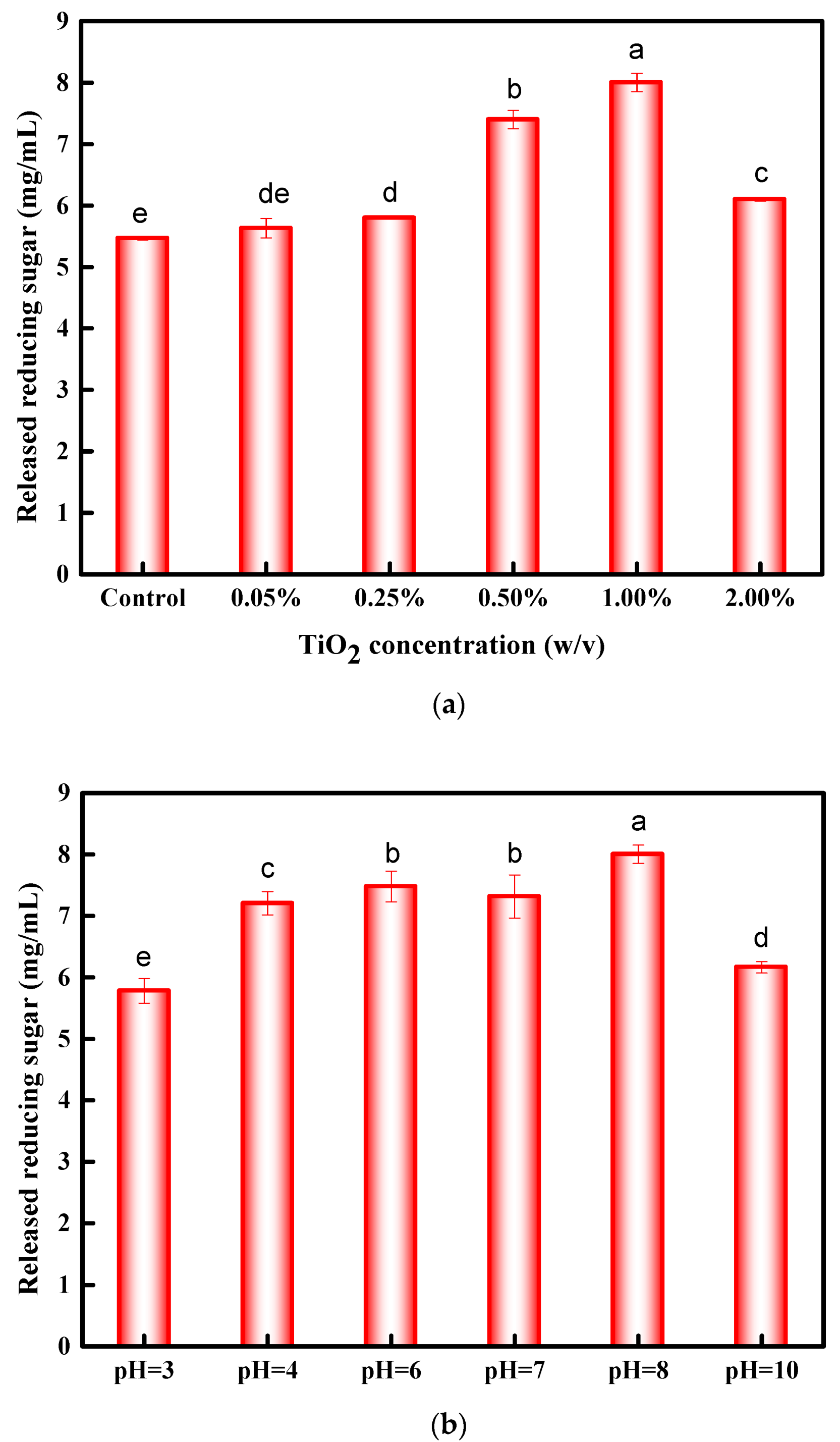
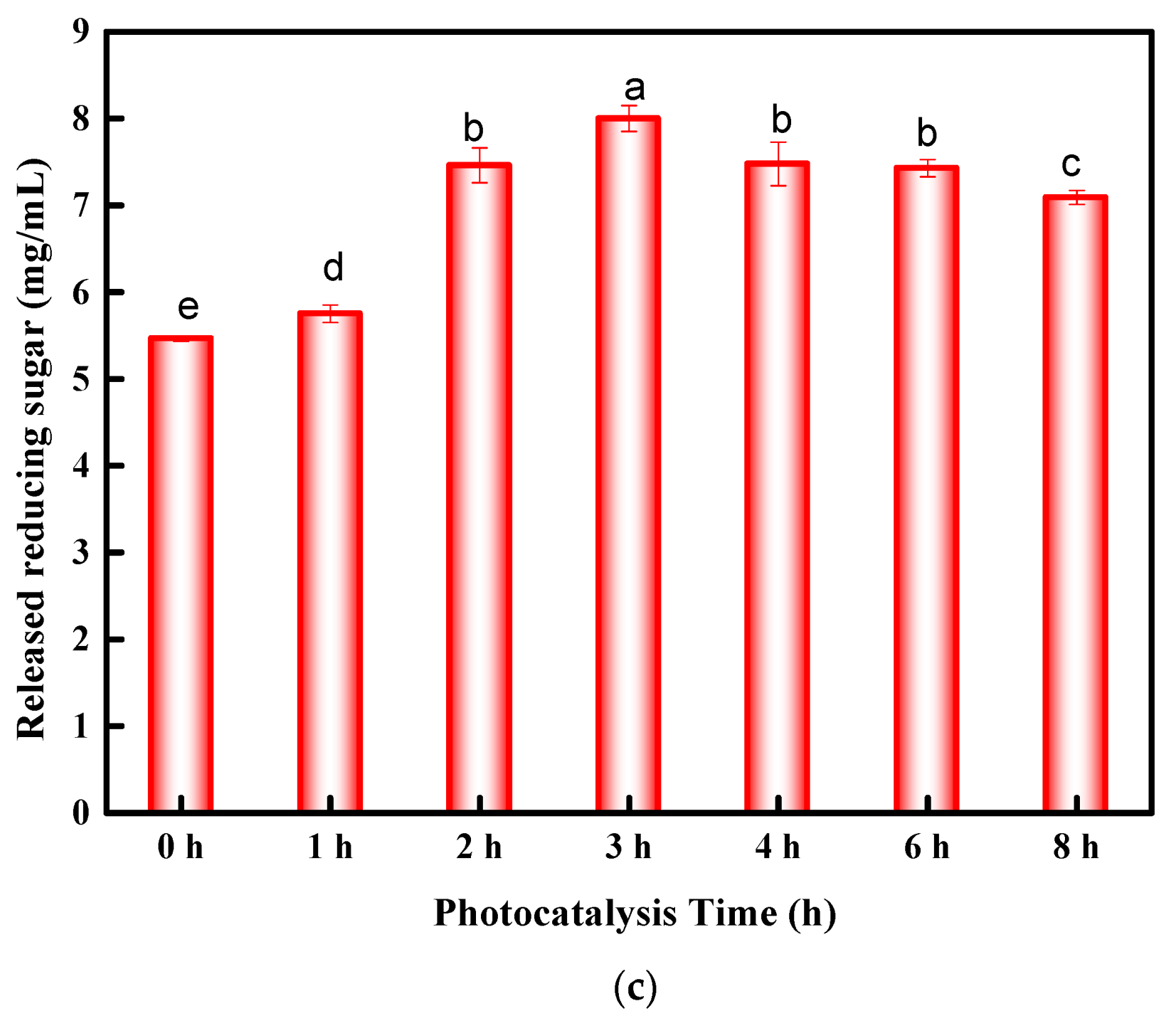
3.1. Optimization of Conditions for Photocatalysis Pretreatment
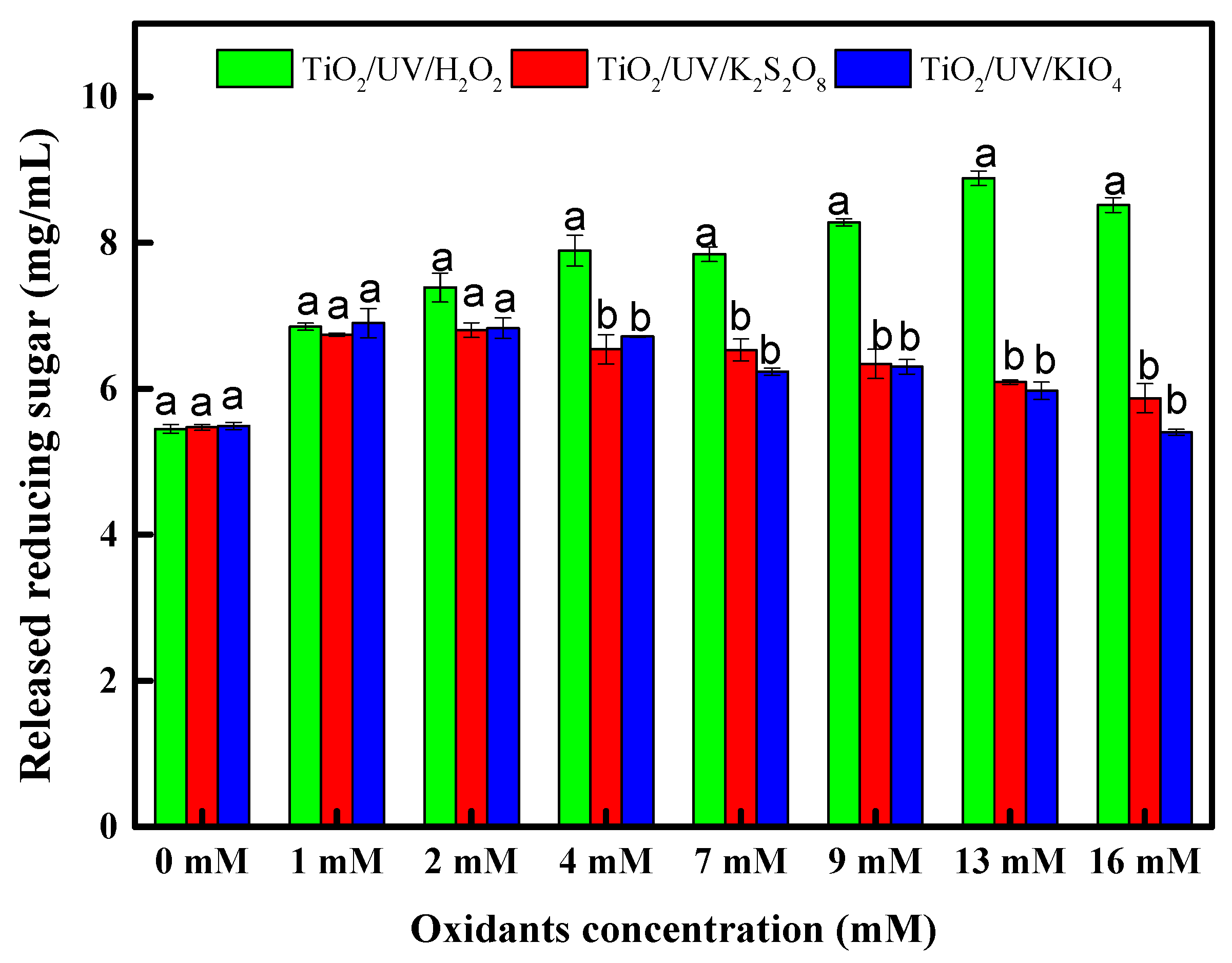
3.2. Effect of Added Oxidants in TiO2/UV Pretreatment on Enzymatic Hydrolysis of Rice Straw
3.3. Composition Analysis of Untreated and Pretreated Rice Straw
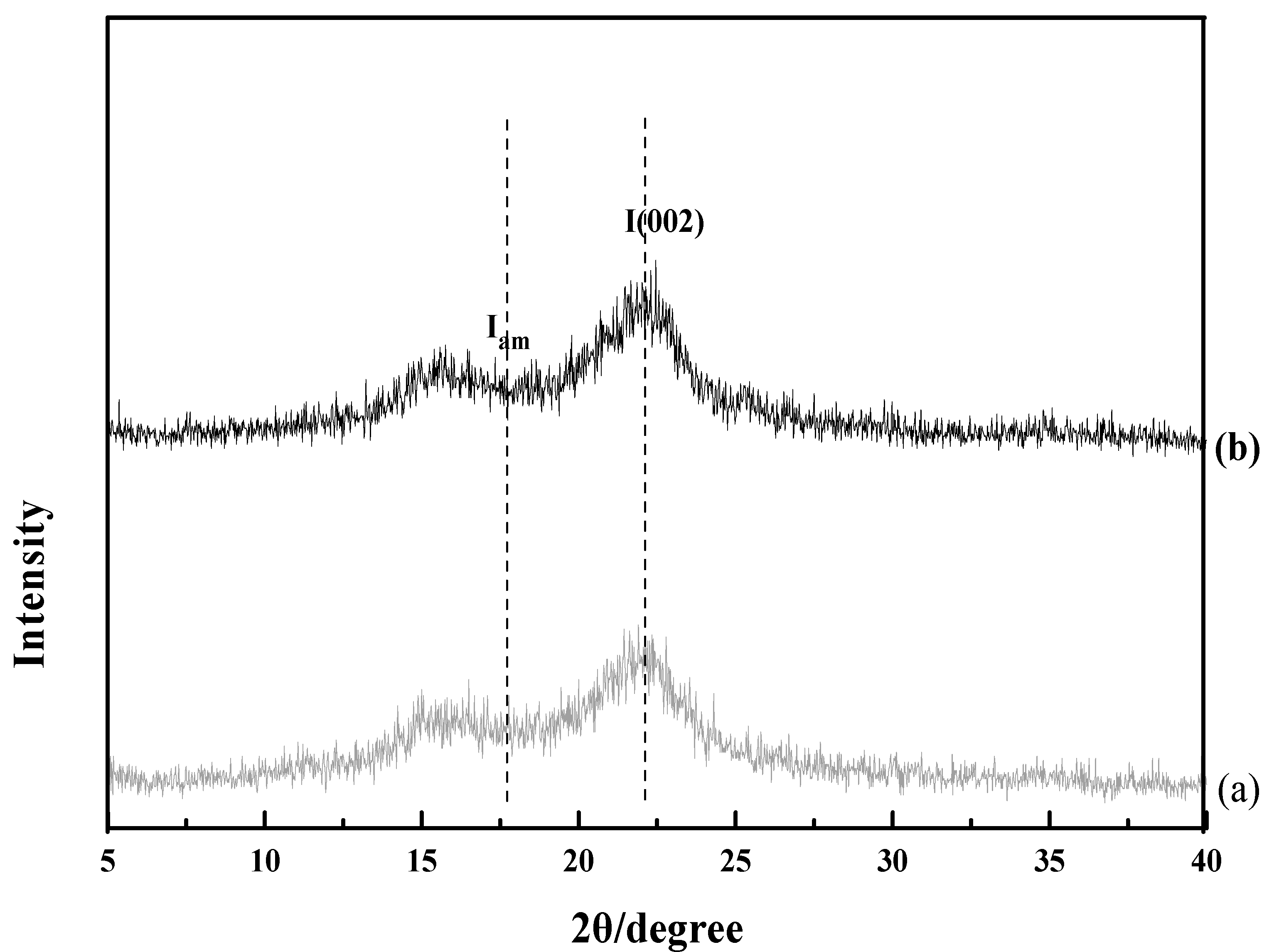
3.4. FE-SEM, XRD and FTIR Profile of Untreated and Pretreated Rice Straw
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, S.H.; Liu, S.; Colmenares, J.C.; Xu, Y.J. A sustainable approach for lignin valorization by heterogeneous photocatalysis. Green Chem. 2016, 18, 594–607. [Google Scholar] [CrossRef]
- Raghavi, S.; Sindhu, R.; Binod, P.; Gnansounou, E.; Pandey, A. Development of a novel sequential pretreatment strategy for the production of bioethanol from sugarcane trash. Bioresour. Technol. 2016, 199, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Jeongheo, K.; Kang, H.S.; Sang, B.I.; Yunje, K.; Min, J.; Mitchell, R.J.; Jinhyung, L. Feasibility of a facile butanol bioproduction using planetary mill pretreatment. Bioresour. Technol. 2016, 199, 283–287. [Google Scholar]
- Himmel, M.E.; Ding, S.Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass recalcitrance: Engineering plants and enzymes for biofuels production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Ren, J.; Liu, L.; Zheng, Z.; Zhu, J.; Yong, Q.; Ouyang, J. A new magnesium bisulfite pretreatment (mbsp) development for bio-ethanol production from corn stover. Bioresour. Technol. 2016, 199, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Wen, J.L.; Yuan, T.Q.; Sun, S.L.; Xu, F.; Sun, R.C. Understanding the chemical transformations of lignin during ionic liquid pretreatment. Green Chem. 2013, 16, 181–190. [Google Scholar]
- Mood, S.H.; Golfeshan, A.H.; Tabatabaei, M.; Jouzani, G.S.; Najafi, G.H.; Gholami, M.; Ardjmand, M. Lignocellulosic biomass to bioethanol, a comprehensive review with a focus on pretreatment. Biomass Bull. 2014, 27, 77–93. [Google Scholar]
- Li, S.; Questellsantiago, Y.M.; Luterbacher, J.S. A mild biomass pretreatment using γ-valerolactone for concentrated sugar production. Green Chem. 2016, 18, 937–943. [Google Scholar]
- Chang, K.L.; Chen, X.M.; Han, Y.J.; Wang, X.Q.; Potprommanee, L.; Ning, X.A.; Liu, J.Y.; Sun, J.; Peng, Y.P.; Sun, S.Y. Synergistic effects of surfactant-assisted ionic liquid pretreatment rice straw. Bioresour. Technol. 2016, 214, 371–375. [Google Scholar] [PubMed]
- Alvaradomorales, M.; Tsapekos, P.; Awais, M.; Gulfraz, M.; Angelidaki, I. TiO2/UV based photocatalytic pretreatment of wheat straw for biogas production. Anaerobe 2016, 46, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Kamwilaisak, K.; Wright, P.C. Investigating laccase and titanium dioxide for lignin degradation. Energy Fuels 2012, 26, 2400–2406. [Google Scholar] [CrossRef]
- Ma, Y.S.; Chang, C.N.; Chiang, Y.P.; Sung, H.F.; Chao, A.C. Photocatalytic degradation of lignin using pt/TiO2 as the catalyst. Chemosphere 2008, 71, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Lanzalunga, O.; Bietti, M. Photo- and radiation chemical induced degradation of lignin model compounds. J. Photochem. Photobiol. B 2000, 56, 85–108. [Google Scholar] [CrossRef]
- Núñez, O.; Rivas, C.; Vargas, R. Minimizing electron-hole recombination in modified tio2 photocatalysis: Electron transfer to solution as rate-limiting step in organic compounds degradation. J. Phys. Org. Chem. 2017, 30, e3659. [Google Scholar] [CrossRef]
- Cortés, J.A.; Alarcón-Herrera, M.T.; Villicaña-Méndez, M.; González-Hernández, J.; Pérez-Robles, J.F. Impact of the kind of ultraviolet light on the photocatalytic degradation kinetics of the TiO2/UV process. Environ. Progress Sustain. Energy 2011, 30, 318–325. [Google Scholar] [CrossRef]
- Hussain, M.; Russo, N.; Saracco, G. Photocatalytic abatement of vocs by novel optimized TiO2 nanoparticles. Chem. Eng. J. 2011, 166, 138–149. [Google Scholar] [CrossRef]
- Badawy, M.I.; Elgohary, F.; Ghaly, M.Y.; Ali, M.E.M. Enhancement of olive mill wastewater biodegradation by homogeneous and heterogeneous photocatalytic oxidation. J. Hazard. Mater. 2009, 169, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.H.; Wu, C.H.; Ho, T.H.; Hong, P.K.A. Decolorization of C.I. Reactive black 5 in UV/TiO2, UV/oxidant and UV/TiO2/oxidant systems: A comparative study. Chem. Eng. J. 2010, 158, 578–583. [Google Scholar] [CrossRef]
- Gözmen, B.; Turabi̇K, M.; Hesenov, A. Photocatalytic degradation of basic red 46 and basic yellow 28 in single and binary mixture by UV/TiO2/periodate system. J. Hazard. Mater. 2009, 164, 1487–1495. [Google Scholar] [CrossRef] [PubMed]
- Seyed-Dorraji, M.S.; Daneshvar, N.; Aber, S. Influence of inorganic oxidants and metal ions on photocatalytic activity of prepared zinc oxide nanocrystals. Glob. Nest J. 2009, 11, 535–545. [Google Scholar]
- Lan, W.; Liu, C.F.; Yue, F.X.; Sun, R.C.; Kennedy, J.F. Ultrasound-assisted dissolution of cellulose in ionic liquid. Carbohydr. Polym. 2011, 86, 672–677. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Biochem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Chang, K.L.; Chen, X.M.; Wang, X.Q.; Han, Y.J.; Potprommanee, L.; Liu, J.Y.; Liao, Y.L.; Ning, X.A.; Sun, S.Y.; Huang, Q. Impact of surfactant type for ionic liquid pretreatment on enhancing delignification of rice straw. Bioresour. Technol. 2016, 227, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Segal, L.; Creely, J.J.; Martin, E.M., Jr.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Gupta, V.K.; Jain, R.; Mittal, A.; Saleh, T.A.; Nayak, A.; Agarwal, S.; Sikarwar, S. Photo-catalytic degradation of toxic dye amaranth on TiO2/UV in aqueous suspensions. Mater. Sci. Eng. C 2012, 32, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Muruganandham, M.; Swaminathan, M. TiO2-UV photocatalytic oxidation of reactive yellow 14: Effect of operational parameters. J. Hazard. Mater. 2006, 135, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Selvam, K.; Muruganandham, M.; Muthuvel, I.; Swaminathan, M. The influence of inorganic oxidants and metal ions on semiconductor sensitized photodegradation of 4-fluorophenol. Chem. Eng. J. 2007, 128, 51–57. [Google Scholar] [CrossRef]
- Nie, X.N.; Liu, J.; She, D.; Sun, R.C.; Xu, F. Physicochemical and structural characterization of hemicelluloses isolated by different alcohols from rice straw. Bioresources 2013, 8, 3817–3832. [Google Scholar] [CrossRef]
- Binod, P.; Sindhu, R.; Singhania, R.R.; Vikram, S.; Devi, L.; Nagalakshmi, S.; Kurien, N.; Sukumaran, R.K.; Pandey, A.; Pandey, A. Bioethanol production from rice straw: An overview. Bioresour. Technol. 2010, 101, 4767–4774. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.; Wang, W.; Gu, F.; Cao, T.; Jin, Y. Comparison of the substrate enzymatic digestibility and lignin structure of wheat straw stems and leaves pretreated by green liquor. Bioresour. Technol. 2016, 199, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Öhgren, K.; Bura, R.; Saddler, J.; Zacchi, G. Effect of hemicellulose and lignin removal on enzymatic hydrolysis of steam pretreated corn stover. Bioresour. Technol. 2007, 98, 2503–2510. [Google Scholar] [CrossRef] [PubMed]
- Wi, S.G.; Cho, E.J.; Lee, D.S.; Lee, S.J.; Lee, Y.J.; Bae, H.J. Lignocellulose conversion for biofuel: A new pretreatment greatly improves downstream biocatalytic hydrolysis of various lignocellulosic materials. Biotechnol. Biofuels 2015, 8, 228. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.L.; Han, Y.J.; Wang, X.Q.; Chen, X.M.; Leu, S.Y.; Liu, J.Y.; Peng, Y.P.; Liao, Y.L.; Potprommanee, L. The effect of surfactant-assisted ultrasound-ionic liquid pretreatment on the structure and fermentable sugar production of a water hyacinth. Bioresour. Technol. 2017, 237, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Hu, J.; Shen, F.; Mei, Z.; Yang, G.; Zhang, Y.; Hu, Y.; Zhang, J.; Deng, S. Pretreating wheat straw by the concentrated phosphoric acid plus hydrogen peroxide (PHP): Investigations on pretreatment conditions and structure changes. Bioresour. Technol. 2015, 199, 245–257. [Google Scholar] [CrossRef] [PubMed]


| Pretreatment | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|
| Untreated | 37.47 ± 0.57 | 32.98 ± 1.10 | 18.68 ± 0.30 |
| H2O2 | 39.68 ± 0.64 | 32.49 ± 0.62 | 17.04 ± 0.74 |
| H2O2/UV | 40.59 ± 1.27 | 32.16 ± 1.14 | 17.45 ± 0.99 |
| TiO2/UV/H2O2 | 42.00 ± 0.70 | 30.14 ± 1.12 | 16.10 ± 0.67 |
| K2S2O8 | 40.21 ± 0.15 | 31.04 ± 0.81 | 18.12 ± 0.74 |
| UV/K2S2O8 | 40.58 ± 0.39 | 31.05 ± 1.40 | 18.70 ± 0.10 |
| TiO2/UV/K2S2O8 | 40.77 ± 0.66 | 31.46 ± 0.28 | 18.41 ± 0.33 |
| KIO4 | 40.21 ± 0.81 | 31.90 ± 0.60 | 17.75 ± 0.23 |
| UV/KIO4 | 41.81 ± 0.10 | 31.76 ± 0.25 | 18.05 ± 0.59 |
| TiO2/UV/KIO4 | 39.72 ± 0.69 | 32.63 ± 0.31 | 17.69 ± 0.54 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, K.-L.; Wang, X.-Q.; Han, Y.-J.; Deng, H.; Liu, J.-y.; Lin, Y.-C. Enhanced Enzymatic Hydrolysis of Rice Straw Pretreated by Oxidants Assisted with Photocatalysis Technology. Materials 2018, 11, 802. https://doi.org/10.3390/ma11050802
Chang K-L, Wang X-Q, Han Y-J, Deng H, Liu J-y, Lin Y-C. Enhanced Enzymatic Hydrolysis of Rice Straw Pretreated by Oxidants Assisted with Photocatalysis Technology. Materials. 2018; 11(5):802. https://doi.org/10.3390/ma11050802
Chicago/Turabian StyleChang, Ken-Lin, Xiao-Qin Wang, Ye-Ju Han, Hao Deng, Jing-yong Liu, and Yuan-Chung Lin. 2018. "Enhanced Enzymatic Hydrolysis of Rice Straw Pretreated by Oxidants Assisted with Photocatalysis Technology" Materials 11, no. 5: 802. https://doi.org/10.3390/ma11050802
APA StyleChang, K.-L., Wang, X.-Q., Han, Y.-J., Deng, H., Liu, J.-y., & Lin, Y.-C. (2018). Enhanced Enzymatic Hydrolysis of Rice Straw Pretreated by Oxidants Assisted with Photocatalysis Technology. Materials, 11(5), 802. https://doi.org/10.3390/ma11050802







