Highly-Stable Li4Ti5O12 Anodes Obtained by Atomic-Layer-Deposited Al2O3
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the LTO Powder and Al2O3-Coated LTO Electrode
2.2. Physical Characterization
2.3. Electrochemical Tests
3. Results and Discussion
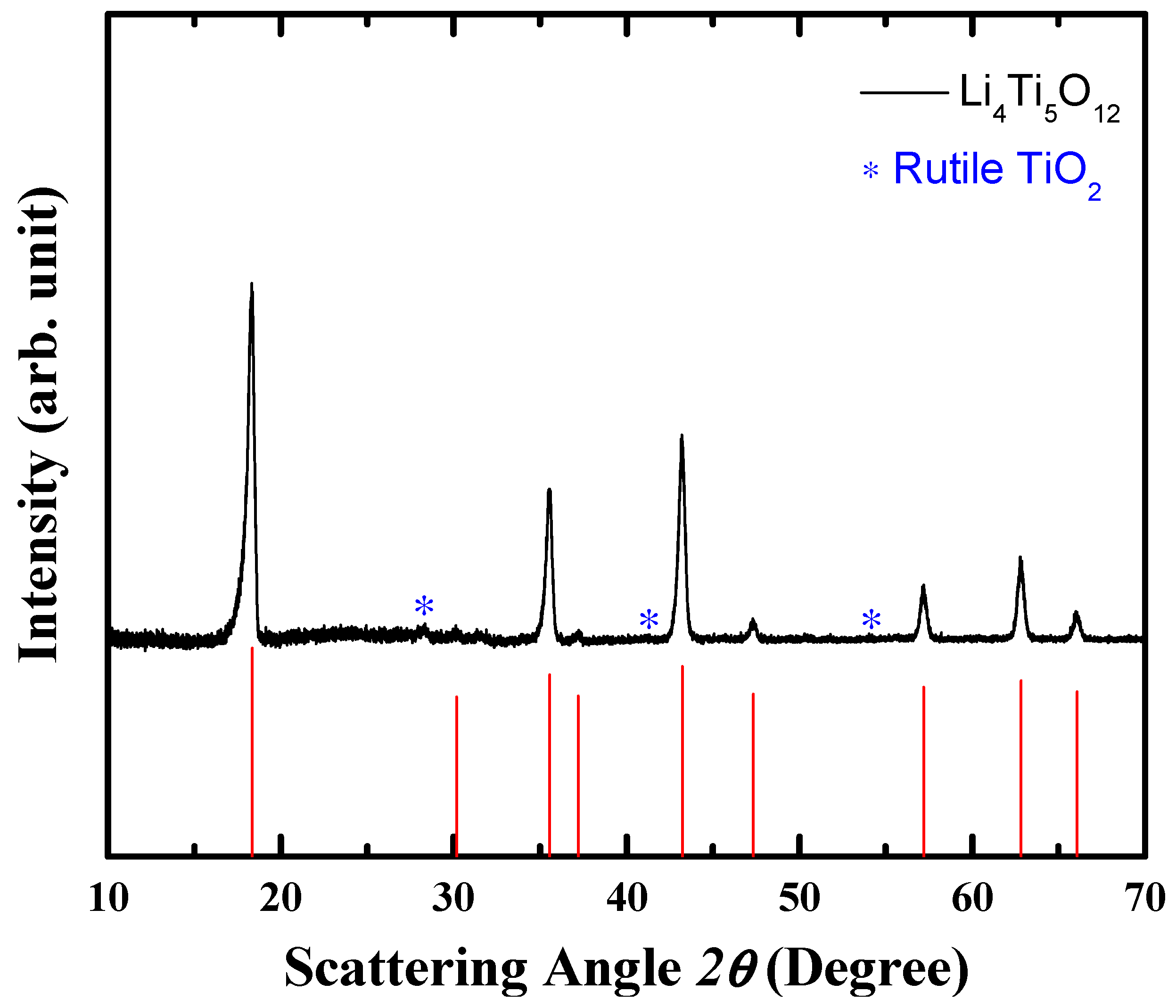
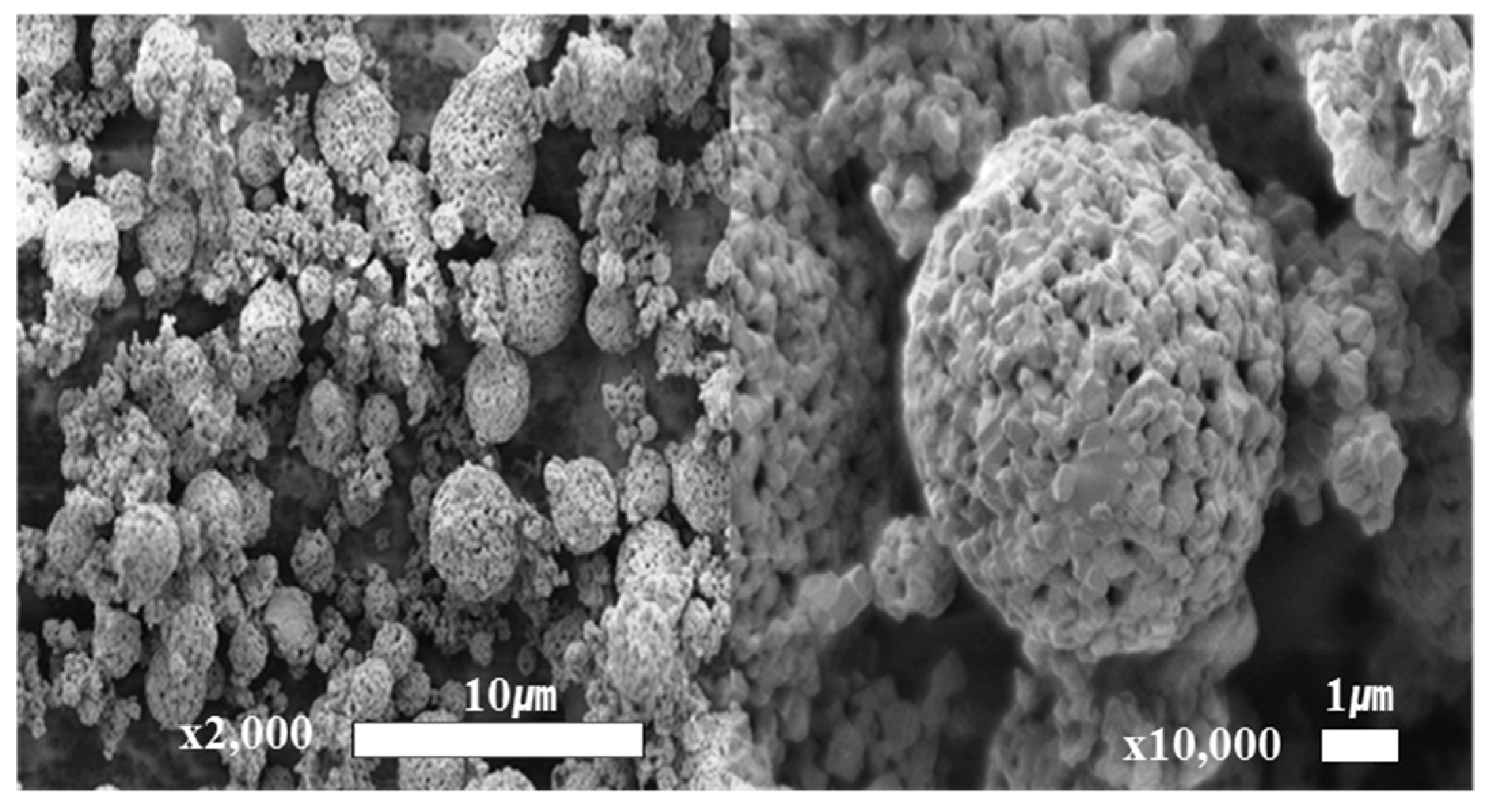
3.1. Synthesis of LTO
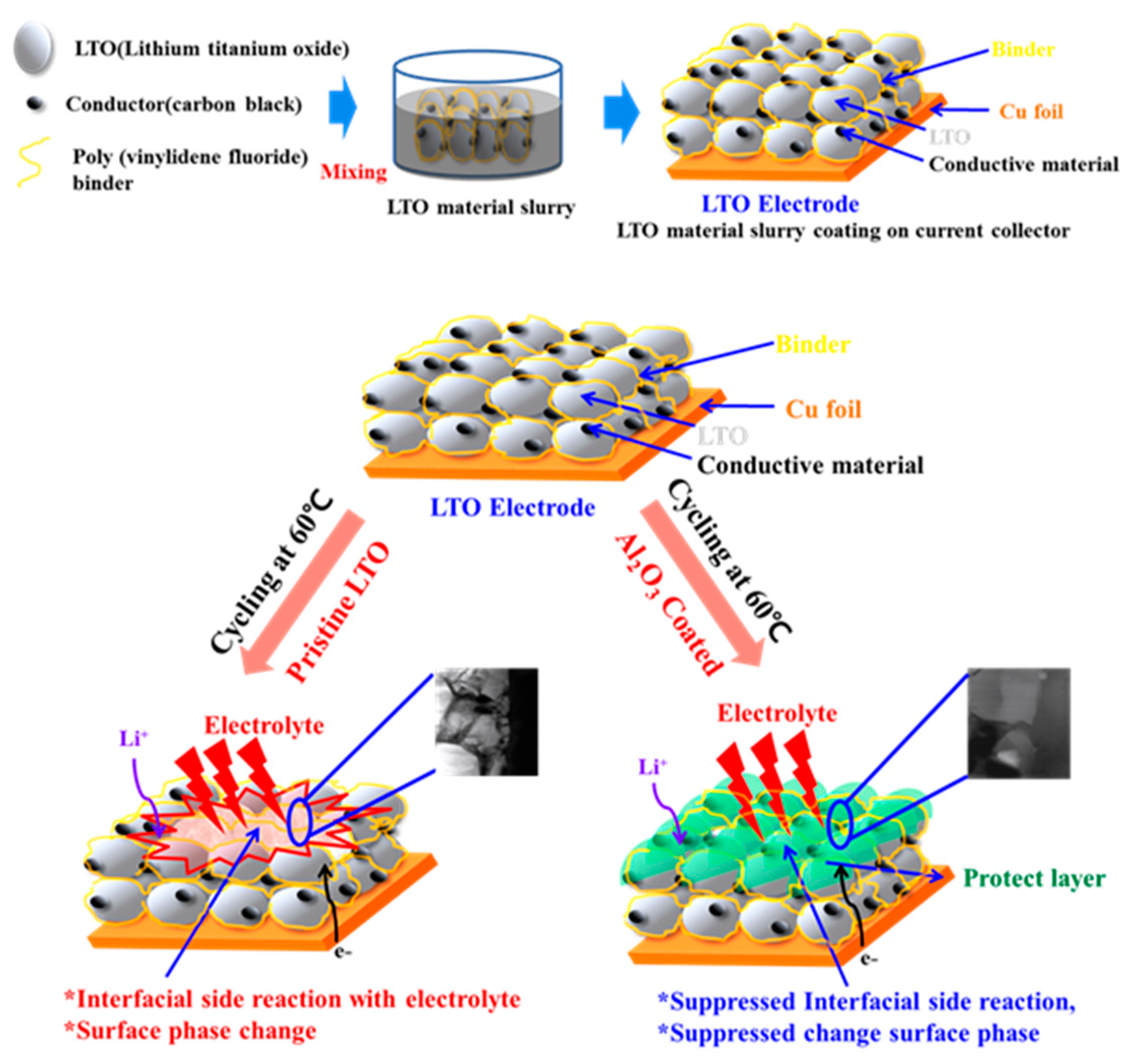
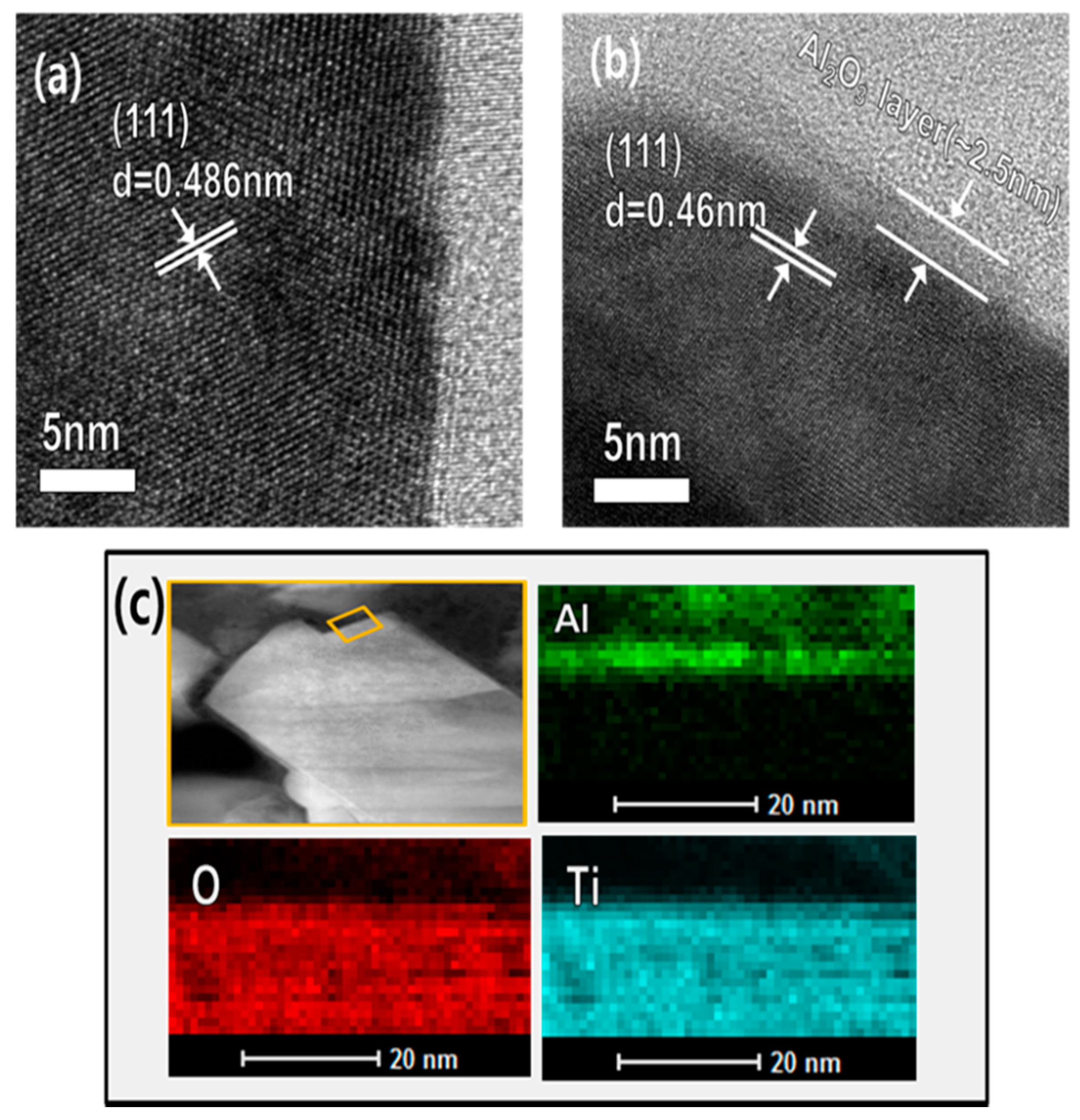
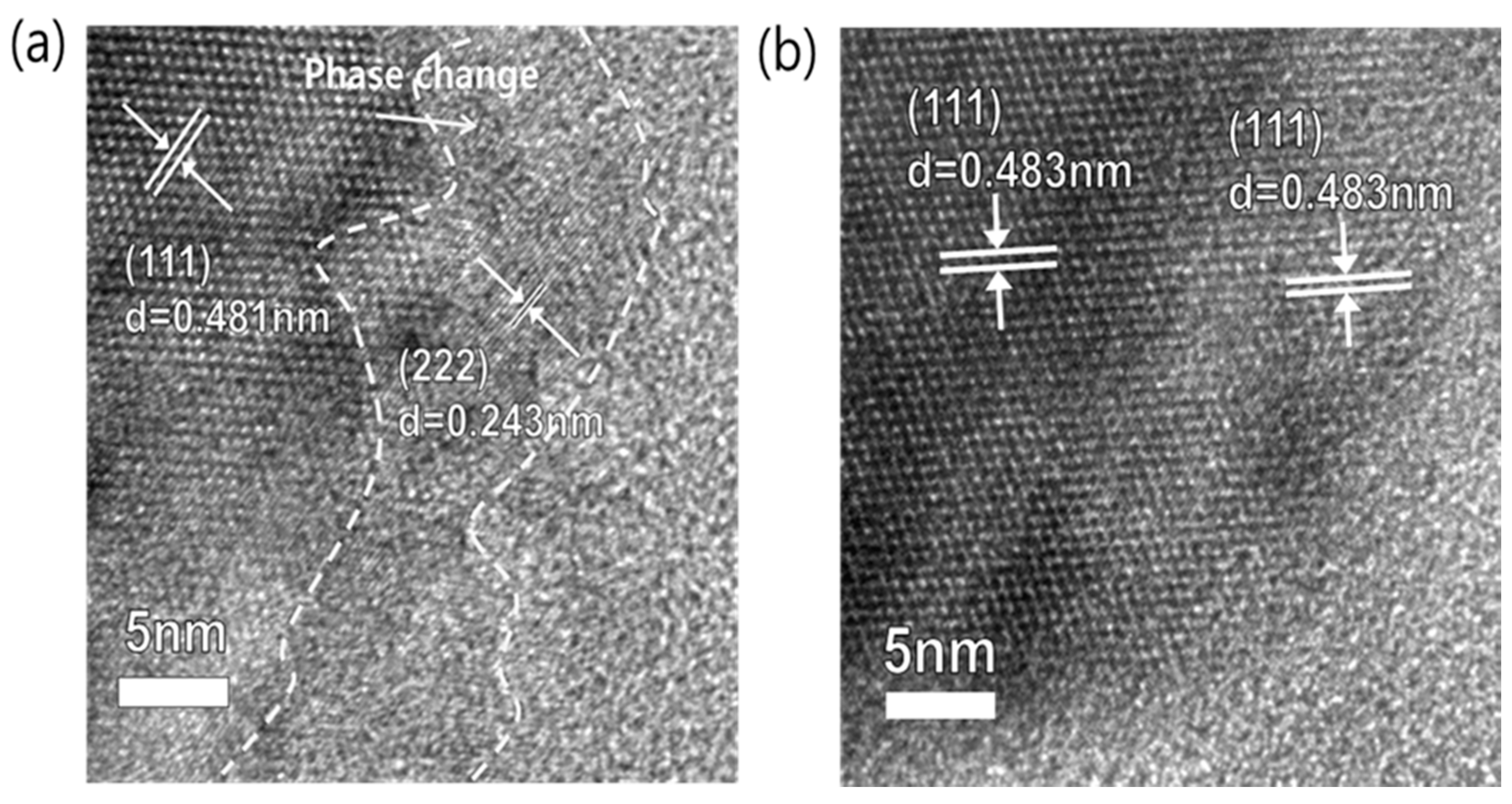
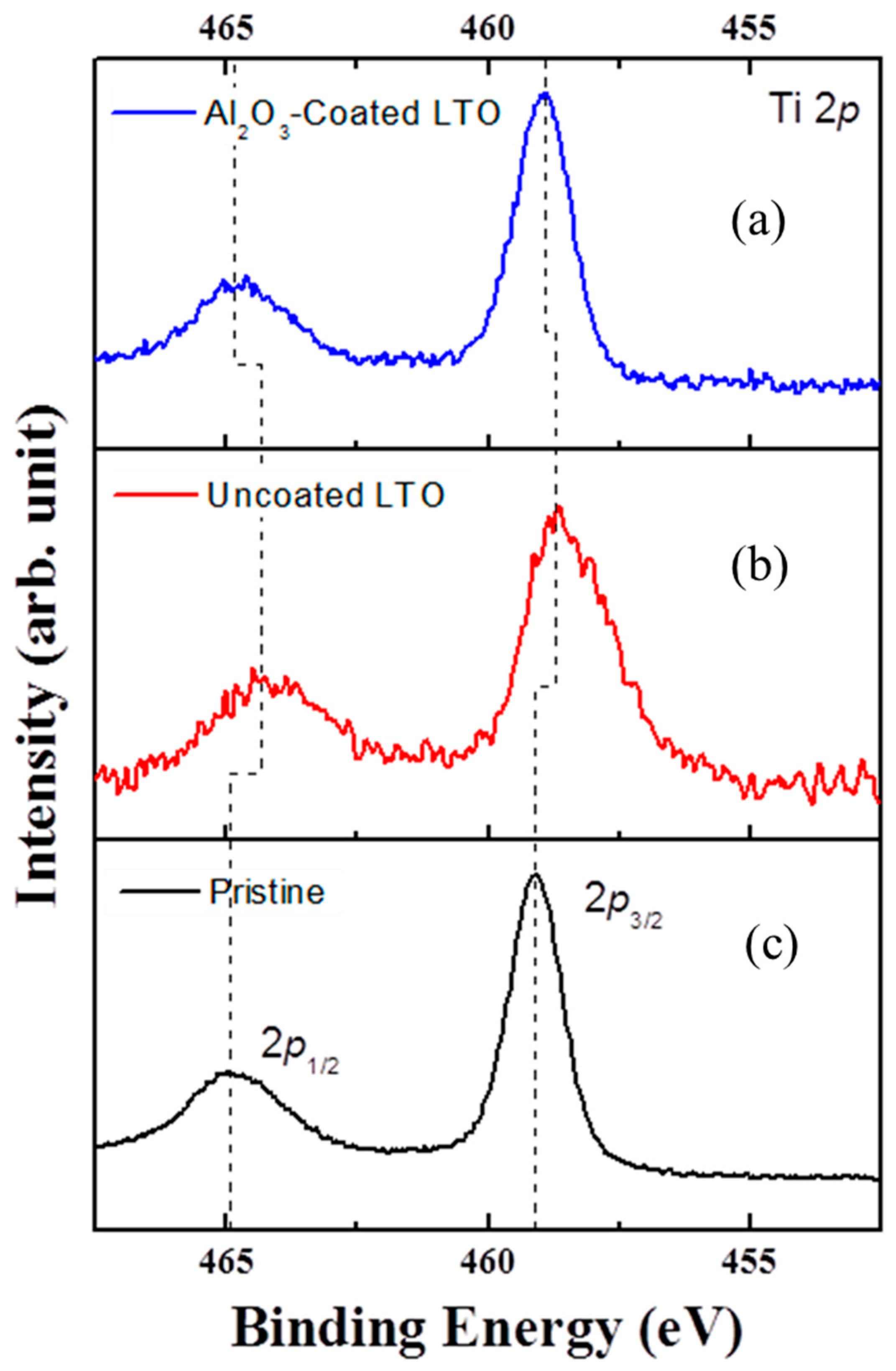
3.2. Schematic Diagram and Morphology of Uncoated and Al2O3-Coated LTO Electrodes
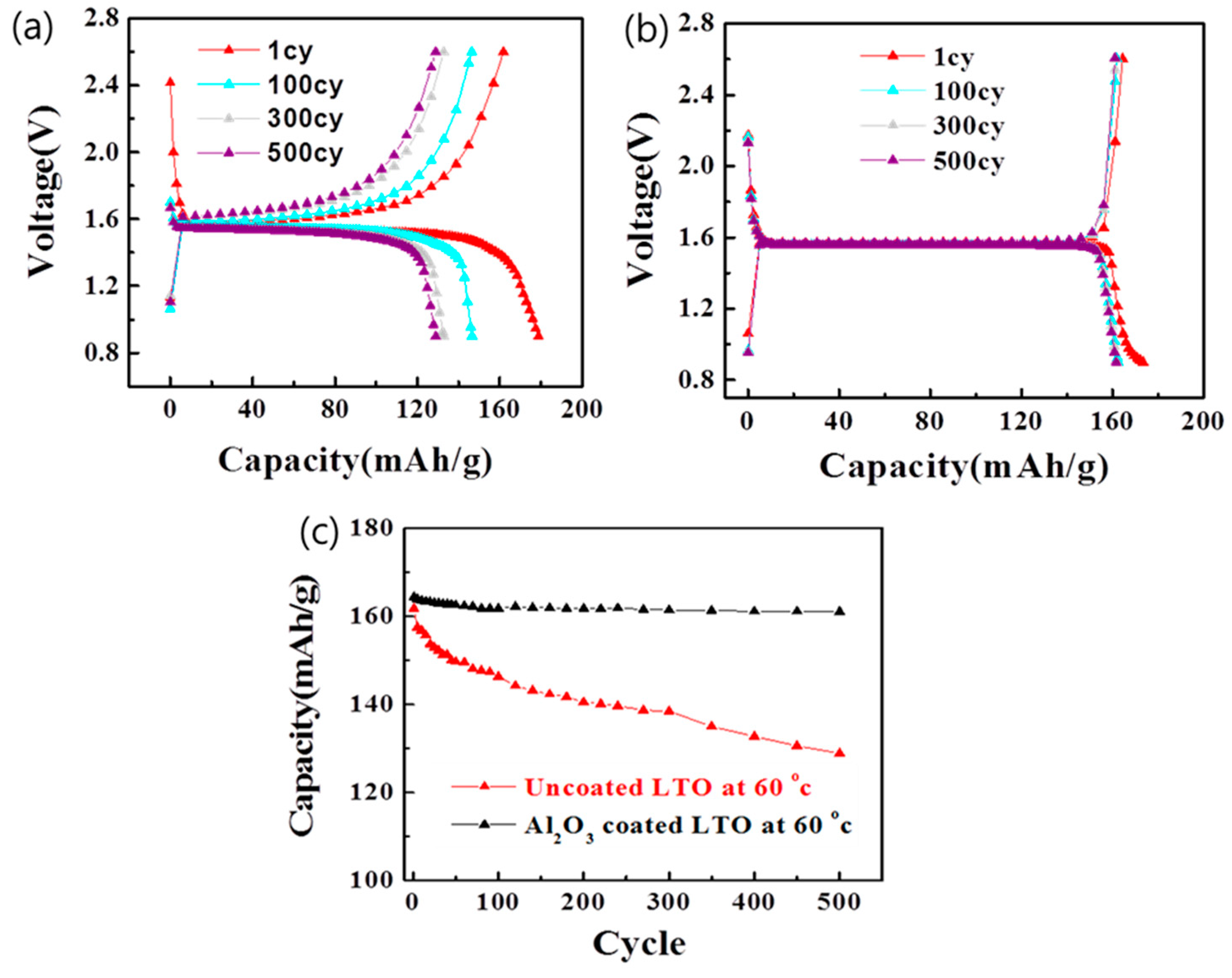
3.3. Electrochemical Properties of the Uncoated and Al2O3-Coated LTO after Cycles at a 60 °C
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Belharouak, I.; Koenig, G.M., Jr.; Amine, K. Electrochemistry and safety of Li4Ti5O12 and graphite anodes paired with LiMn2O4 for hybrid electric vehicle Li-ion battery applications. J. Power Sources 2011, 196, 10344–10350. [Google Scholar] [CrossRef]
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Bruce, P.G.; Scrosati, B.; Tarascon, J.-M. Nanomaterials for rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 2930–2946. [Google Scholar] [CrossRef] [PubMed]
- Belharouak, I.; Amine, K. Li2MTi6O14 (M = Sr, Ba): New anodes for lithium-ion batteries. Electrochem. Commun. 2003, 5, 435–438. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Sun, X.; Radovanovic, P.V.; Cui, B. Advances in spinel Li4Ti5O12 anode materials for lithium-ion batteries. New J. Chem. 2015, 39, 38–63. [Google Scholar] [CrossRef]
- Wu, F.; Wang, Z.; Li, X.; Guo, H. Synthesis of high performance Li4Ti5O12 microspheres and TiO2 nanowires from natural ilmenite. RSC Adv. 2014, 4, 40111–40119. [Google Scholar] [CrossRef]
- Aricò, A.S.; Bruce, P.; Scrosati, B.; Tarascon, J.-M.; Schalkwijk, W.V. Nanostructured materials for advanced energy conversion and storage devices. Nat. Mater. 2005, 4, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Takami, N.; Inagaki, H.; Kishi, T.; Harada, Y.; Fujita, Y.; Hoshina, K. Electrochemical kinetics and safety of 2-volt class Li-ion battery system using lithium titanium oxide anode. J. Electrochem. Soc. 2009, 156, A128–A132. [Google Scholar] [CrossRef]
- Zaghib, K.; Simoneau, M.; Armand, M.; Gauthier, M. Electrochemical study of Li4Ti5O12 as negative electrode for Li-ion polymer rechargeable batteries. J. Power Sources 1999, 81, 300–305. [Google Scholar] [CrossRef]
- Wagemaker, M.; van Eck, E.R.H.; Kentgens, A.P.; Mulder, F.M. Li-Ion Diffusion in the Equilibrium Nanomorphology of Spinel Li4+xTi5O12. J. Phys. Chem. B 2009, 113, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Huang, Y.; Wang, X.; Jia, D.; Pang, W.K.; Guo, Z.; Tang, X. High rate capability core–shell lithium titanate@ ceria nanosphere anode material synthesized by one-pot co-precipitation for lithium-ion batteries. J. Power Sources 2014, 257, 280–285. [Google Scholar] [CrossRef]
- Lin, C.; Fan, X.; Xin, Y.; Cheng, F.; Lai, M.O.; Zhou, H.; Lu, L. Li4Ti5O12-based anode materials with low working potentials, high rate capabilities and high cyclability for high-power lithium-ion batteries: A synergistic effect of doping, incorporating a conductive phase and reducing the particle size. J. Mater. Chem. A 2014, 2, 9982–9993. [Google Scholar] [CrossRef]
- Ouyang, C.Y.; Zhong, Z.Y.; Lei, M.S. Ab initio studies of structural and electronic properties of Li4Ti5O12 spinel. Electrochem. Commun. 2007, 9, 1107–1112. [Google Scholar] [CrossRef]
- Yin, Y.H.; Li, S.Y.; Fan, Z.J.; Ding, X.L.; Yang, S.T. Synthesis of novel anode Li4Ti5O12/C with PAN as carbon source and its electrochemical performance. Mater. Chem. Phys. 2011, 130, 186–190. [Google Scholar] [CrossRef]
- Wang, G.J.; Gao, J.; Fu, L.J.; Zhao, N.H.; Wu, Y.P.; Takamura, T. Preparation and characteristic of carbon-coated Li4Ti5O12 anode material. J. Power Sources 2007, 174, 1109–1112. [Google Scholar] [CrossRef]
- Huang, S.H.; Wen, Z.Y.; Zhu, X.J.; Yang, X.L. Research on Li4Ti5O12/CuxO Composite Anode Materials for Lithium-Ion Batteries. J. Electrochem. Soc. 2005, 152, A1301–A1305. [Google Scholar] [CrossRef]
- Oh, Y.; Nam, S.; Wi, S.; Kang, J.H.; Hwang, T.H.; Lee, S.H.; Park, H.H.; Cabana, J.; Kim, C.; Park, B. Effective wrapping of graphene on individual Li4Ti5O12 grains for high-rate Li-ion batteries. J. Mater. Chem. A 2014, 2, 2023–2027. [Google Scholar] [CrossRef]
- Liu, J.; Li, X.F.; Cai, M.; Li, R.; Sun, X.L. Ultrathin atomic layer deposited ZrO2 coating to enhance the electrochemical performance of Li4Ti5O12 as an anode material. Electrochim. Acta 2013, 93, 195–201. [Google Scholar] [CrossRef]
- Snyder, M.Q.; Trebukhova, S.A.; Ravdel, B.; Wheeler, M.C.; Carlo, J.D.; Tripp, C.P.; DeSisto, W.J. Synthesis and characterization of atomic layer deposited titanium nitride thin films on lithium titanate spinel powder as a lithium-ion battery anode. J. Power Sources 2007, 165, 379–385. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.E.; Kim, K.H.; Wi, S.; Lee, S.; Nam, S.; Kim, C.; Kim, S.O.; Park, B. Single-layer graphene-wrapped Li4Ti5O12 anode with superior lithium storage capability. Carbon 2017, 114, 275–283. [Google Scholar] [CrossRef]
- Liu, J.; Bian, P.; Li, J.; Ji, W.; Hao, H.; Yu, A. Gassing behavior of lithium titanate based lithium ion batteries with different types of electrolytes. J. Power Sources 2015, 286, 380–387. [Google Scholar] [CrossRef]
- Belharouak, I.; Koening, G.M., Jr.; Tan, T.; Yumoto, H.; Ota, N.; Amine, K. Performance degradation and gassing of Li4Ti5O12/LiMn2O4 lithium-ion cells. J. Electrochem. Soc. 2012, 159, A1165–A1170. [Google Scholar] [CrossRef]
- Amine, K.; Belharouak, I.; Chen, Z.; Tran, T.; Yumoto, H.; Ota, N.; Myung, S.T.; Sun, Y.K. Nanostructured anode material for high-power battery system in electric vehicles. Adv. Mater. 2010, 22, 3052–3057. [Google Scholar] [CrossRef] [PubMed]
- He, M.L.; Castel, E.; Laumann, A.; Nuspl, G.; Novák, P.; Berga, E.J. In situ gas analysis of Li4Ti5O12 based electrodes at elevated temperatures. J. Electrochem. Soc. 2015, 162, A870–A876. [Google Scholar] [CrossRef]
- Wu, K.; Yang, J.; Zhang, Y.; Wang, C.; Wang, D. Investigation on Li4Ti5O12 batteries developed for hybrid electric vehicle. J. Appl. Electrochem. 2012, 42, 989–995. [Google Scholar] [CrossRef]
- He, Y.B.; Li, B.; Liu, M.; Zhang, C.; Lv, W.; Yang, C.; Li, J.; Du, H.; Zhang, B.; Yang, Q.H.; Kim, J.K.; Kang, F. Gassing in Li4Ti5O12-based batteries and its remedy. Sci. Rep. 2012, 2, 913. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J.; Hwang, P.; Yang, W.; Wang, Z.; Wang, M.; Huang, Y.; Zhou, Y.; Qu, M.; Yu, Z.; Lin, Y. In-situ carbon coating to enhance the rate capability of the Li4Ti5O12 anode material and suppress the electrolyte reduction decomposition on the electrode. Electrochim. Acta 2016, 190, 69–75. [Google Scholar] [CrossRef]
- Xiao, L.J.; Higgins, X.; Lee, D.C.; Hassan, F.; Chen, Z.W. Hierarchical Li4Ti5O12-TiO2 composite microsphere consisting of nanocrystals for high power Li-ion batteries. Electrochim. Acta 2013, 108, 104–111. [Google Scholar]
- Schwartzberg, A.M.; Olynick, D. Complex materials by atomic layer deposition. Adv. Mater. 2015, 27, 5778–5784. [Google Scholar] [CrossRef] [PubMed]
- Marichy, C.; Bechelany, M.; Pinna, N. Atomic layer deposition of nanostructured materials for energy and environmental applications. Adv. Mater. 2012, 24, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Seo, J.; Park, S.; Shin, S.S.; Kim, Y.C.; Jeon, N.J.; Shin, H.-W.; Ahn, T.K.; Noh, J.H.; Yoon, S.C.; Hwang, C.S.; Seok, S.I. Efficient CH3NH3PbI3 Perovskite Solar Cells Employing Nanostructured p-Type NiO Electrode Formed by a Pulsed Laser Deposition. Adv. Electron. Mater. 2015, 27, 4013–4019. [Google Scholar] [CrossRef] [PubMed]
- George, S.M. Atomic layer deposition: An overview. Chem. Rev. 2010, 110, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; RamLee, H.B.; Maeng, W.J. Applications of atomic layer deposition to nanofabrication and emerging nanodevices. Thin Solid Films 2009, 517, 2563–2580. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Gu, L.; Guo, Y.-G.; Li, H.; He, X.-Q.; Tsukimoto, S.; Ikuhara, Y.; Wan, L.J. Rutile-TiO2 nanocoating for a high-rate Li4Ti5O12 anode of a lithium-ion battery. J. Am. Chem. Soc. 2012, 134, 7874–7879. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, J.K.; Nam, S.; Shim, H.C.; Park, K.; Yoon, T.; Park, H.S.; Hyun, S. Highly-Stable Li4Ti5O12 Anodes Obtained by Atomic-Layer-Deposited Al2O3. Materials 2018, 11, 803. https://doi.org/10.3390/ma11050803
Yoon JK, Nam S, Shim HC, Park K, Yoon T, Park HS, Hyun S. Highly-Stable Li4Ti5O12 Anodes Obtained by Atomic-Layer-Deposited Al2O3. Materials. 2018; 11(5):803. https://doi.org/10.3390/ma11050803
Chicago/Turabian StyleYoon, Jae Kook, Seunghoon Nam, Hyung Cheoul Shim, Kunwoo Park, Taeho Yoon, Hyung Sang Park, and Seungmin Hyun. 2018. "Highly-Stable Li4Ti5O12 Anodes Obtained by Atomic-Layer-Deposited Al2O3" Materials 11, no. 5: 803. https://doi.org/10.3390/ma11050803
APA StyleYoon, J. K., Nam, S., Shim, H. C., Park, K., Yoon, T., Park, H. S., & Hyun, S. (2018). Highly-Stable Li4Ti5O12 Anodes Obtained by Atomic-Layer-Deposited Al2O3. Materials, 11(5), 803. https://doi.org/10.3390/ma11050803




