Early Age Carbonation Heat and Products of Tricalcium Silicate Paste Subject to Carbon Dioxide Curing
Abstract
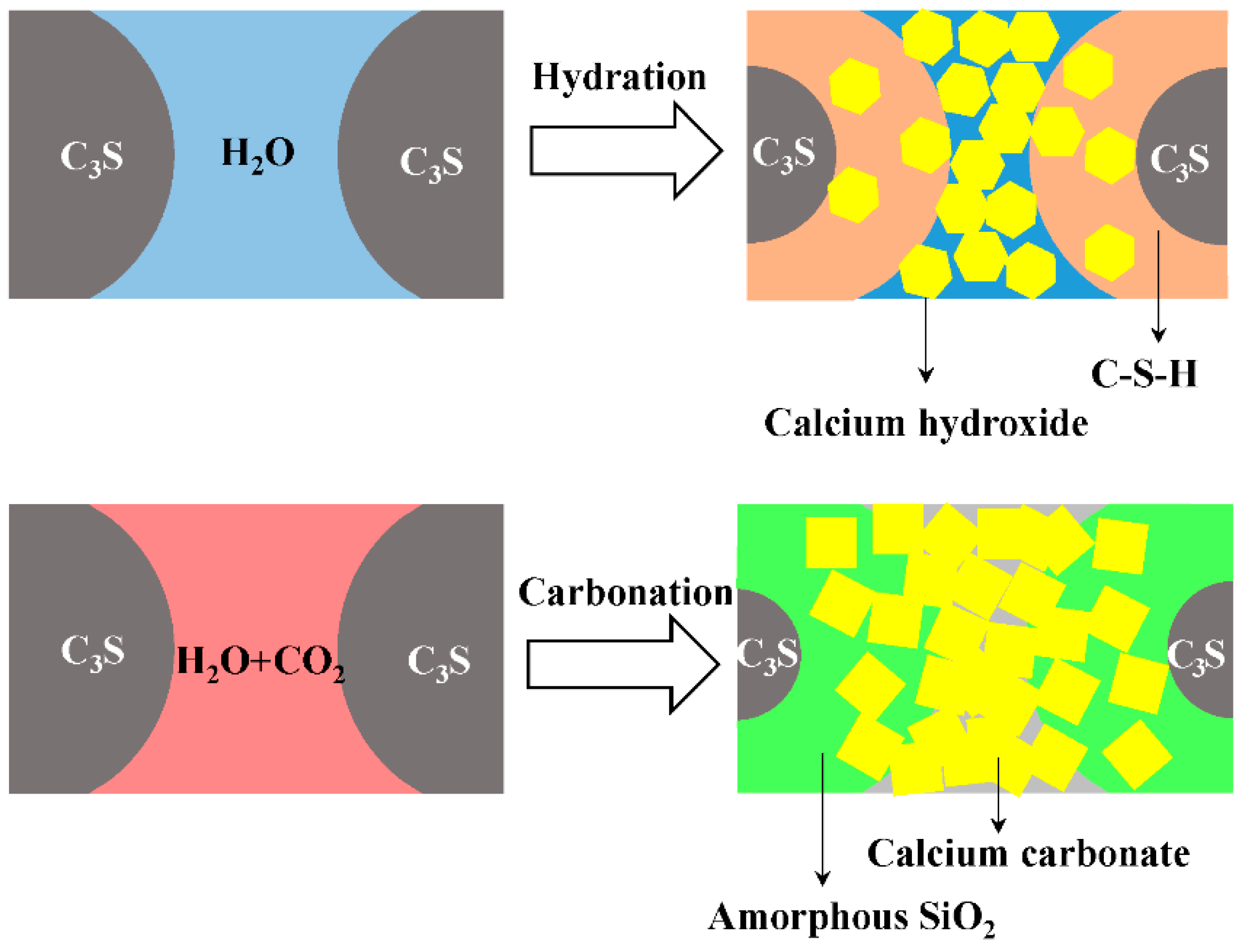
:1. Introduction
2. Materials and Methods
3. Results
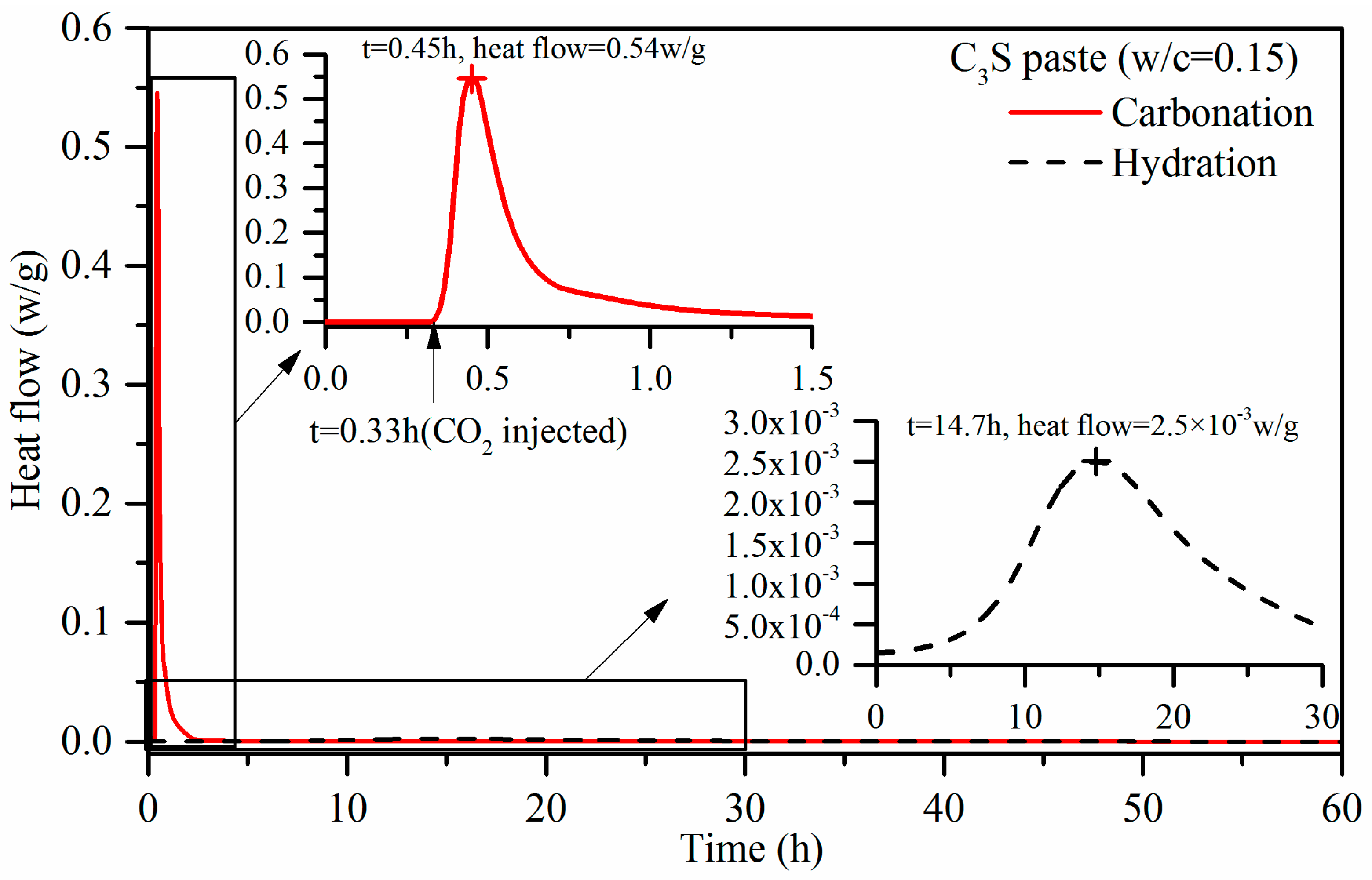
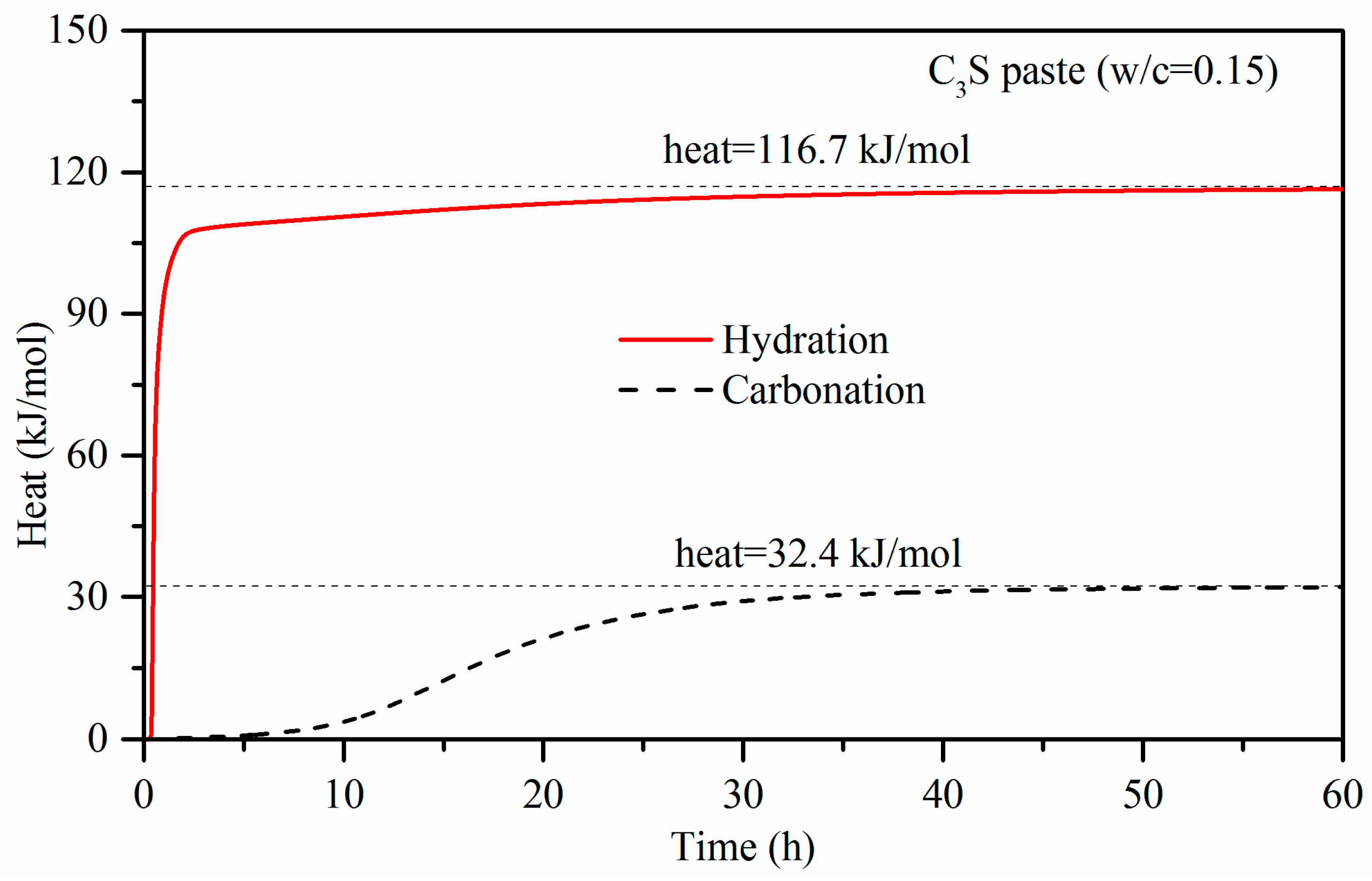
3.1. Carbonation Reaction Heat
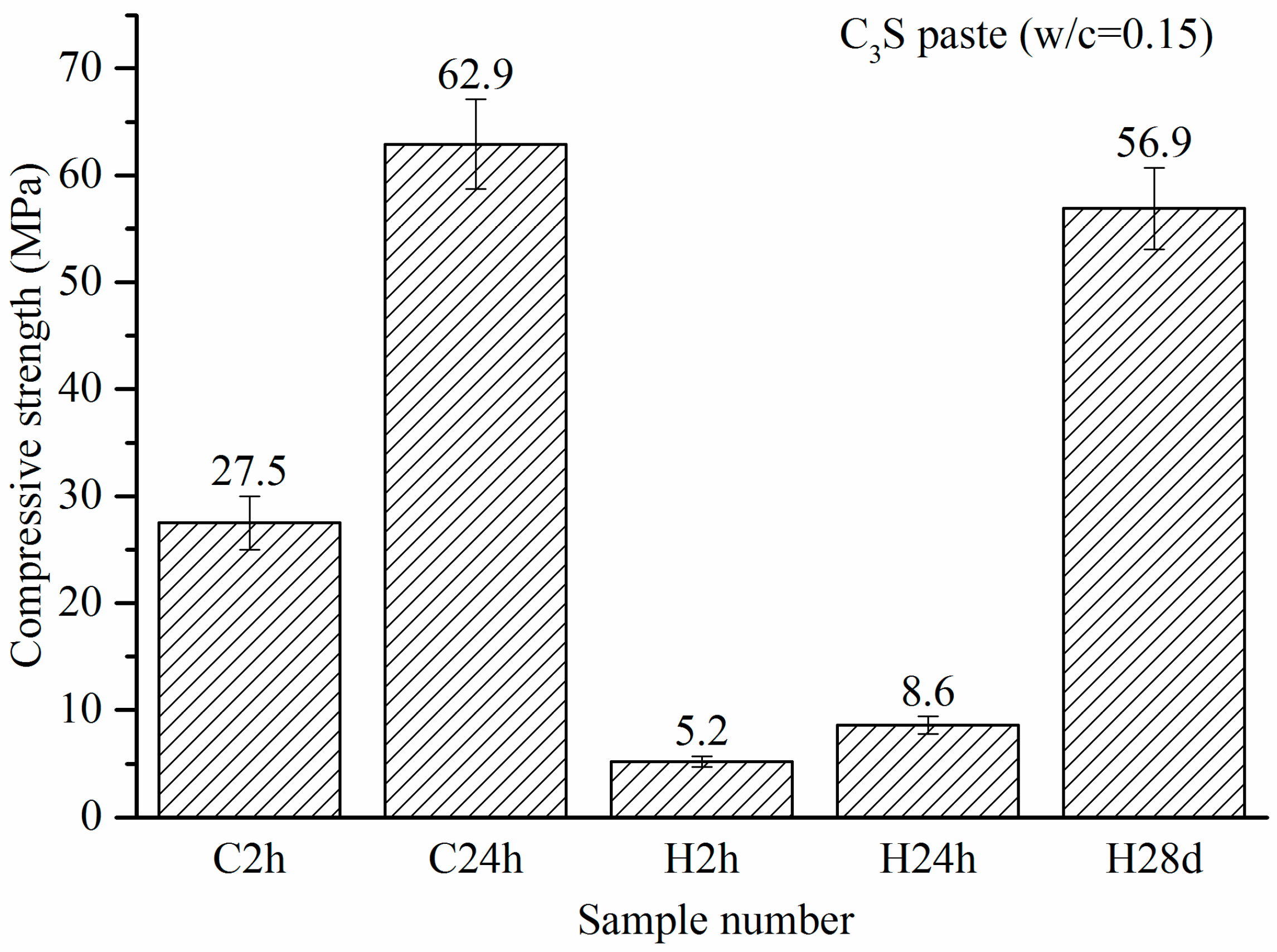
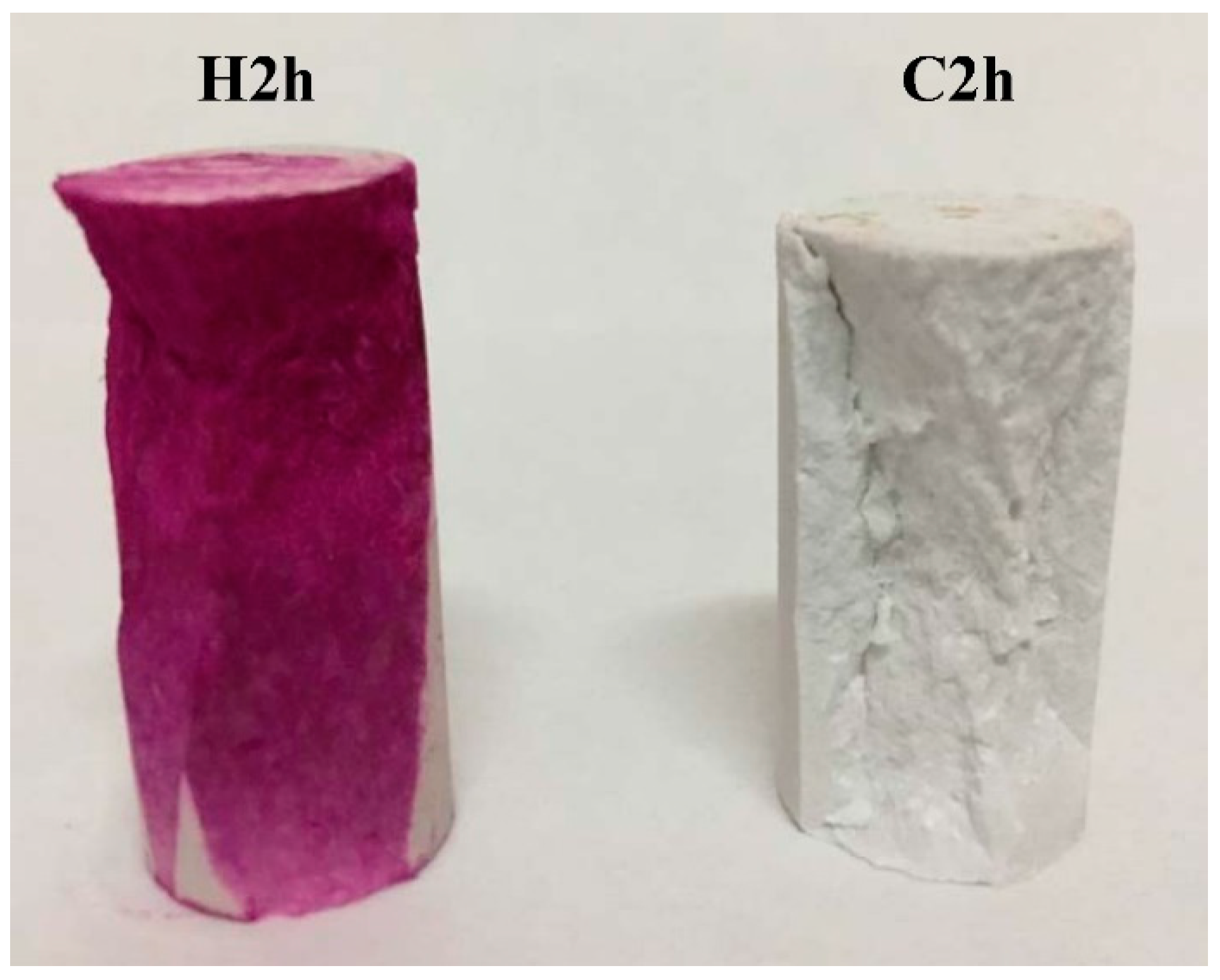
3.2. Compressive Strength
3.3. Carbonation Depth
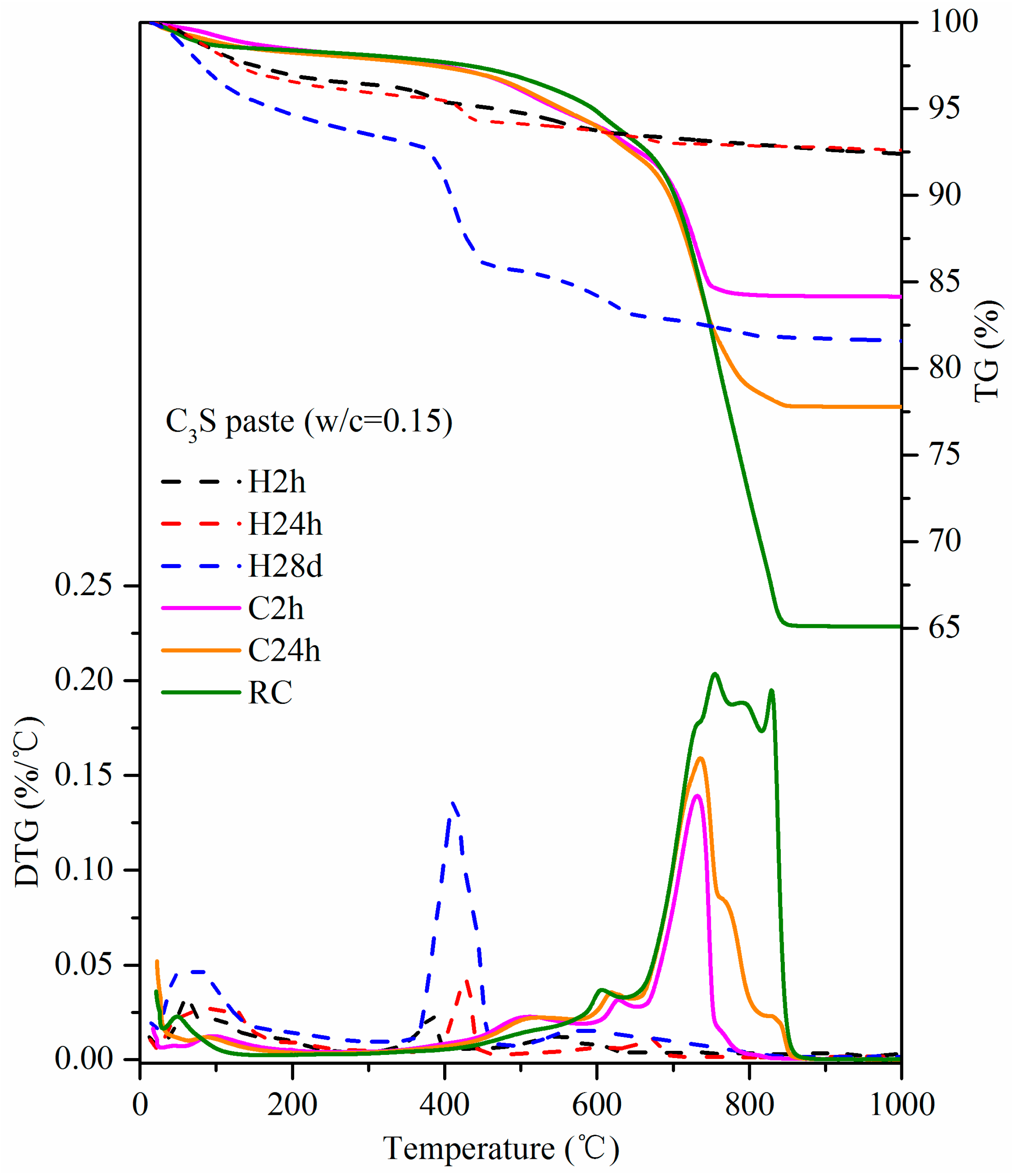
3.4. TG-DTG
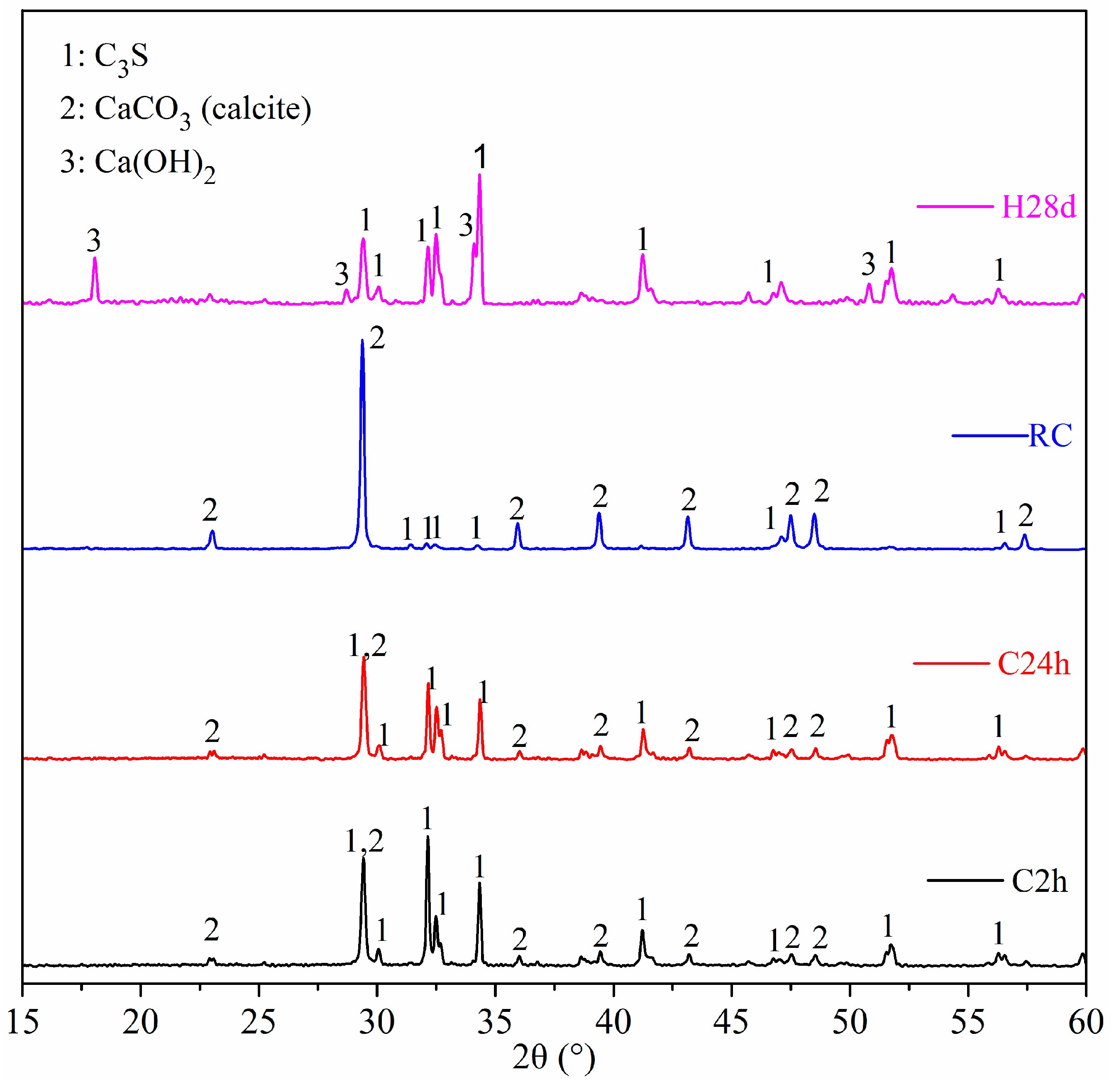
3.5. XRD
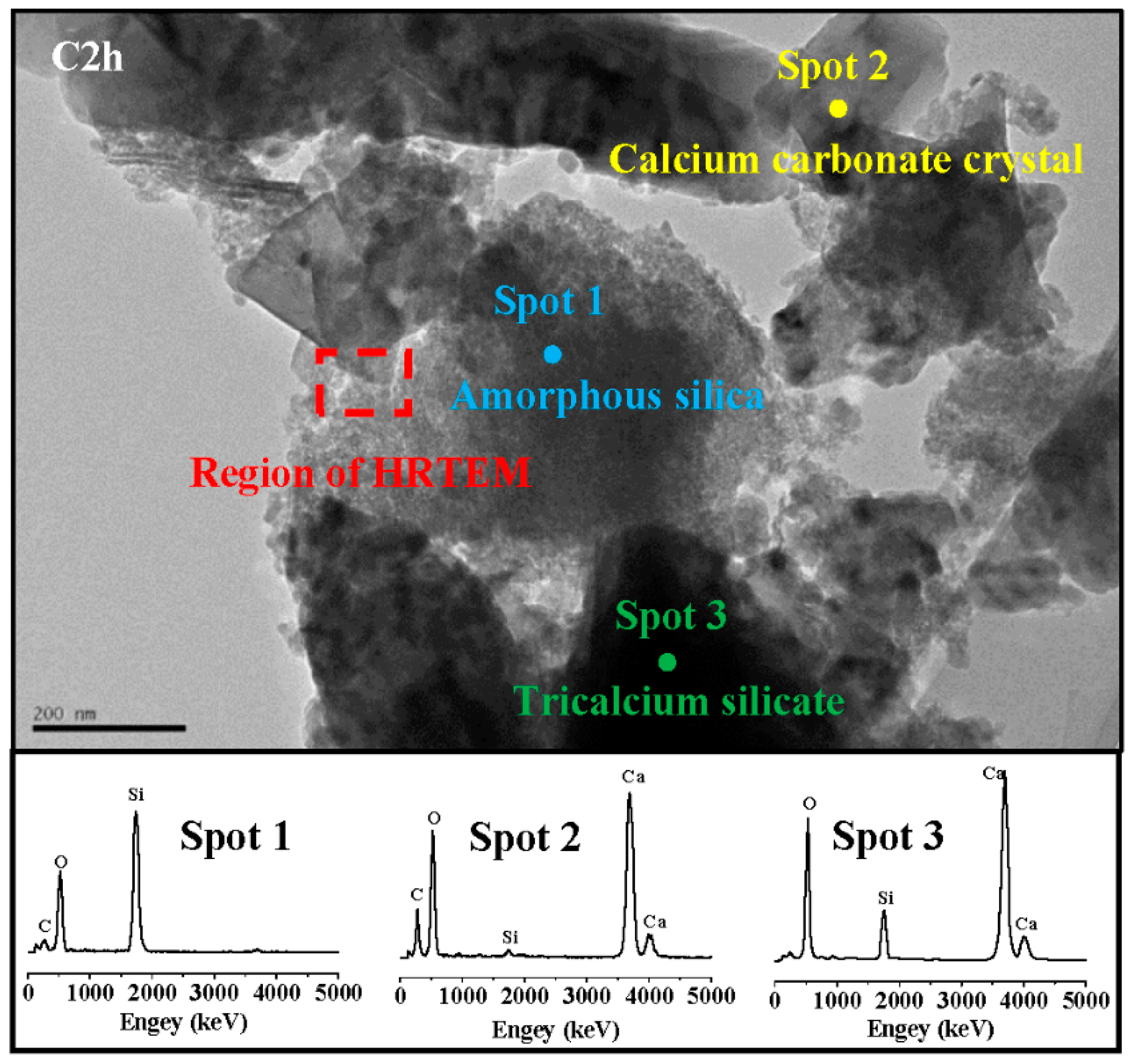
3.6. TEM-EDS
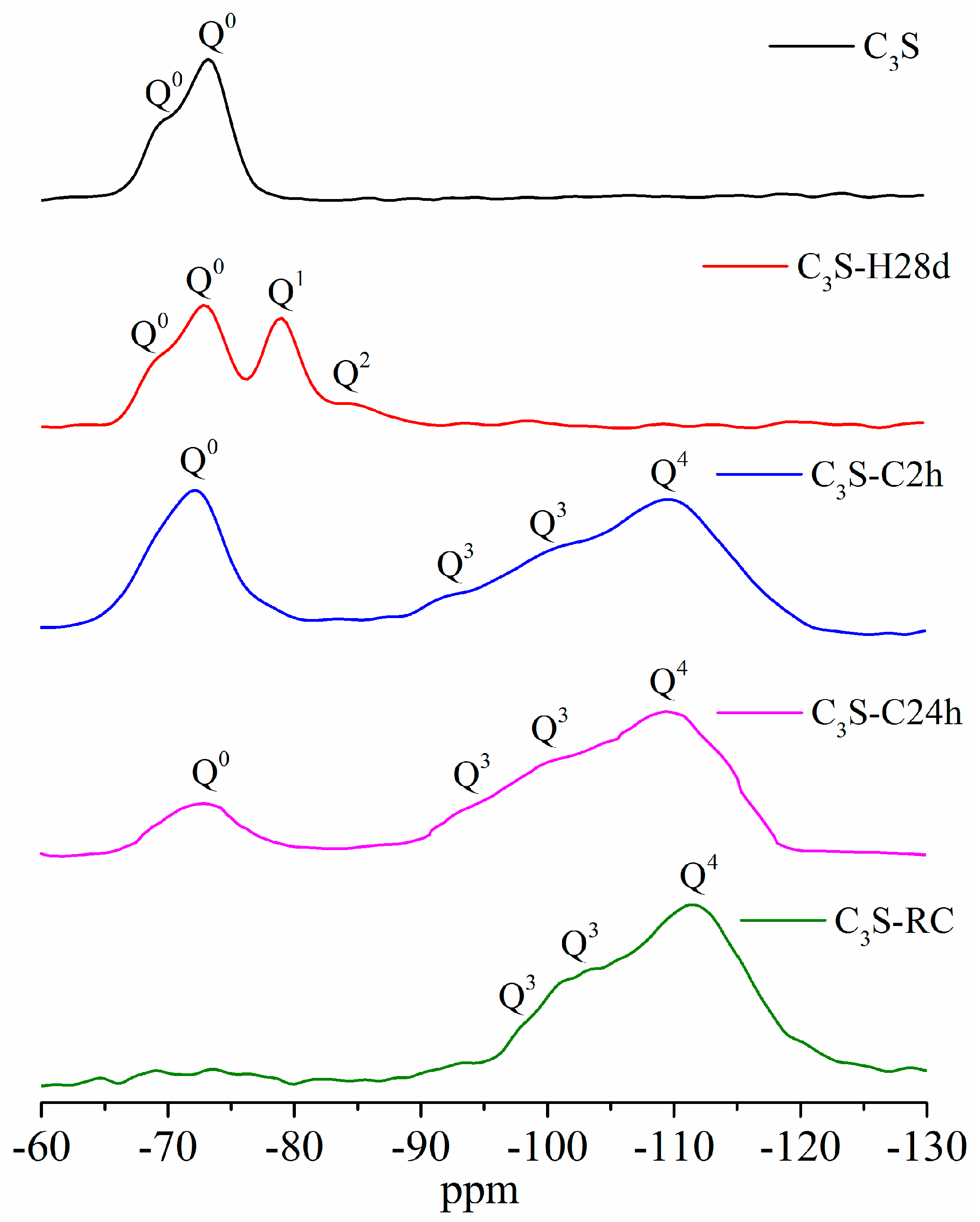
3.7. 29Si MAS NMR
4. Discussion
5. Conclusions
- With the use of a micro-calorimeter under pressure of 0.5 bar, the maximum heat flow of C3S paste activated by carbonation was measured at 0.54 W/g. This is 200 times higher than that of the hydration reaction with the same w/b of 0.15. The carbonation heat of C3S is 116.7 kJ/mol, which is more than three times than that of hydration. It is indicative that the carbonation reaction is more rapid than the hydration for C3S paste with 0.15 w/b, and it also has a higher reaction degree than that in the hydration case. The experimental carbonation heat of C3S is lower than that of the calculated value. This is mainly because C3S does not completely react under experimental conditions.
- Under 4-bar reaction pressure, the compressive strength of C3S paste reached 27.5 MPa by 2 h of carbonation, and 62.9 MPa by 24 h of carbonation. The compressive strength of the sample with 24 h of carbonation exceeded that of the 24-h hydration reference, and even exceeded that of the sample with 28 days of hydration.
- Based on the TG-DTG data, the CO2 uptake by C3S paste carbonated for 2 h and 24 h reached 17.17% and 26.32%, respectively. This is evident that the materials containing C3S can have a high potential to absorb carbon dioxide through curing for the application of low-carbon products. It applies to Portland cement-based products.
- The maximum CO2 uptake of C3S paste is tested under experimental conditions. Through the addition of water and repeated carbonation, the CO2 uptake of C3S paste can go up to 51.11%.
- The carbonation products of C3S paste are mainly calcite crystal and amorphous SiO2, which is confirmed by TG-DTG, XRD, TEM-EDS, and 29Si MAS NMR. They are independent from carbonation degree and duration.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rostami, V.; Shao, Y.; Boyd, A.J. Durability of concrete pipes subjected to combined steam and carbonation curing. Constr. Build. Mater. 2011, 25, 3345–3355. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Effect of early carbonation curing on chloride penetration and weathering carbonation in concrete. Constr. Build. Mater. 2016, 123, 516–526. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shao, Y. Carbon storage through concrete block carbonation. J. Clean Energy Technol. 2014, 2, 287–291. [Google Scholar] [CrossRef]
- El-Hassan, H.; Shao, Y. Early carbonation curing of concrete masonry units with portland limestone cement. Cem. Concr. Compos. 2015, 62, 168–177. [Google Scholar] [CrossRef]
- Zhang, D.; Cai, X.; Shao, Y. Carbonation curing of precast fly ash concrete. J. Mater. Civ. Eng. 2016, 28, 04016127. [Google Scholar] [CrossRef]
- Zhang, D.; Shao, Y. Early age carbonation curing for precast reinforced concretes. Constr. Build. Mater. 2016, 113, 134–143. [Google Scholar] [CrossRef]
- He, Z.; Li, Z.; Shao, Y. Effect of carbonation mixing on CO2 uptake and strength gain in concrete. J. Mater. Civ. Eng. 2017, 29, 04017176. [Google Scholar] [CrossRef]
- Goodbrake, C.J.; Young, J.F.; Berger, R.L. Reaction of beta-dicalcium silicate and tricalcium silicate with carbon dioxide and water vapor. J. Am. Ceram. Soc. 1979, 62, 168–171. [Google Scholar] [CrossRef]
- Berger, R.L.; Young, J.F.; Leung, K. Acceleration of hydration of calcium silicates by carbon dioxide treatment. Nat. Phys. Sci. 1972, 240, 16–18. [Google Scholar] [CrossRef]
- Berger, R.L.; Klemm, W. Accelerated curing of cementitious systems by carbon dioxide: Part ii. Hydraulic calcium silicates and aluminates. Cem. Concr. Res. 1972, 2, 647–652. [Google Scholar] [CrossRef]
- Young, J.F.; Berger, R.L.; Breese, J. Accelerated curing of compacted calcium silicate mortars on exposure to CO2. J. Am. Ceram. Soc. 1974, 57, 394–397. [Google Scholar] [CrossRef]
- Goto, S.; Suenaga, K.; Kado, T.; Fukuhara, M. Calcium silicate carbonation products. J. Am. Ceram. Soc. 1995, 78, 2867–2872. [Google Scholar] [CrossRef]
- Shtepenko, O.; Hills, C.; Brough, A.; Thomas, M. The effect of carbon dioxide on β-dicalcium silicate and portland cement. Chem. Eng. J. 2006, 118, 107–118. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Carbonation behavior of hydraulic and non-hydraulic calcium silicates: Potential of utilizing low-lime calcium silicates in cement-based materials. J. Mater. Sci. 2016, 51, 6173–6191. [Google Scholar] [CrossRef]
- Falke, M.; Platen, A.V. Nanoscience in sem and tem: Energy dispersive x-ray analysis with high spatial resolution. Imaging Microsc. 2010, 11, 36–39. [Google Scholar] [CrossRef]
- Wesselsky, A.; Jensen, O.M. Synthesis of pure portland cement phases. Cem. Concr. Res. 2009, 39, 973–980. [Google Scholar] [CrossRef]
- Dunstetter, F.; de Noirfontaine, M.N.; Courtial, M. Polymorphism of tricalcium silicate, the major compound of portland cement clinker. Cem. Concr. Res. 2006, 36, 39–53. [Google Scholar] [CrossRef]
- Mumme, W.G. Crystal-structure of tricalcium silicate from a Portland-cement clinker and its application to quantitative XRD analysis. Neues Jahrb. Fur Miner. -Monatshefte 1995, 145–160. [Google Scholar]
- Klemm, W.; Berger, R. Accelerated curing of cementitious systems by carbon dioxide: Part i. Portland cement. Cem. Concr. Res. 1972, 2, 567–576. [Google Scholar] [CrossRef]
- Fang, Y.; Chang, J. Rapid hardening β-C2S mineral and microstructure changes activated by accelerated carbonation curing. J. Therm. Anal. Calorim. 2017, 129, 681–689. [Google Scholar] [CrossRef]
- Bahafid, S.; Ghabezloo, S.; Duc, M.; Faure, P.; Sulem, J. Effect of the hydration temperature on the microstructure of Class G cement: C–S–H composition and density. Cem. Concr. Res. 2017, 95, 270–281. [Google Scholar] [CrossRef]
- Alarcon-Ruiz, L.; Platret, G.; Massieu, E.; Ehrlacher, A. The use of thermal analysis in assessing the effect of temperature on a cement paste. Cem. Concr. Res. 2005, 35, 609–613. [Google Scholar] [CrossRef]
- Sha, W.; Pereira, G.B. Differential scanning calorimetry study of ordinary portland cement paste containing metakaolin and theoretical approach of metakaolin activity. Cem. Concr. Compos. 2001, 23, 455–461. [Google Scholar] [CrossRef]
- Han, S.; Yan, P.; Liu, R. Study on the hydration product of cement in early age using TEM. Sci. China Technol. Sci. 2012, 55, 2284–2290. [Google Scholar] [CrossRef]
- Richardson, I.G.; Skibsted, J.; Black, L.; Kirkpatrick, R.J. Characterisation of cement hydrate phases by TEM, NMR and Raman spectroscopy. Adv. Cem. Res. 2010, 22, 233–248. [Google Scholar] [CrossRef]
- Brinker, C.J.; Kirkpatrick, R.J.; Tallant, D.R.; Bunker, B.C.; Montez, B. NMR confirmation of strained “defects” in amorphous silica. J. Non-Cryst. Solids 1988, 99, 418–428. [Google Scholar] [CrossRef]
- Groves, G.W.; Brough, A.; Richardson, I.G.; Dobson, C.M. Progressive changes in the structure of hardened C3S cement pastes due to carbonation. J. Am. Ceram. Soc. 1991, 74, 2891–2896. [Google Scholar] [CrossRef]
- Johansson, K.; Larsson, C.; Antzutkin, O.N.; Forsling, W.; Kota, H.R.; Ronin, V. Kinetics of the hydration reactions in the cement paste with mechanochemically modified cement 29Si magic-angle-spinning NMR study. Cem. Concr. Res. 1999, 29, 1575–1581. [Google Scholar] [CrossRef]
- Lippmaa, E.; Maegi, M.; Samoson, A.; Engelhardt, G.; Grimmer, A.R. Structural studies of silicates by solid-state high-resolution silicon-29 NMR. J. Am. Chem. Soc. 1980, 102, 4889–4893. [Google Scholar] [CrossRef]
- Gopi, S.; Subramanian, V.K.; Palanisamy, K. Aragonite–calcite–vaterite: A temperature influenced sequential polymorphic transformation of CaCO3 in the presence of DTPA. Mater. Res. Bull. 2013, 48, 1906–1912. [Google Scholar] [CrossRef]
- Gmira, A.; Zabat, M.; Pellenq, R.J.M.; Van Damme, H. Microscopic physical basis of the poromechanical behavior of cement-based materials. Mater. Struct. 2004, 37, 3–14. [Google Scholar] [CrossRef]
- Powers, T.C. Structure and physical properties of hardened portland cement paste. J. Am. Ceram. Soc. 1958, 41, 1–6. [Google Scholar] [CrossRef]
- Powers, T.C.; Brownyard, T.L. Studies of the physical properties of hardened portland cement paste. ACI J. Proc. 1946, 43, 101–132. [Google Scholar]
- Richardson, I.G.; Groves, G.W. Microstructure and microanalysis of hardened ordinary portland cement pastes. J. Mater. Sci. 1993, 28, 265–277. [Google Scholar] [CrossRef]
- Kumar, A.; Bishnoi, S.; Scrivener, K.L. Modelling early age hydration kinetics of alite. Cem. Concr. Res. 2012, 42, 903–918. [Google Scholar] [CrossRef]
- Lu, L.; Xiang, C.; He, Y.; Wang, F.; Hu, S. Early hydration of C3S in the presence of Cd2+, Pb2+ and Cr3+ and the immobilization of heavy metals in pastes. Constr. Build. Mater. 2017, 152, 923–932. [Google Scholar] [CrossRef]
- Bishnoi, S.; Scrivener, K.L. Studying nucleation and growth kinetics of alite hydration using μic. Cem. Concr. Res. 2009, 39, 849–860. [Google Scholar] [CrossRef]
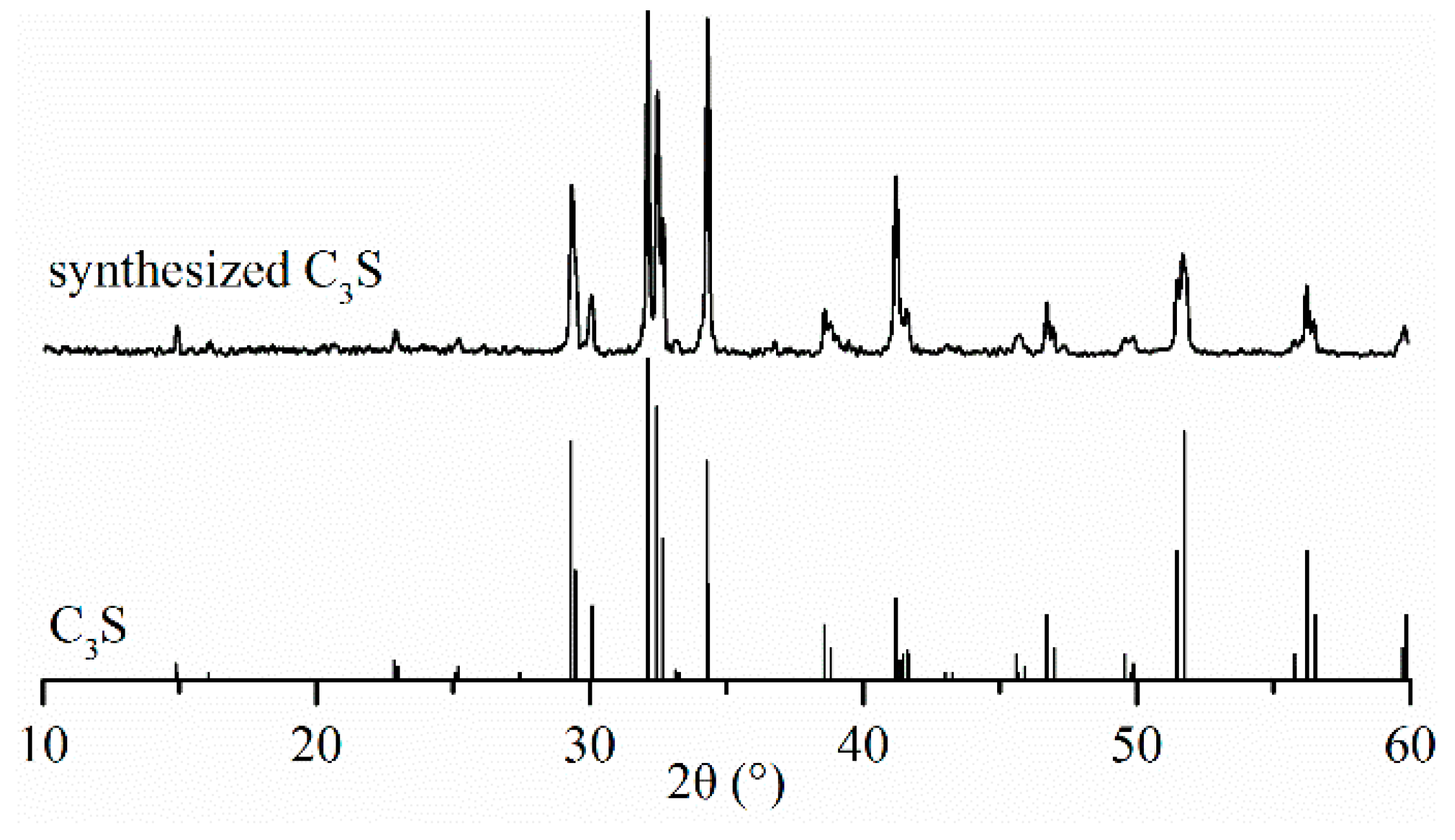
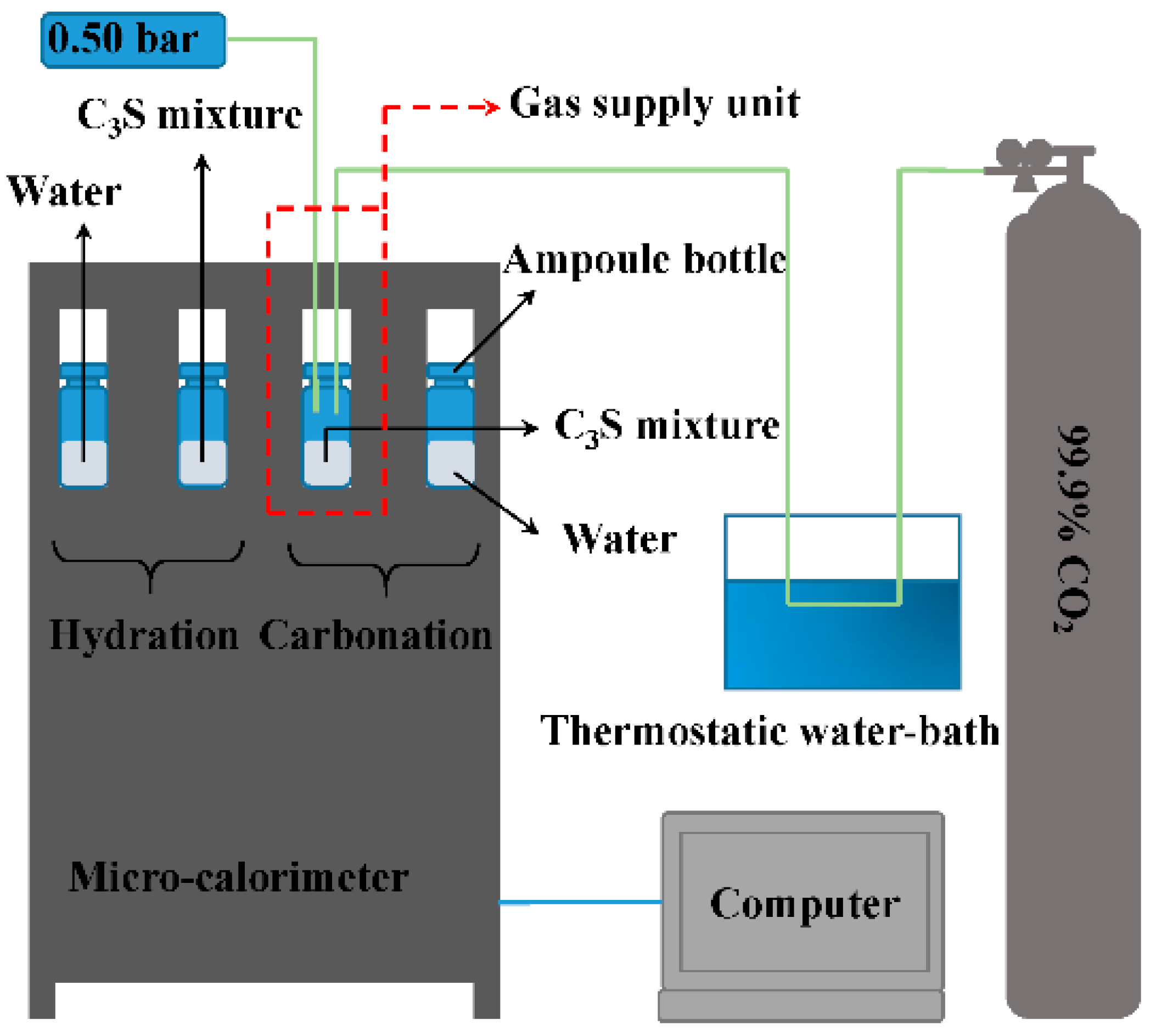
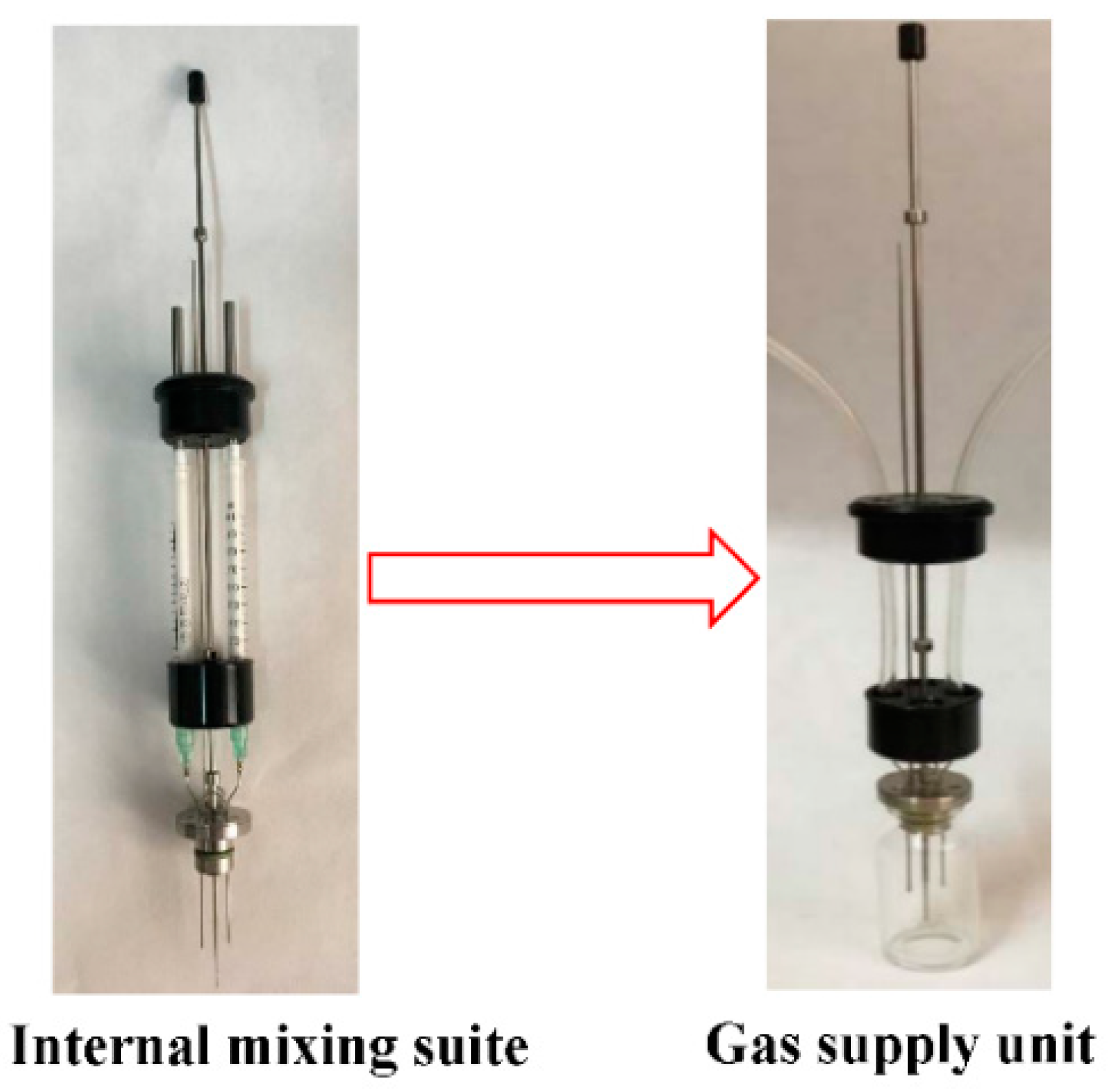
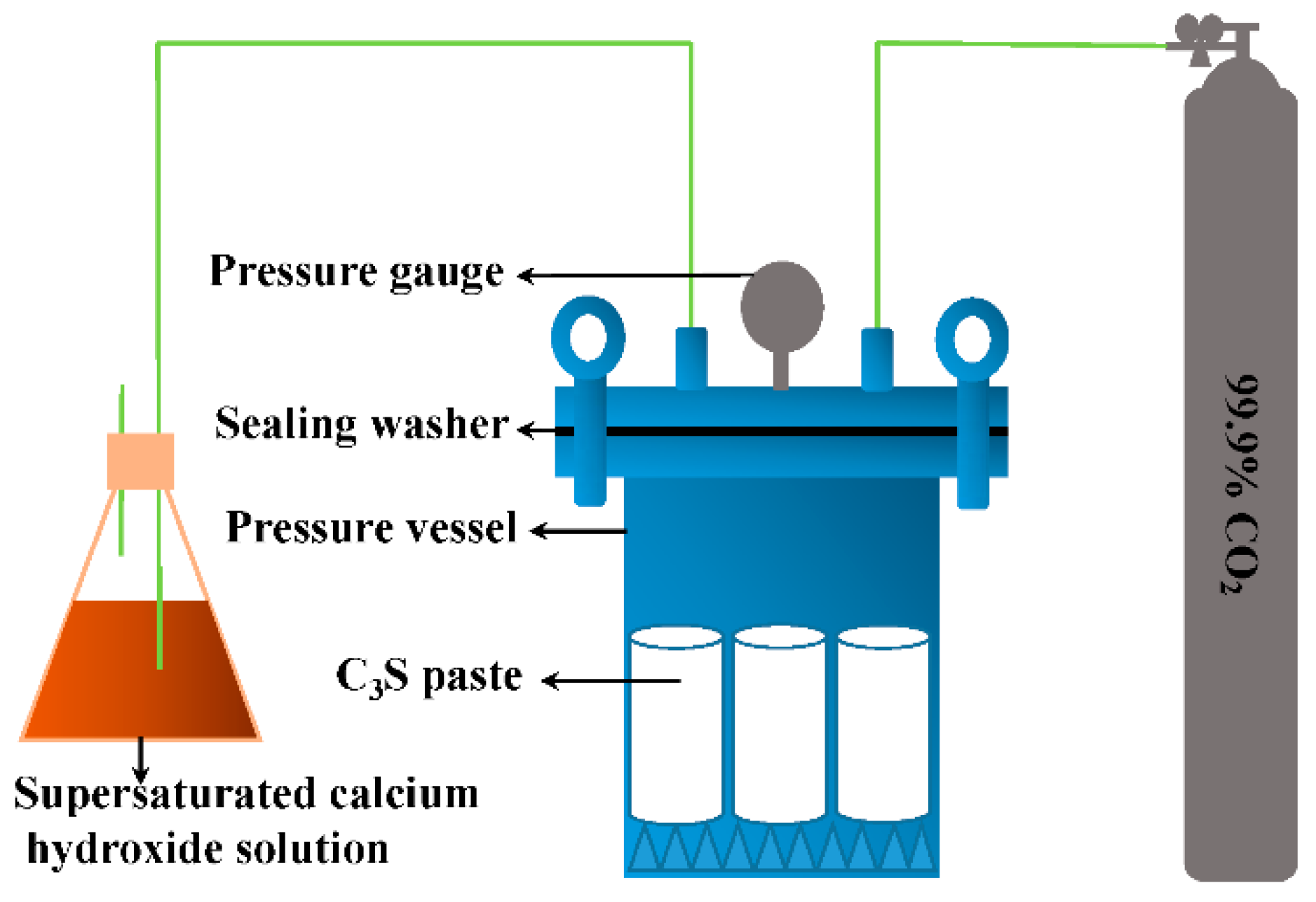




| Chemical Composition | Content (%) |
|---|---|
| CaO | 73.15 |
| SiO2 | 26.06 |
| Others | 0.79 |
| Sample Number | Curing Conditions | Sample Form | Curing Age |
|---|---|---|---|
| H2h | Hydration curing; RH ≥ 95%; temperature = 20 °C. | Compact cylinder | 2 h |
| H24h | 24 h | ||
| H28d | 28 days | ||
| C2h | Carbonation curing; purity of CO2 = 99.9%; curing pressure = 4 bar; RH ≥75%; temperature = 20 ± 3 °C. | 2 h | |
| C24h | 24 h | ||
| RC | Powder | 24 h × 4 times |
| Sample Number | Weight Loss (%) | CO2 Uptake (%) | Carbonation Degree (%) | ||
|---|---|---|---|---|---|
| Decarbonation | Total | Experimental | Theoretical | ||
| C2h | 14.21 | 15.85 | 17.17 | 57.84 | 29.71 |
| C24h | 20.47 | 22.22 | 26.32 | 45.53 | |
| RC | 31.28 | 34.89 | 51.11 | 88.43 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; He, Z.; Shao, Y. Early Age Carbonation Heat and Products of Tricalcium Silicate Paste Subject to Carbon Dioxide Curing. Materials 2018, 11, 730. https://doi.org/10.3390/ma11050730
Li Z, He Z, Shao Y. Early Age Carbonation Heat and Products of Tricalcium Silicate Paste Subject to Carbon Dioxide Curing. Materials. 2018; 11(5):730. https://doi.org/10.3390/ma11050730
Chicago/Turabian StyleLi, Zhen, Zhen He, and Yixin Shao. 2018. "Early Age Carbonation Heat and Products of Tricalcium Silicate Paste Subject to Carbon Dioxide Curing" Materials 11, no. 5: 730. https://doi.org/10.3390/ma11050730
APA StyleLi, Z., He, Z., & Shao, Y. (2018). Early Age Carbonation Heat and Products of Tricalcium Silicate Paste Subject to Carbon Dioxide Curing. Materials, 11(5), 730. https://doi.org/10.3390/ma11050730




