Synthesis of Hydroxyapatite, β-Tricalcium Phosphate and Biphasic Calcium Phosphate Particles to Act as Local Delivery Carriers of Curcumin: Loading, Release and In Vitro Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental
2.2. Synthesis
2.2.1. Method 1
2.2.2. Method 2
2.3. Curcumin Loading
2.4. Curcumin Release
2.5. Cell Cytotoxicity—MTT Assay
2.6. Alkaline Phosphatase Activity Assay
3. Results and Discussion
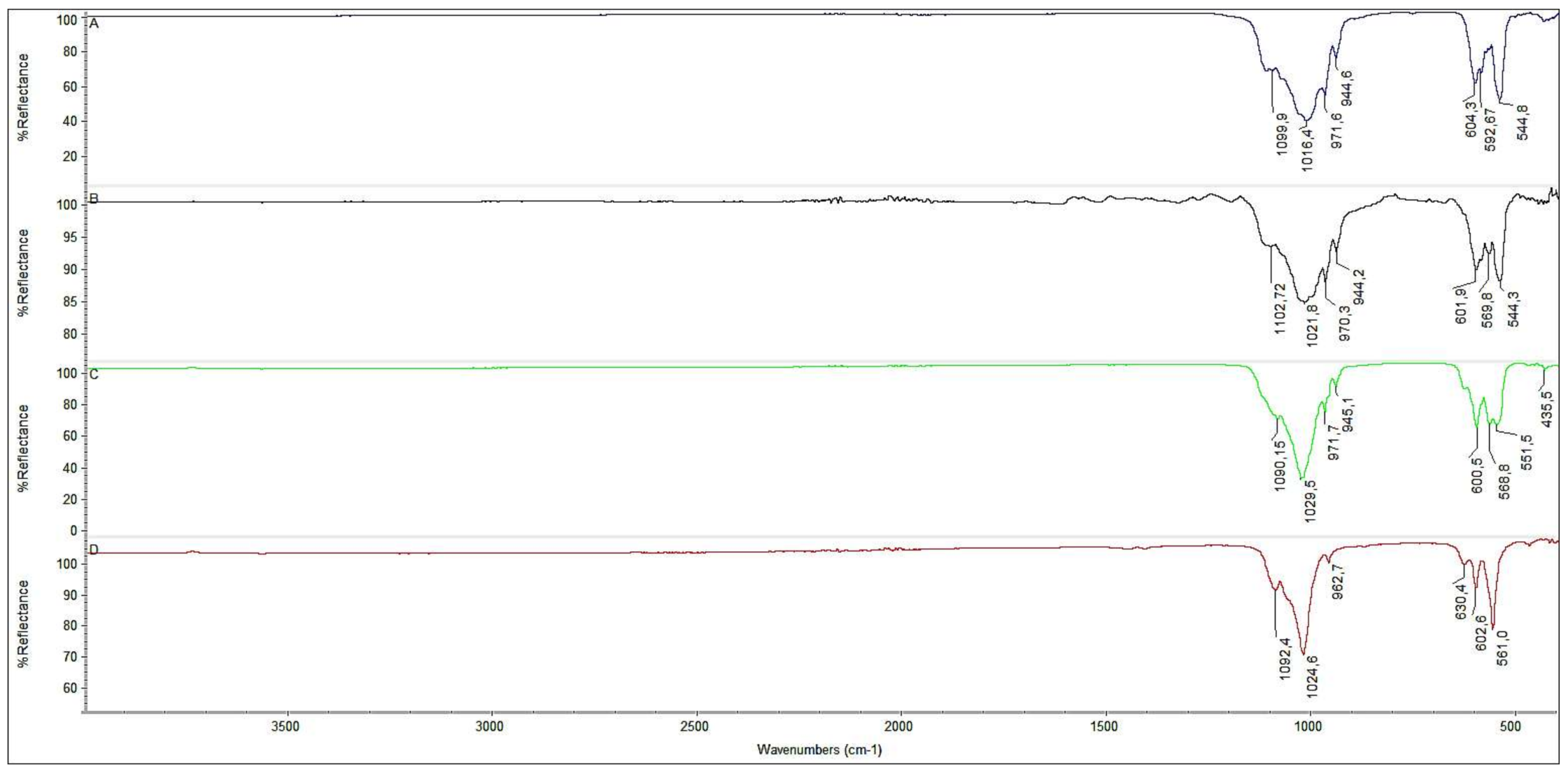
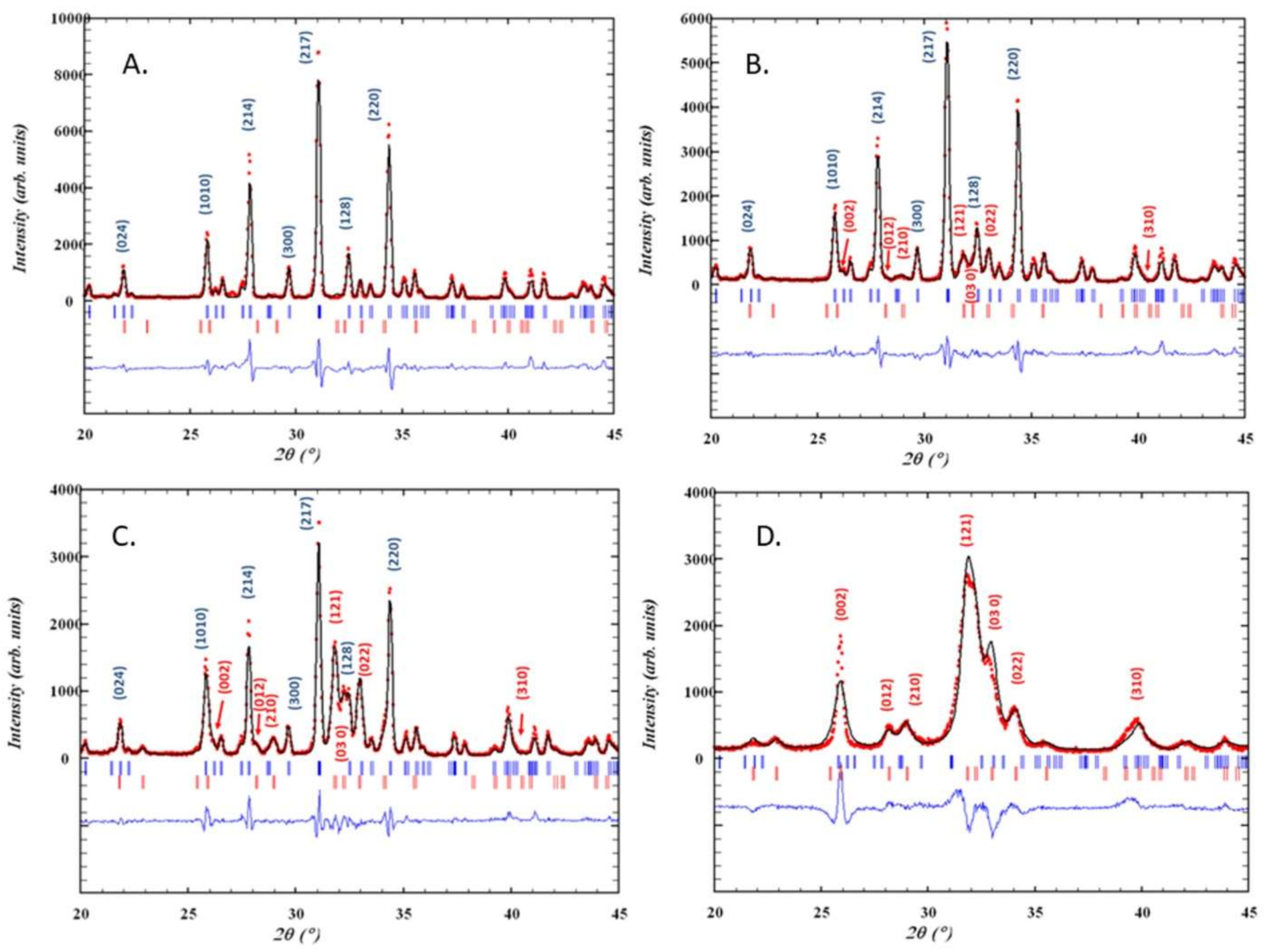
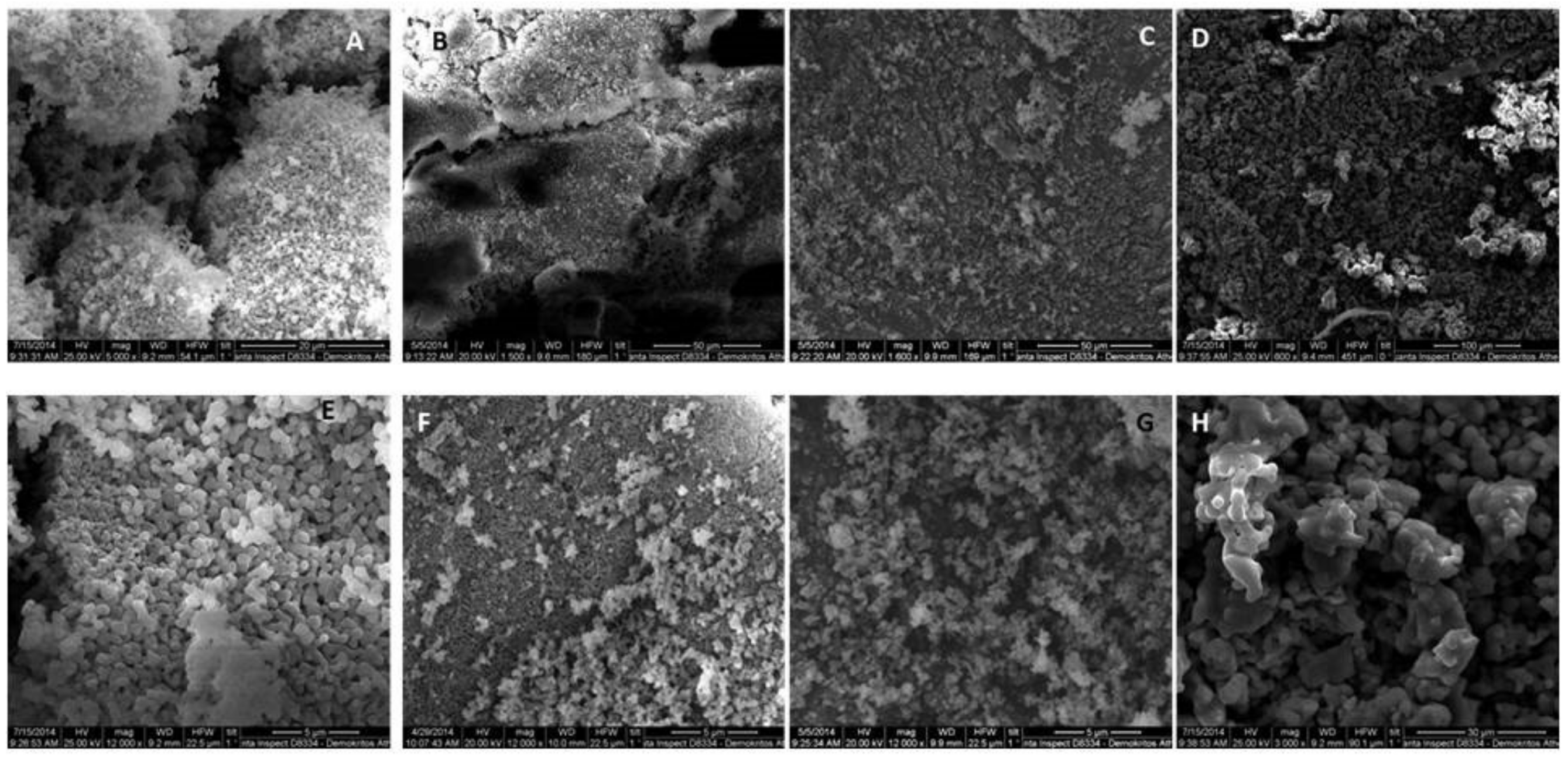
3.1. Synthesis and Characterization
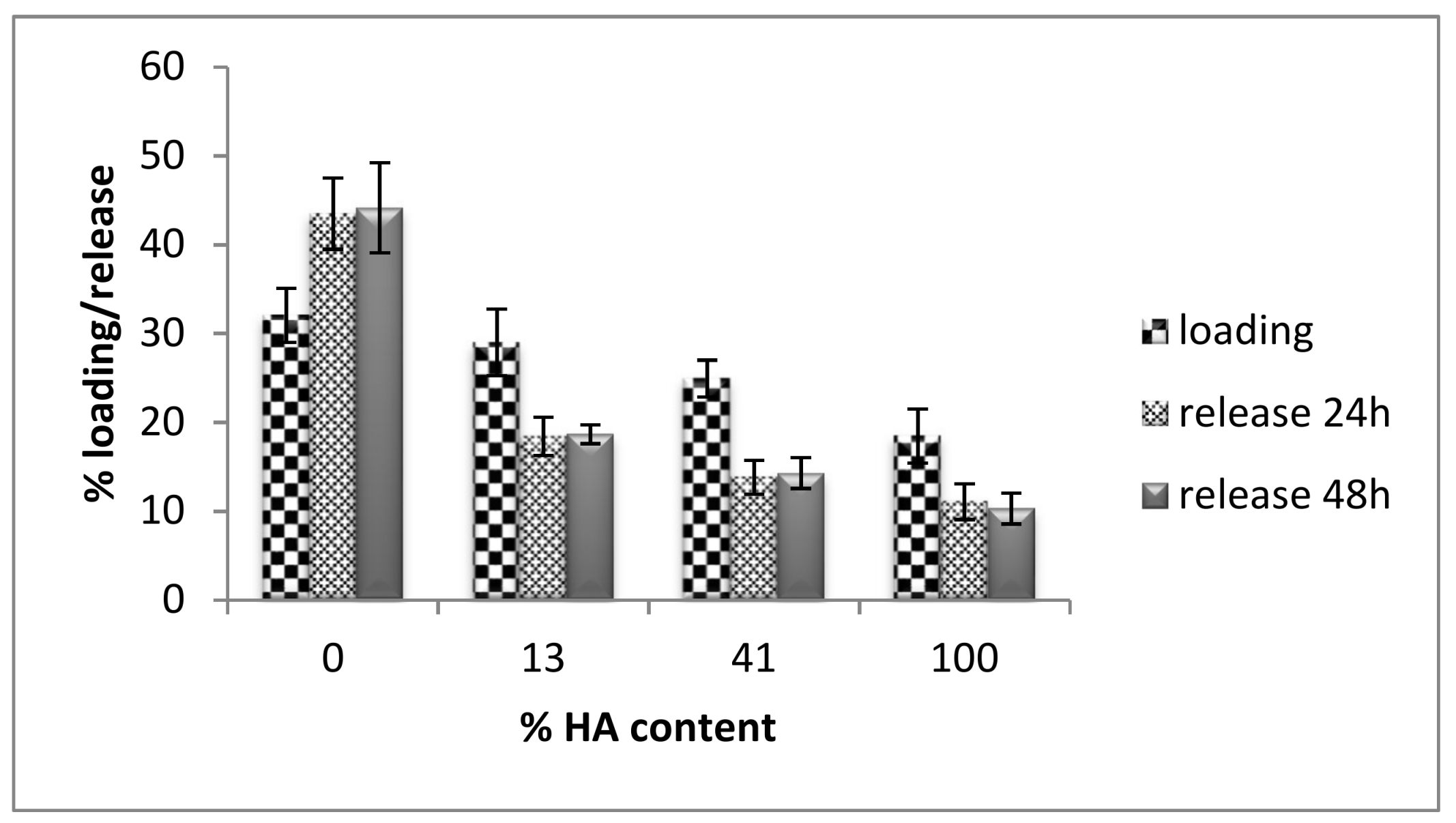
3.2. Curcumin Loading and Release
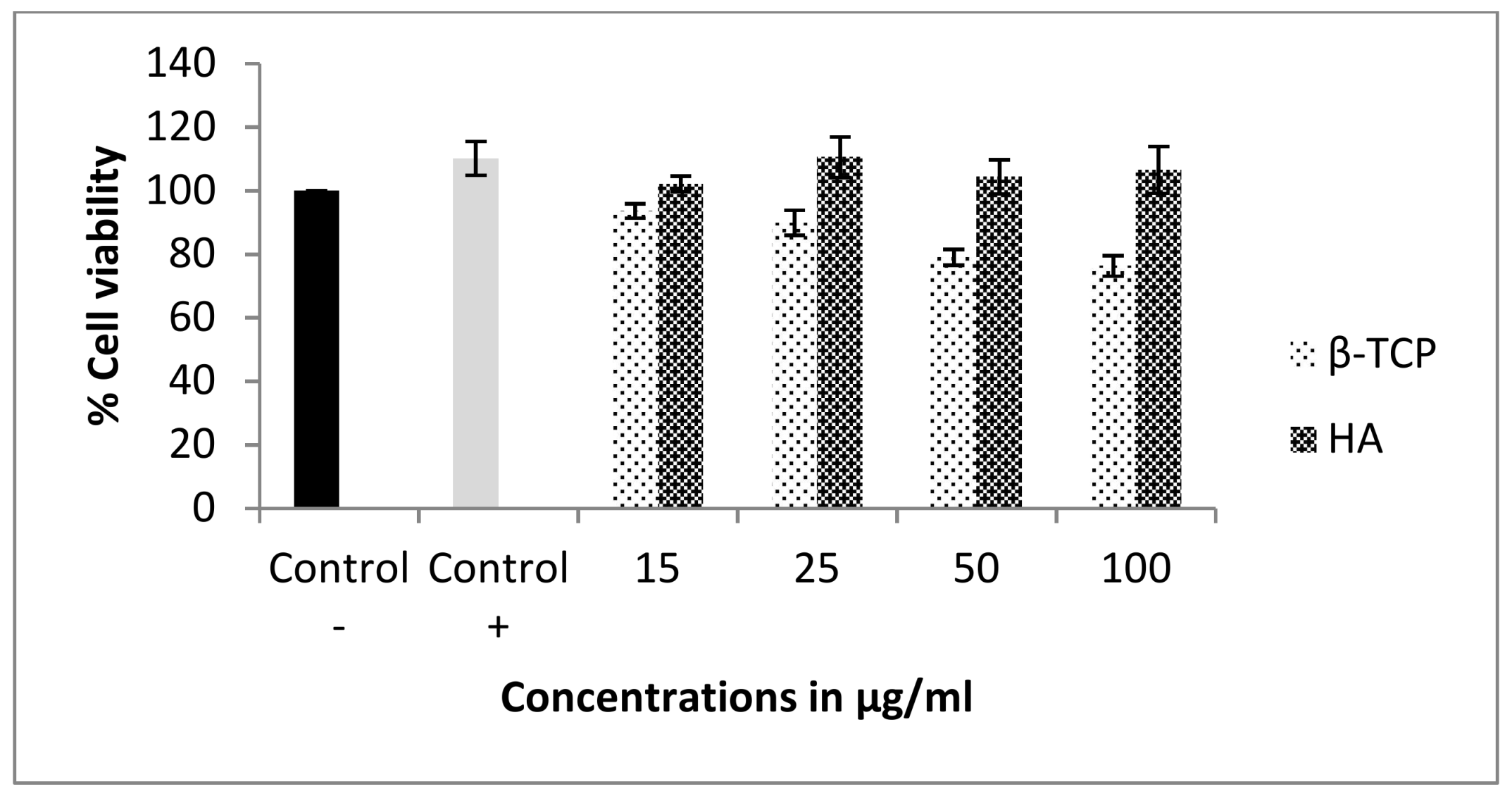
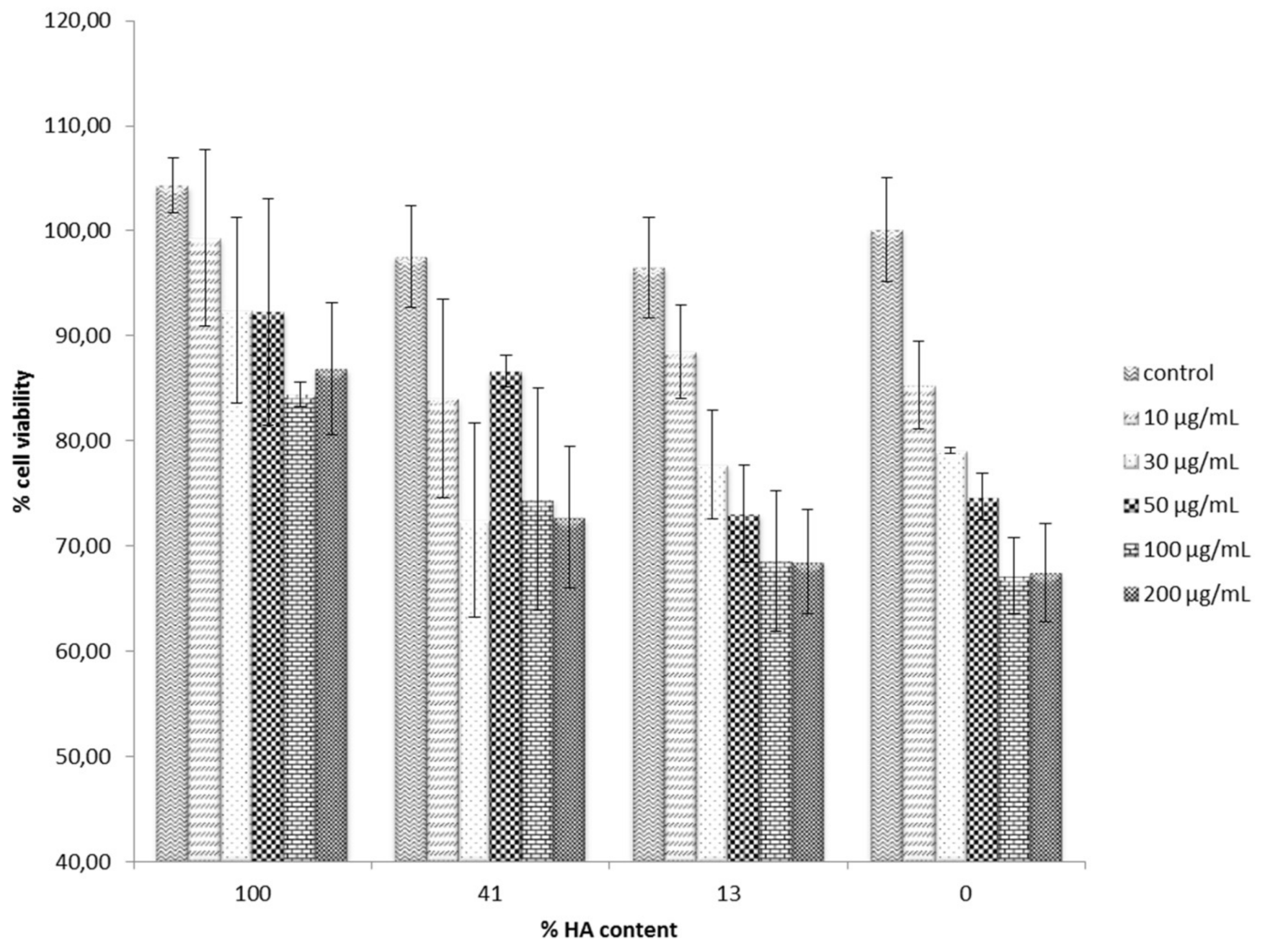
3.3. Cell Cytotoxicity
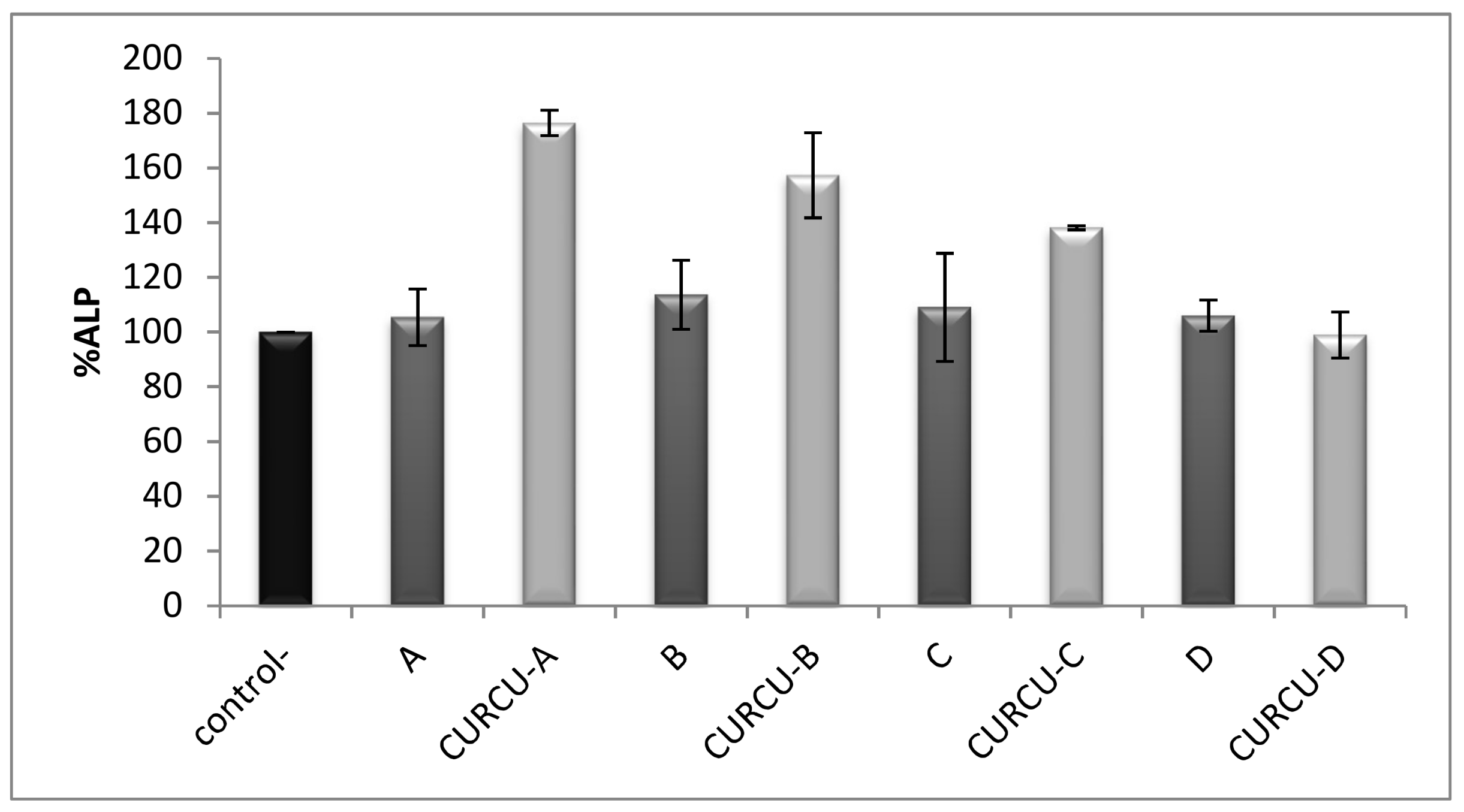
3.4. Alkaline Phosphatase Activity Assay
4. Conclusions
Author Contributions
Conflicts of Interest
Funding
References
- Ducheyne, P.; Qiu, Q. Bioactive ceramics: The effect of surface reactivity on bone formation and bone cell function. Biomaterials 1999, 20, 2287–2303. [Google Scholar] [CrossRef]
- Kim, H.-M. Ceramic bioactivity and related biomimetic strategy. Solid State Mater. Sci. 2003, 7, 289–299. [Google Scholar] [CrossRef]
- Rompen, E.; Domken, O.; Degidi, M.; Farias Pontes, A.E.; Piattelli, A. The effect of material characteristics, of surface topography and of implant components and connections on soft tissue integration: A literature review. Clin. Oral Implants Res. 2006, 17, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.D.; Zhang, H.J.; Fan, H.S.; Li, W.; Zhang, X.D. Effect of phase composition and microstructure of calcium phosphate ceramic particles on protein adsorption. Acta Biomater. 2010, 6, 1536–1541. [Google Scholar] [CrossRef] [PubMed]
- Yoganand, C.P.; Selvarajan, V.; Cannillo, V.; Sola, A.; Roumeli, E.; Goudouri, O.M.; Paraskevopoulos, K.M.; Rouabhia, M. Characterization and in vitro-bioactivity of natural hydroxyapatite based bio-glass–ceramics synthesized by thermal plasma processing. Ceram. Int. 2010, 36, 1757–1766. [Google Scholar] [CrossRef]
- Ni, S.; Chang, J.; Chou, L.; Zhai, W. Comparison of osteoblast-like cell responses to calcium silicate and tricalcium phosphate ceramics in vitro. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 80, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Le Nihouannen, D.; Duval, L.; Lecomte, A.; Julien, M.; Guicheux, J.; Daculsi, G.; Layrolle, P. Interactions of total bone marrow cells with increasing quantities of macroporous calcium phosphate ceramic granules. J. Mater. Sci. Mater. Med. 2007, 18, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Hing, K.A.; Wilson, L.F.; Buckland, T. Comparative performance of three ceramic bone graft substitutes. Spine J. 2007, 7, 475–490. [Google Scholar] [CrossRef] [PubMed]
- Julien, M.; Khairoun, I.; LeGeros, R.Z.; Delplace, S.; Pilet, P.; Weiss, P.; Daculsi, G.; Bouler, J.M.; Guicheux, J. Physico-chemical-mechanical and in vitro biological properties of calcium phosphate cements with doped amorphous calcium phosphates. Biomaterials 2007, 28, 956–965. [Google Scholar] [CrossRef] [PubMed]
- LeGeros, R.Z.; Lin, S.; Rohanizadeh, R.; Mijares, D.; LeGeros, J.P. Biphasic calcium phosphate bioceramics: Preparation, properties and applications. J. Mater. Sci. Mater. Med. 2003, 14, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Kasten, P.; Beyen, I.; Niemeyer, P.; Luginbuhl, R.; Bohner, M.; Richter, W. Porosity and pore size of beta-tricalcium phosphate scaffold can influence protein production and osteogenic differentiation of human mesenchymal stem cells: An in vitro and in vivo study. Acta Biomater. 2008, 4, 1904–1915. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.L.; Faria, P.E.; Grisi, M.F.; Souza, S.L.; Taba, M.J.; Palioto, D.B.; Novaes, A.B.; Fraga, A.F.; Ozyegin, L.S.; Oktar, F.N.; et al. Effects of granule size on the osteoconductivity of bovine and synthetic hydroxyapatite: A histologic and histometric study in dogs. J. Oral Implantol. 2007, 33, 267–276. [Google Scholar] [CrossRef]
- Maté Sánchez de Val, J.E.; Calvo-Guirado, J.L.; Gomez-Moreno, G.; Perez-Albacete, C.; Mazon, P.; De Aza, P.N. Influence of hydroxyapatite granule size, porosity and crystallinity on tissue reaction in vivo. Part A: Synthesis, characterization of the materials and SEM analysis. Clin. Oral Implants Res. 2016, 27, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, P.K.; Benghuzzi, H.A. Ceramic systems for long delivery of chemicals and biological. J. Biomed. Mater. Res. 1988, 22, 1245–1266. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.P.; Song, W.; Esquivel, A.O.; Jackson, N.M.; Nelson, M.; Flynn, J.C.; Markel, D.C. Effect of erythromycin-doped calcium polyphosphate scaffold composite in a mouse pouch infection model. J. Biomed. Mater. Res. Part B Appl. Biomater. 2014, 102, 1140–1147. [Google Scholar] [CrossRef] [PubMed]
- Loca, D.; Sokolova, M.; Locs, J.; Smirnova, A.; Irbe, Z. Calcium phosphate bone cements for local vancomycin delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 49, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Kundu, B.; Soundrapandian, C.; Nandi, S.K.; Mukherjee, P.; Dandapat, N.; Roy, S.; Datta, B.K.; Mandal, T.K.; Basu, D.; Bhattacharya, R.N. Development of new localized drug delivery system based on ceftriaxone-sulbactam composite drug impregnated porous hydroxyapatite: A systematic approach for in vitro and in vivo animal trial. Pharm. Res. 2010, 27, 1659–1676. [Google Scholar] [CrossRef] [PubMed]
- Aneja, A.; Woodall, J.; Wingerter, S.; Tucci, M.; Benghuzzi, H. Analysis of tobramycin release from beta tricalcium phosphate drug delivery system. Biomed. Sci. Instrum. 2008, 44, 88–93. [Google Scholar] [PubMed]
- Gao, S.; Shiota, M.; Fujii, M.; Chen, K.; Shimogishi, M.; Sato, M.; Kasugai, S. Combination of simvastatin and hydroxyapatite fiber induces bone augmentation. Open J. Regen. Med. 2013, 2, 53–60. [Google Scholar] [CrossRef]
- Ozeki, K.; Aoki, H.; Fukui, Y. The effect of adsorbed vitamin D and K to hydroxyapatite on ALP activity of MC3T3-E1 cell. J. Mater. Sci. Mater. Med. 2008, 19, 1753–1757. [Google Scholar] [CrossRef] [PubMed]
- Barroug, A.; Glimcher, M.J. Hydroxyapatite crystals as a local delivery system for cisplatin: Adsorption and release of cisplatin in vitro. J. Orthop. Res. 2002, 20, 274–280. [Google Scholar] [CrossRef]
- Otsuka, M.; Matsuda, Y.; Fox, J.L.; Higuchi, W.I. A novel skeletal drug delivery system using self-setting calcium phosphate cement. 9: Effects of the mixing solution volume on anticancer drug release from homogeneous drug-loaded cement. J. Pharm. Sci. 1995, 6, 733–736. [Google Scholar] [CrossRef]
- Ammon, H.P.; Wahl, M.A. Pharmacology of Curcuma longa. Planta Med. 1991, 57, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lampe, V.; Milobedzka, J. Studien über Curcumin. Ber. Dtsch. Chem. Ges. 1913, 46, 2235–2240. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Harikumar, K.B. Potential therapeutic effects of curcumin, the anti-inflammatory agent, against neurodegenerative, cardiovascular, pulmonary, metabolic, autoimmune and neoplastic diseases. Int. J. Biochem. Cell Biol. 2009, 41, 40–59. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, M.; Sood, S. Role of curcumin in systemic and oral health: An Overview. J. Nat. Sci. Biol. Med. 2013, 4, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Najafi, S.; Khayamzadeh, M.; Paknejad, M.; Poursepanj, G.; KharaziFard, M.J.; Bahador, A. An in vitro comparison of antimicrobial effects of curcumin-based photodynamic therapy and chlorhexidine, on aggregatibacter actinomycetemcomitans. J. Lasers Med. Sci. 2016, 7, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Panhóca, V.H.; Esteban Florez, F.L.; Corrêa, T.Q.; Paolillo, F.R.; de Souza, C.W.; Bagnato, V.S. Oral Decontamination of Orthodontic Patients Using Photodynamic Therapy Mediated by Blue-Light Irradiation and Curcumin Associated with Sodium Dodecyl Sulfate. Photomed. Laser Surg. 2016, 34, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Quishida, C.C.; De Oliveira Mima, E.G.; Jorge, J.H.; Vergani, C.E.; Bagnato, V.S.; Pavarina, A.C. Photodynamic inactivation of a multispecies biofilm using curcumin and LED light. Lasers Med. Sci. 2016, 31, 997–1009. [Google Scholar] [CrossRef] [PubMed]
- Santezi, C.; Tanomaru, J.M.; Bagnato, V.S.; Júnior, O.B.; Dovigo, L.N. Potential of curcumin-mediated photodynamic inactivation to reduce oral colonization. Photodiagn. Photodyn. Ther. 2016, 15, 46–52. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pellissari, C.V.; Pavarina, A.C.; Bagnato, V.S.; Mima, E.G.; Vergani, C.E.; Jorge, J.H. Cytotoxicity of antimicrobial photodynamic inactivation on epithelial cells when co-cultured with Candida albicans. Photochem. Photobiol. Sci. 2016, 15, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, M.; Urolagin, S.S.; Pentyala, K.B.; Urolagin, S.B.; KB, M.; Bhoi, S. Novel Therapeutic approach for the treatment of periodontitis by curcumin. J. Clin. Diagn. Res. 2014, 8, ZC65–ZC69. [Google Scholar] [CrossRef] [PubMed]
- Hugar, S.S.; Patil, S.; Metgud, R.; Nanjwade, B.; Hugar, S.M. Influence of application of chlorhexidine gel and curcumin gel as an adjunct to scaling and root planing: A interventional study. J. Nat. Sci. Biol. Med. 2016, 7, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Hosadurga, R.R.; Rao, S.N.; Jose, J.; Rompicharla, N.C.; Shakil, M.; Shashidhara, R. Evaluation of the efficacy of 2% curcumin gel in the treatment of experimental periodontitis. Pharmacogn. Res. 2014, 6, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Wichitnithad, W.; Nimmannit, U.; Wacharasindhu, S.; Rojsitthisak, P. Synthesis, Characterization and Biological Evaluation of Succinate Prodrugs of Curcuminoids for Colon Cancer Treatment. Molecules 2011, 16, 1888–1900. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. J. Phys. B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Lee, D.S.H.; Pai, Y.; Chang, S. Effect of pH control of mixture solution on the fabrication of the highly pure β-tricalcium phosphate powders synthesized by liquid–solid mixture precipitation method. Mater. Lett. 2013, 102–103, 76–79. [Google Scholar] [CrossRef]
- Slosakczyz, A.; Paszkiewicz, Z.; Paluszkiewisz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet method. J. Mol. Struct. 2005, 744–747, 657–661. [Google Scholar]
- Yashima, M.; Sakai, A.; Kamiyama, T.; Hoshikawa, A. Crystal structure analysis of β-tricalcium phosphate Ca3(PO4)2 by neutron powder diffraction. J. Solid State Chem. 2003, 175, 272–277. [Google Scholar] [CrossRef]
- Sudarsanan, K.; Young, R.A. Significant Precision in Crystal Structural Details: Holly Springs Hydroxyapatite. Acta Crystallogr. Sect. B 1969, 25, 1534–1543. [Google Scholar] [CrossRef]
- França, R.; Samani, T.D.; Bayade, G.; Yahia, L.; Sacher, E. Nanoscale surface characterization of biphasic calcium phosphate, with comparisons to calcium hydroxyapatite and b-tricalcium phosphate bioceramics. J. Colloid Interface Sci. 2014, 420, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Nicolini, V.; Caselli, M.; Ferrari, E.; Menabue, L.; Lusvardi, G.; Saladini, M.; Malavasi, G. SiO2-CaO-P2O5 Bioactive Glasses: A Promising Curcuminoids Delivery System. Materials 2016, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhou, K.; Li, Z.; Zhang, D. Preparation, characterization and in vitro gentamicin release of porous HA microspheres. Mater Sci. Eng. C Mater. Biol. Appl. 2014, 45, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wei, J.; Wei, S. Biological Effects of Osteoblast-Like Cells on Nanohydroxyapatite Particles at a Low Concentration Range. J. Nanomater. 2011, 2010, 104747. [Google Scholar] [CrossRef]
- Huang, Y.C.; Hsiao, P.C.; Chai, H.J. Hydroxyapatite extracted from fish scale: Effects on MG63 osteoblast-like cells. Ceram. Int. 2011, 37, 1825–1831. [Google Scholar] [CrossRef]
- Gu, Q.; Cai, Y.; Huang, C.; Shi, Q.; Yang, H. Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation. Pharmacogn. Mag. 2012, 8, 202–208. [Google Scholar] [PubMed]
| Sample (% HA) | Specific Surface Area BET (m2/g) |
|---|---|
| 0 | 16.779 |
| 13 | 12.547 |
| 41 | 9.742 |
| 100 | 5.077 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xidaki, D.; Agrafioti, P.; Diomatari, D.; Kaminari, A.; Tsalavoutas-Psarras, E.; Alexiou, P.; Psycharis, V.; Tsilibary, E.C.; Silvestros, S.; Sagnou, M. Synthesis of Hydroxyapatite, β-Tricalcium Phosphate and Biphasic Calcium Phosphate Particles to Act as Local Delivery Carriers of Curcumin: Loading, Release and In Vitro Studies. Materials 2018, 11, 595. https://doi.org/10.3390/ma11040595
Xidaki D, Agrafioti P, Diomatari D, Kaminari A, Tsalavoutas-Psarras E, Alexiou P, Psycharis V, Tsilibary EC, Silvestros S, Sagnou M. Synthesis of Hydroxyapatite, β-Tricalcium Phosphate and Biphasic Calcium Phosphate Particles to Act as Local Delivery Carriers of Curcumin: Loading, Release and In Vitro Studies. Materials. 2018; 11(4):595. https://doi.org/10.3390/ma11040595
Chicago/Turabian StyleXidaki, Despoina, Panagiota Agrafioti, Dimitra Diomatari, Archontia Kaminari, Eleftherios Tsalavoutas-Psarras, Polyxeni Alexiou, Vasilios Psycharis, Effie C. Tsilibary, Spyridon Silvestros, and Marina Sagnou. 2018. "Synthesis of Hydroxyapatite, β-Tricalcium Phosphate and Biphasic Calcium Phosphate Particles to Act as Local Delivery Carriers of Curcumin: Loading, Release and In Vitro Studies" Materials 11, no. 4: 595. https://doi.org/10.3390/ma11040595
APA StyleXidaki, D., Agrafioti, P., Diomatari, D., Kaminari, A., Tsalavoutas-Psarras, E., Alexiou, P., Psycharis, V., Tsilibary, E. C., Silvestros, S., & Sagnou, M. (2018). Synthesis of Hydroxyapatite, β-Tricalcium Phosphate and Biphasic Calcium Phosphate Particles to Act as Local Delivery Carriers of Curcumin: Loading, Release and In Vitro Studies. Materials, 11(4), 595. https://doi.org/10.3390/ma11040595






