Efficient Active Oxygen Free Radical Generated in Tumor Cell by Loading-(HCONH2)·H2O2 Delivery Nanosystem with Soft-X-ray Radiotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of HMSNs
2.2. Fabrication of FPO@HMSNs-PAA/FPO@HMSNs-PAA-FITC
2.3. Evaluation of FPO Loading Capacity and pH-Responsive Release Profiles in Different Buffer Solutions
2.4. Measurement of ROS Produced by FPO@HMSNs-PAA under Soft-X-ray Irradiation
2.5. Intracellular Uptake of FPO@HMSNs-PAA
2.6. In Vitro Cytotoxicity Assay of FPO@HMSNs-PAA with and without Soft-X-ray Irradiation
2.7. Measurement of ROS in Tumor Cell
3. Results and Discussion
3.1. Preparation and Characterization of FPO@HMSNs-PAA
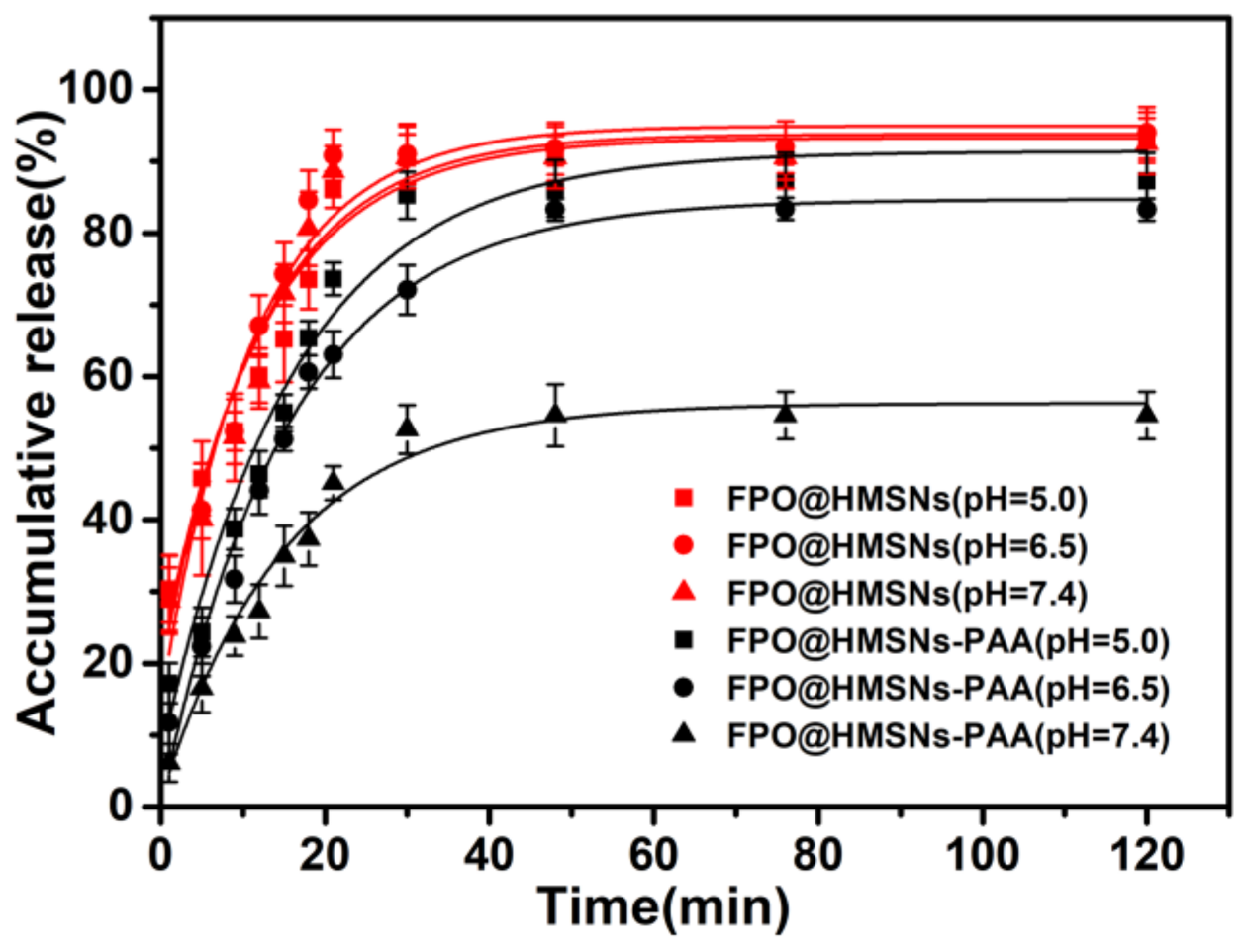
3.2. Evaluation of FPO Loading Capacity and pH-Responsive Release Profiles in Different Buffer Solution (pH 5.0, 6.50 and 7.40)
3.3. ROS Generation of FPO@HMSNs-PAA under Soft-X-ray Irradiation
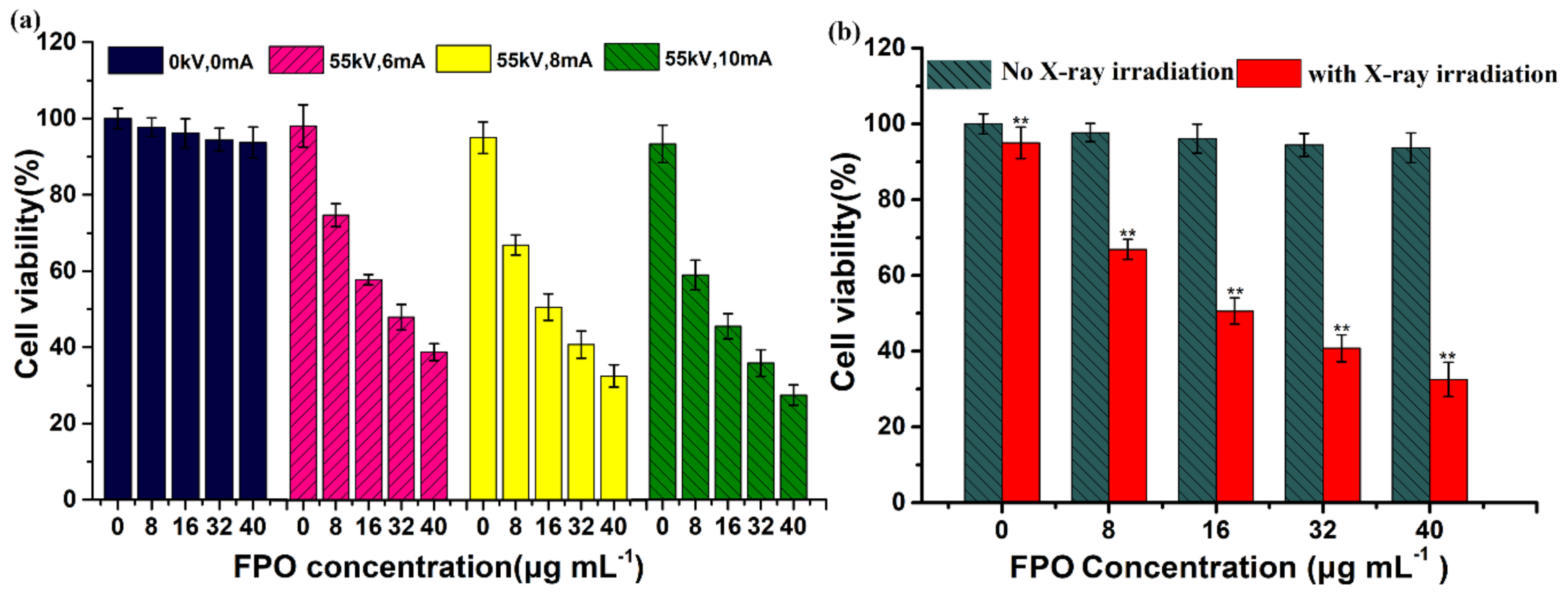
3.4. Cellular Uptake and Cytotoxicity of FPO@HMSNs-PAA
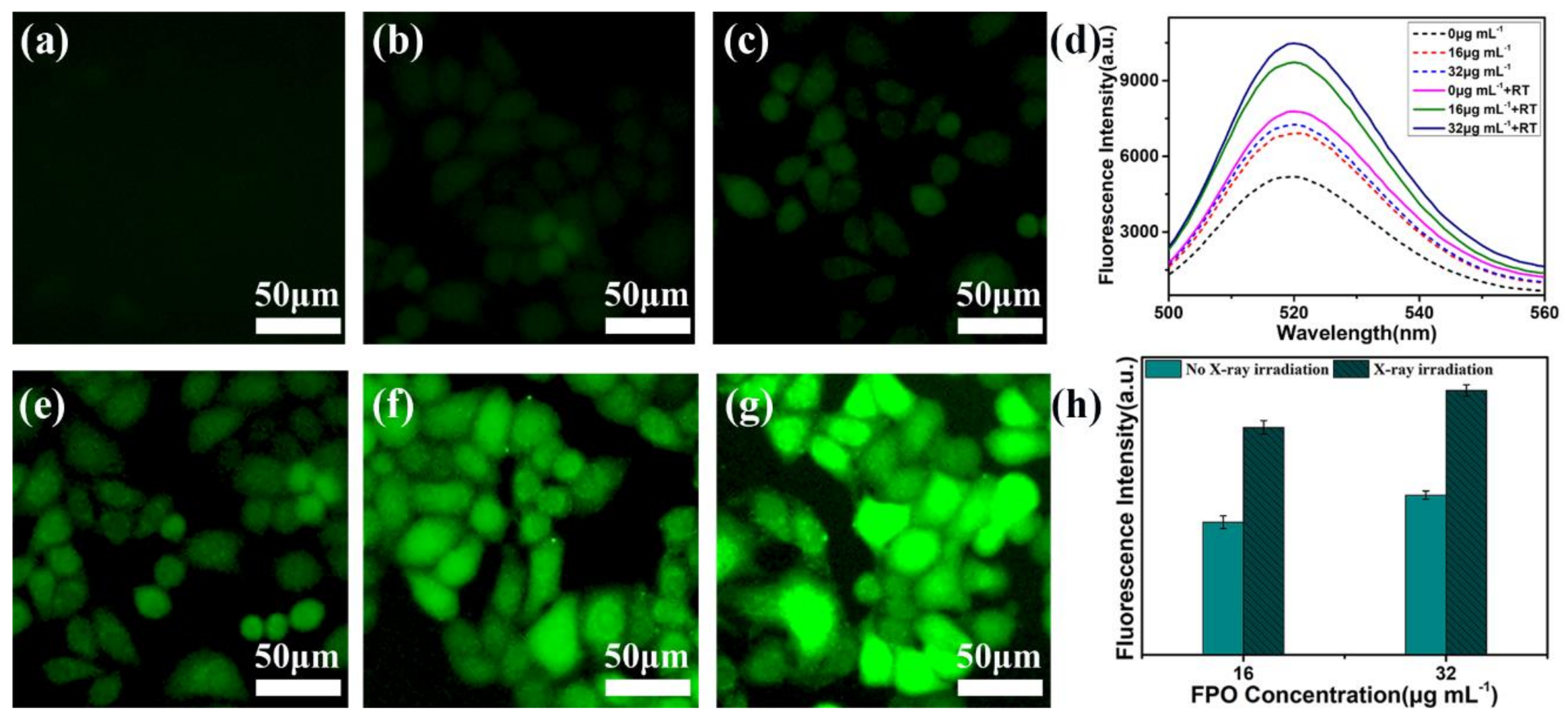
3.5. Observation of Intracellular ROS Generation by Fluorescence Microscopy
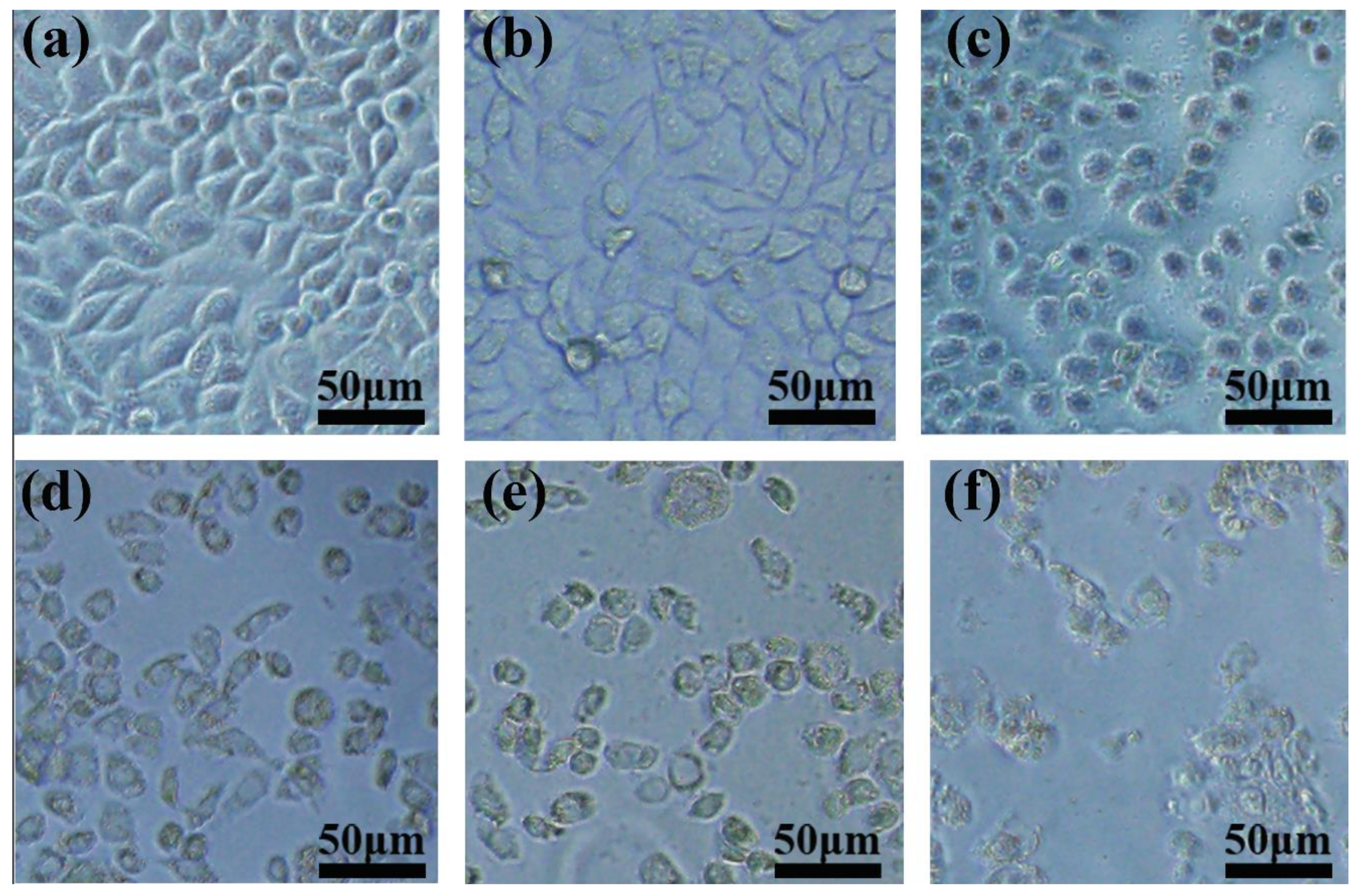
3.6. The Radio-Sensitization of FPO@HMSNs-PAA with Soft-X-ray Irradiation on Tumor Cell
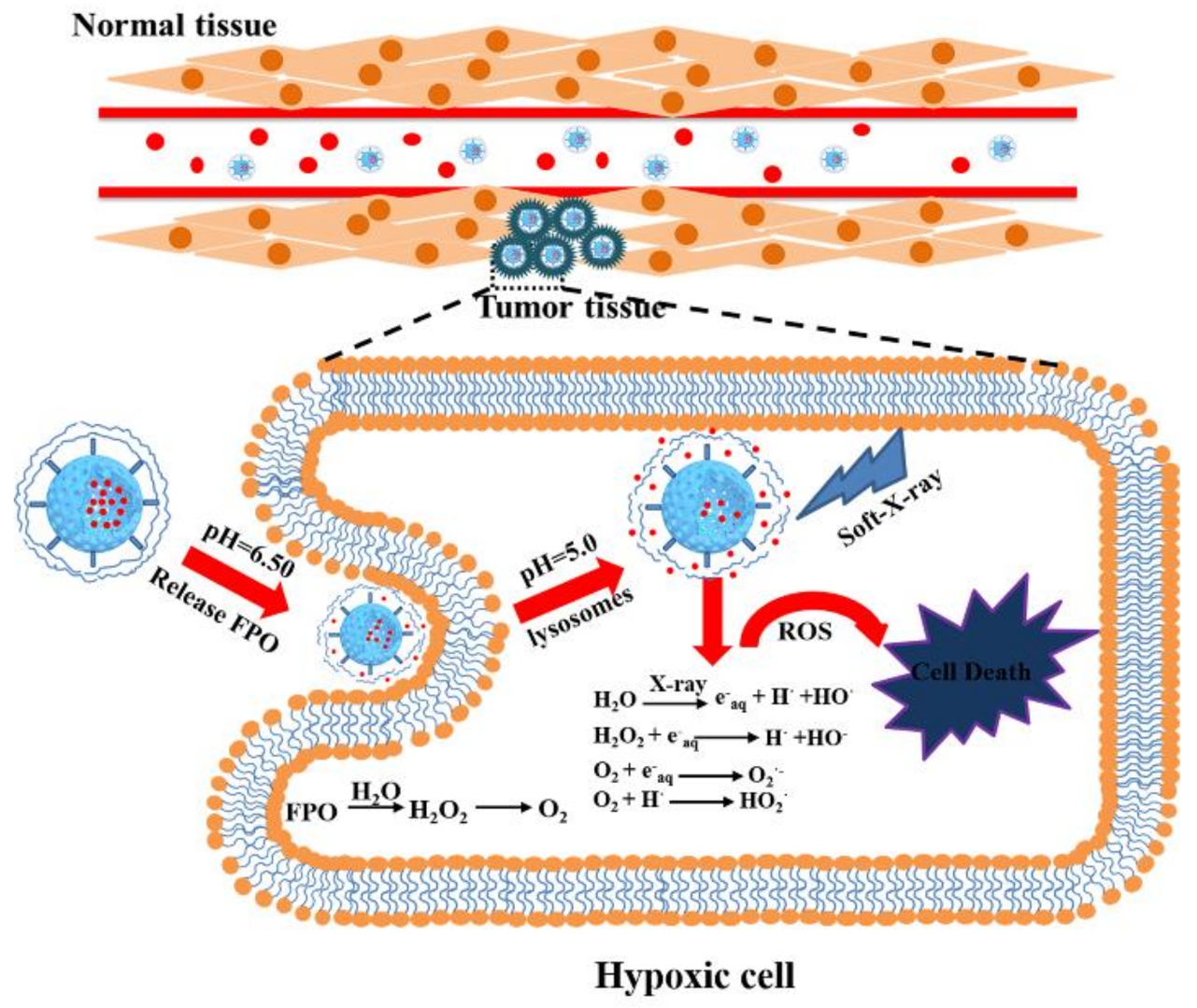
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, Y.; Luo, Y.; Zheng, W.; Chen, T. Rational design of cancer-targeted BSA protein nanoparticles as radiosensitizer to overcome cancer radioresistance. ACS Appl. Mater. Interfaces 2014, 6, 19217–19228. [Google Scholar] [CrossRef] [PubMed]
- Shen, B.; Zhao, K.; Ma, S.; Yuan, D.; Bai, Y. Topotecan-loaded mesoporous silica nanoparticles for reversing multi-drug resistance by synergetic chemoradiotherapy. Chem. Asian J. 2015, 10, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Katakwar, P.; Metgud, R.; Naik, S.; Mittal, R. Oxidative stress marker in oral cancer: A review. J. Cancer Res. Ther. 2016, 12, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Barilla, J.; Lokajicek, M. The role of oxygen in DNA damage by ionizing particles. J. Theor. Biol. 2000, 207, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Koch, C.J. Oxygen effects in radiobiology. Adv. Exp. Med. Biol. 1982, 157, 123–144. [Google Scholar] [PubMed]
- Noman, M.Z.; Hasmim, M.; Messai, Y.; Terry, S.; Kieda, C.; Janji, B.; Chouaib, S. Hypoxia: A key player in antitumor immune response. A review in the theme: Cellular responses to hypoxia. Am. J. Physiol.-Cell Physiol. 2015, 309, C569–C579. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Dai, M.; Liu, Q.; Xiu, R. Oxygen carriers and cancer chemo- and radiotherapy sensitization: Bench to bedside and back. Cancer Treat. Rev. 2007, 33, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Song, X.; Liang, C.; Yi, X.; Song, G.; Chao, Y.; Yang, Y.; Yang, K.; Feng, L.; Liu, Z. Catalase-loaded cisplatin-prodrug-constructed liposomes to overcome tumor hypoxia for enhanced chemo-radiotherapy of cancer. Biomaterials 2017, 138, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Nomura, A.; Iwamoto, Y.; Arakawa, K.; Kashida, A.; Kodera, M.; Hitomi, Y. DNA cleavage through reductive dioxygen activation by iron-bleomycin mimics with carboxamido ligation: Correlation between DNA cleavage efficacy and redox potential. Chem. Lett. 2017, 46, 1109–1111. [Google Scholar] [CrossRef]
- Sinha, B.K.; van’t Erve, T.J.; Kumar, A.; Bortner, C.D.; Motten, A.G.; Mason, R.P. Synergistic enhancement of topotecan-induced cell death by ascorbic acid in human breast mcf-7 tumor cells. Free Radic. Biol. Med. 2017, 113, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Rahbari, M.; Rahlfs, S.; Przyborski, J.M.; Schuh, A.K.; Hunt, N.H.; Fidock, D.A.; Grau, G.E.; Becker, K. Hydrogen peroxide dynamics in subcellular compartments of malaria parasites using genetically encoded redox probes. Sci. Rep. 2017, 7, 10449. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.N.; Bu, W.B.; Shi, J.L. Chemical design and synthesis of functionalized probes for imaging and treating tumor hypoxia. Chem. Rev. 2017, 117, 6160–6224. [Google Scholar] [CrossRef] [PubMed]
- Koukabi, N. Sodium percarbonate: A versatile oxidizing reagent. Synlett 2010, 2010, 2969–2970. [Google Scholar] [CrossRef]
- Pesman, E.; Kalyoncu, E.E.; Kirci, H. Sodium perborate usage instead of hydrogen peroxide for the reinforcement of oxygen delignification. Fibres Text. East. Eur. 2010, 18, 106–109. [Google Scholar]
- Ning, Z.; Qingru, Z.; Lt, A.O.B.; Jieling, J.; Xihong, Z.; Chengjian, Y. Transformation character of percarbamide in acidity and alkalinity soil. J. Agro-Environ. Sci. 2006, 25, 716–721. [Google Scholar]
- Van Elk, M.; Murphy, B.P.; Eufrasio-da-Silva, T.; O’Reilly, D.P.; Vermonden, T.; Hennink, W.E.; Duffy, G.P.; Ruiz-Hernandez, E. Nanomedicines for advanced cancer treatments: Transitioning towards responsive systems. Int. J. Pharm. 2016, 515, 132–164. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Tsai, P.-Y.; Kuthati, Y.; Wei, P.-R.; Liu, C.-L.; Lee, C.-H. Overcoming multidrug resistance through co-delivery of ROS-generating nano-machinery in cancer therapeutics. J. Mater. Chem. B 2017, 5, 1507–1517. [Google Scholar] [CrossRef]
- Zhan, Z.; Zhang, X.; Huang, J.; Huang, Y.; Huang, Z.; Pan, X.; Quan, G.; Liu, H.; Wang, L.; Wu, C. Improved gene transfer with functionalized hollow mesoporous silica nanoparticles of reduced cytotoxicity. Materials 2017, 10, 731. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.X.; Wang, X.; Yang, Y.W. Polymer nanoassembly as delivery systems and anti-bacterial toolbox: From PGMAS to MSN@ PGMAS. Chem. Rec. 2018, 18, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Chen, Z.; Wang, B.; Wang, L.; Lu, T.; Zhang, Z. Reactive oxygen species-manipulated drug release from a smart envelope-type mesoporous titanium nanovehicle for tumor sonodynamic-chemotherapy. ACS Appl. Mater. Interfaces 2015, 7, 28554–28565. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; He, J.M.; Cheng, W.L.; Wu, Y.D.; Hu, Z.; Tian, H.; Huang, Y.D. Redox-responsive nanoreservoirs based on collagen end-capped mesoporous hydroxyapatite nanoparticles for targeted drug delivery. J. Mater. Chem. B 2014, 2, 6089–6096. [Google Scholar] [CrossRef]
- Chen, F.; Zhu, Y.C. Chitosan enclosed mesoporous silica nanoparticles as drug nano-carriers: Sensitive response to the narrow pH range. Microporous Mesoporous Mater. 2012, 150, 83–89. [Google Scholar] [CrossRef]
- Hu, C.L.; Huang, P.; Zheng, Z.; Yang, Z.B.; Wang, X.L. A facile strategy to prepare an enzyme-responsive mussel mimetic coating for drug delivery based on mesoporous silica nanoparticles. Langmuir 2017, 33, 5511–5518. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.L.; Bi, H.T.; Deng, X.R.; Li, C.X.; He, F.; Ma, P.A.; Yang, P.P.; Lin, J. 808 nm near-infrared light controlled dual-drug release and cancer therapy in vivo by upconversion mesoporous silica nanostructures. J. Mater. Chem. B 2017, 5, 2086–2095. [Google Scholar] [CrossRef]
- Kankala, R.K.; Kuthati, Y.; Liu, C.-L.; Mou, C.-Y.; Lee, C.-H. Killing cancer cells by delivering a nanoreactor for inhibition of catalase and catalytically enhancing intracellular levels of ROS. RSC Adv. 2015, 5, 86072–86081. [Google Scholar] [CrossRef]
- Lei, Q.; Qiu, W.-X.; Hu, J.-J.; Cao, P.-X.; Zhu, C.-H.; Cheng, H.; Zhang, X.-Z. Multifunctional mesoporous silica nanoparticles with thermal-responsive gatekeeper for NIR light-triggered chemo/photothermal-therapy. Small 2016, 12, 4286–4298. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Hu, Y.; Cai, K.; Ding, X.; Zhang, Q.; Li, M.; Ma, X.; Zhang, B.; Zeng, Y.; Li, P.; et al. Intracellular redox-activated anticancer drug delivery by functionalized hollow mesoporous silica nanoreservoirs with tumor specificity. Biomaterials 2014, 35, 7951–7962. [Google Scholar] [CrossRef] [PubMed]
- Slowing, I.I.; Trewyn, B.G.; Giri, S.; Lin, V.S.Y. Mesoporous silica nanoparticles for drug delivery and biosensing applications. Adv. Funct. Mater. 2007, 17, 1225–1236. [Google Scholar] [CrossRef]
- Chen, J.; Xue, Z.; Feng, S.; Tu, B.; Zhao, D. Synthesis of mesoporous silica hollow nanospheres with multiple gold cores and catalytic activity. J. Colloid Interface Sci. 2014, 429, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, Z.; Zhang, J.; Luo, T.; Zhou, J.; Zhao, X.; Cai, K. Hollow mesoporous silica nanoparticles facilitated drug delivery via cascade pH stimuli in tumor microenvironment for tumor therapy. Biomaterials 2016, 83, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, H.; Wu, M.; Zhong, Y.; Wang, D.; Jiao, Z. A soft–hard template approach towards hollow mesoporous silica nanoparticles with rough surfaces for controlled drug delivery and protein adsorption. J. Mater. Chem. B 2015, 3, 6480–6489. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, Y.; Wang, J.; Yang, Y.; Li, Y.; Yuan, Y.; Liu, C. Charge-reversal aptes-modified mesoporous silica nanoparticles with high drug loading and release controllability. ACS Appl. Mater. Interfaces 2016, 8, 17166–17175. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.R.; Wang, L.Y.; Fu, J.K.; Shi, C.; Xu, J.Q.; Zhu, Y.C. Efficient free radical generation against cancer cells by low-dose X-ray irradiation with a functional SPC delivery nanosystem. J. Mater. Chem. B 2016, 4, 5863–5872. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y. Synergistic cytotoxicity of low-energy ultrasound and innovative mesoporous silica-based sensitive nanoagents. J. Mater. Sci. 2014, 49, 3665–3673. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Shi, J. Inorganic nanoparticle-based drug codelivery nanosystems to overcome the multidrug resistance of cancer cells. Mol. Pharm. 2014, 11, 2495–2510. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.K.; Shao, Y.R.; Wang, L.Y.; Zhu, Y.C. Lysosome-controlled efficient ROS overproduction against cancer cells with a high pH-responsive catalytic nanosystem. Nanoscale 2015, 7, 7275–7283. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Zhu, Y.; Zhao, Y. Controlled free radical generation against tumor cells by pH-responsive mesoporous silica nanocomposite. J. Mater. Chem. B 2014, 2, 3538. [Google Scholar] [CrossRef]
- Luo, Z.; Cai, K.; Hu, Y.; Zhao, L.; Liu, P.; Duan, L.; Yang, W. Mesoporous silica nanoparticles end-capped with collagen: Redox-responsive nanoreservoirs for targeted drug delivery. Angew. Chem. Int. Ed. 2011, 50, 640–643. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhu, Y.C.; Fu, J.K. Manageable cytotoxicity of nanocapsules immobilizing D-amino acid oxidase via exogenous administration of nontoxic prodrug. Appl. Surf. Sci. 2014, 293, 109–115. [Google Scholar] [CrossRef]
- Cheng, N.; Kang, Q.; Xiao, J.H.; Du, N.; Yu, L. Supramolecular gels: Using an amide-functionalized imidazolium-based surfactant. J. Colloid Interface Sci. 2018, 511, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Nakano, M.; Wada, T.; Koga, N. Exothermic behavior of thermal decomposition of sodium percarbonate: Kinetic deconvolution of successive endothermic and exothermic processes. J. Phys. Chem. A 2015, 119, 9761–9769. [Google Scholar] [CrossRef] [PubMed]
- Bennewitz, M.F.; Lobo, T.L.; Nkansah, M.K.; Ulas, G.; Brudvig, G.W.; Shapiro, E.M. Biocompatible and pH-sensitive PLGA encapsulated MnO nanocrystals for molecular and cellular MRI. ACS Nano 2011, 5, 3438–3446. [Google Scholar] [CrossRef] [PubMed]
- Crayton, S.H.; Tsourkas, A. Ph-titratable superparamagnetic iron oxide for improved nanoparticle accumulation in acidic tumor microenvironments. ACS Nano 2011, 5, 9592–9601. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhou, H.; Zhou, X.; Gong, S.; Xu, H.; Chen, H. Preparation and properties of pH-responsive control release system of paa/chlorpyrifos/amino functionalized mesoporous silica. CIESC J. 2016, 67, 4500–4507. [Google Scholar]
- Illés, E.; Tegze, A.; Kovács, K.; Sági, G.; Papp, Z.; Takács, E.; Wojnárovits, L. Hydrogen peroxide formation during radiolysis of aerated aqueous solutions of organic molecules. Radiat. Phys. Chem. 2017, 134, 8–13. [Google Scholar] [CrossRef]
- Kohanoff, J.; Artacho, E. Water radiolysis by low-energy carbon projectiles from first-principles molecular dynamics. PLoS ONE 2017, 12, e0171820. [Google Scholar] [CrossRef] [PubMed]
- Le Caer, S. Water radiolysis: Influence of oxide surfaces on h-2 production under ionizing radiation. Water 2011, 3, 235–253. [Google Scholar] [CrossRef]
- Zhou, W.; Qin, K.M.; Shan, J.J.; Ju, W.Z.; Liu, S.J.; Cai, B.C.; Di, L.Q. Improvement of intestinal absorption of forsythoside a in weeping forsythia extract by various absorption enhancers based on tight junctions. Phytomedicine 2012, 20, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Meng, H.; Xue, M.; Xia, T.; Ji, Z.X.; Tarn, D.Y.; Zink, J.I.; Nel, A.E. Use of size and a copolymer design feature to improve the biodistribution and the enhanced permeability and retention effect of doxorubicin-loaded mesoporous silica nanoparticles in a murine xenograft tumor model. ACS Nano 2011, 5, 4131–4144. [Google Scholar] [CrossRef] [PubMed]
- Nanda, S.S.; An, S.S.A.; Yi, D.K. Measurement of creatinine in human plasma using a functional porous polymer structure sensing motif. Int. J. Nanomed. 2015, 10, 93–99. [Google Scholar]
- Kankala, R.K.; Liu, C.-G.; Chen, A.-Z.; Wang, S.-B.; Xu, P.-Y.; Mende, L.K.; Liu, C.-L.; Lee, C.-H.; Hu, Y.-F. Overcoming multidrug resistance through the synergistic effects of hierarchical pH-sensitive, ROS-generating nanoreactors. ACS Biomater. Sci. Eng. 2017, 3, 2431–2442. [Google Scholar] [CrossRef]
- Waris, G.; Ahsan, H. Reactive oxygen species: Role in the development of cancer and various chronic conditions. J. Carcinog. 2006, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Cerda, C.; Sanchez, C.; Climent, B.; Vazquez, A.; Iradi, A.; El Amrani, F.; Bediaga, A.; Saez, G.T. Oxidative stress and DNA damage in obesity-related tumorigenesis. In Oxidative Stress and Inflammation in Non-Communicable Diseases—Molecular Mechanisms and Perspectives in Therapeutics; Camps, J., Ed.; Springer: Cham, Switzerland, 2014; Volume 824, pp. 5–17. [Google Scholar]

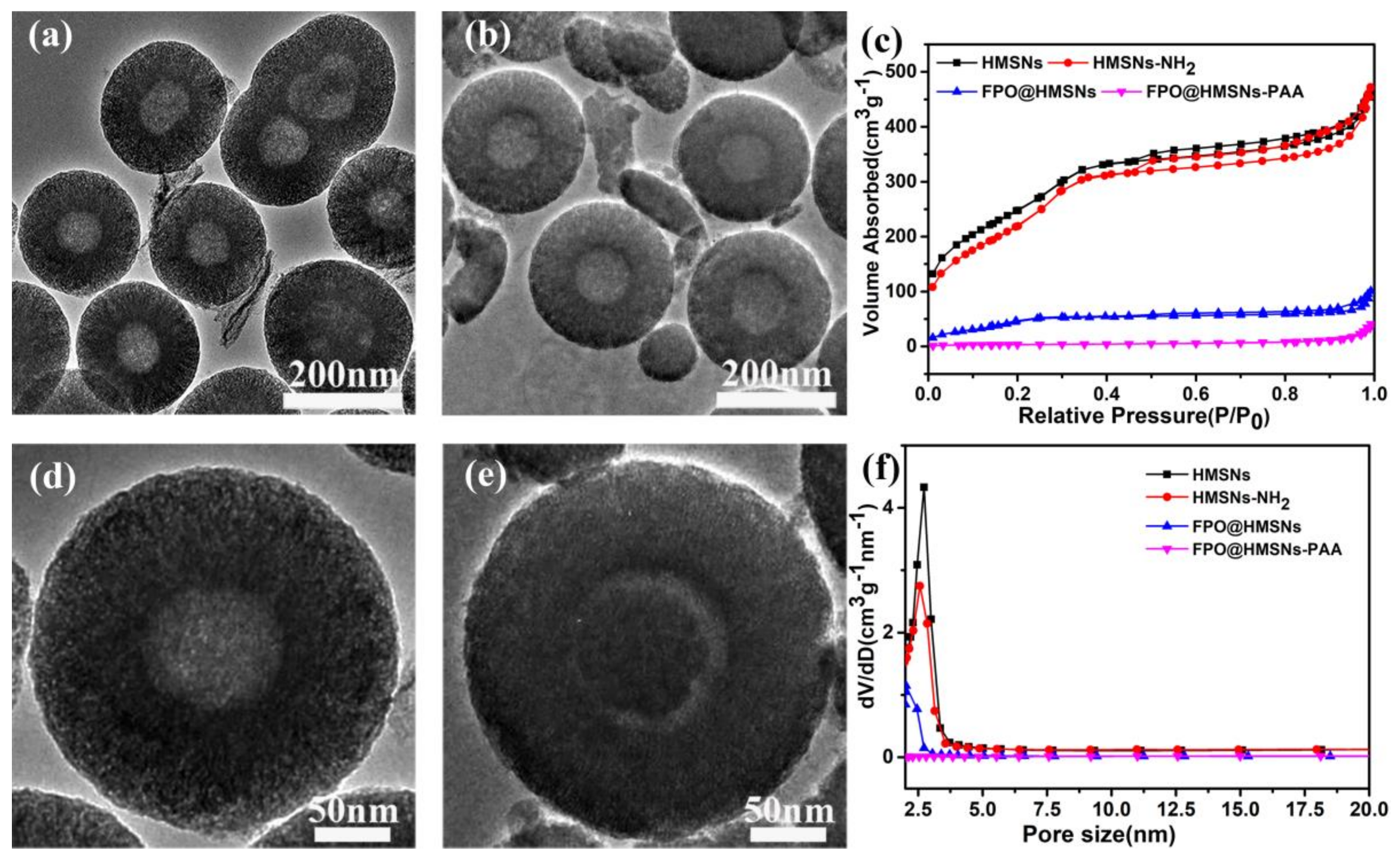
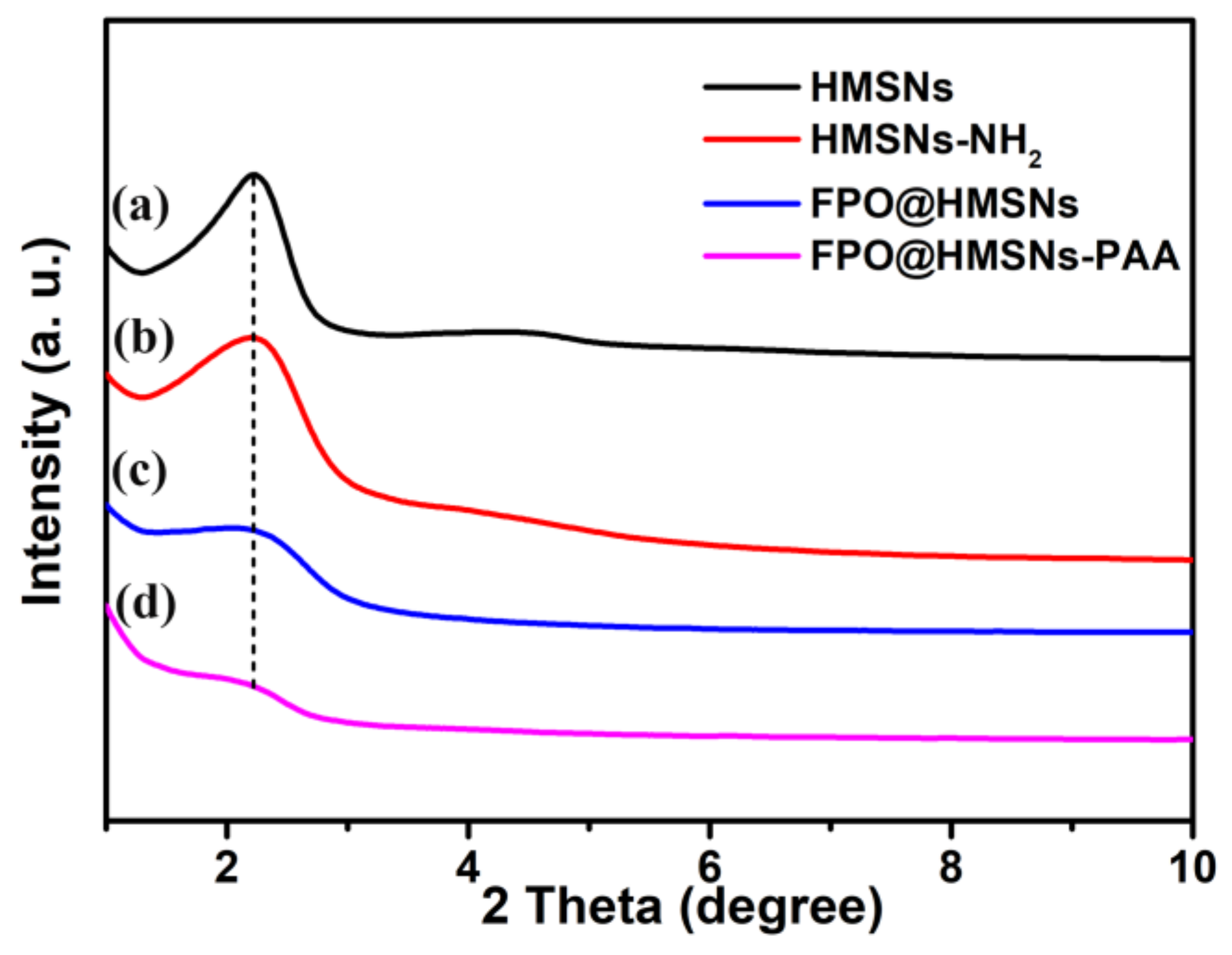
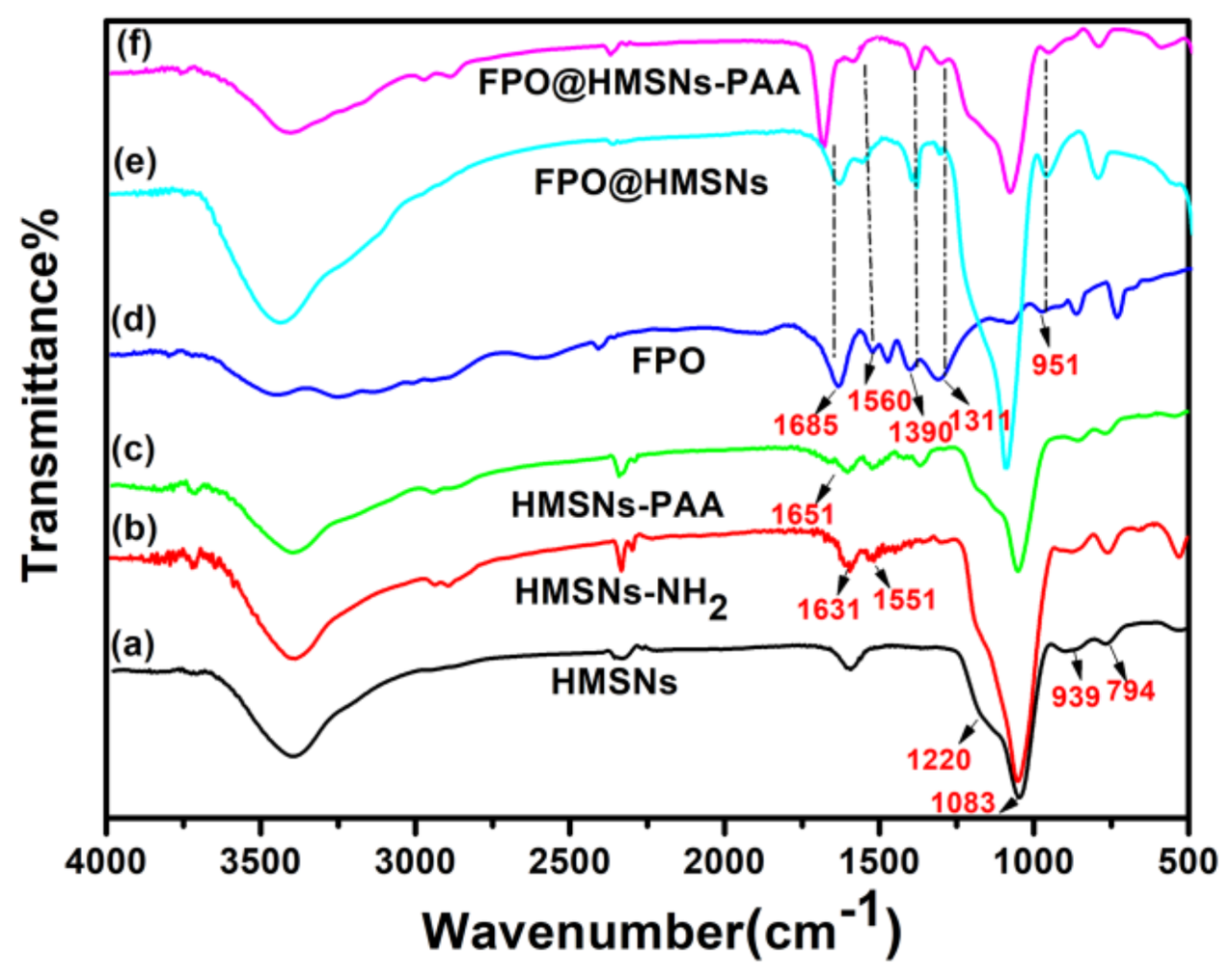
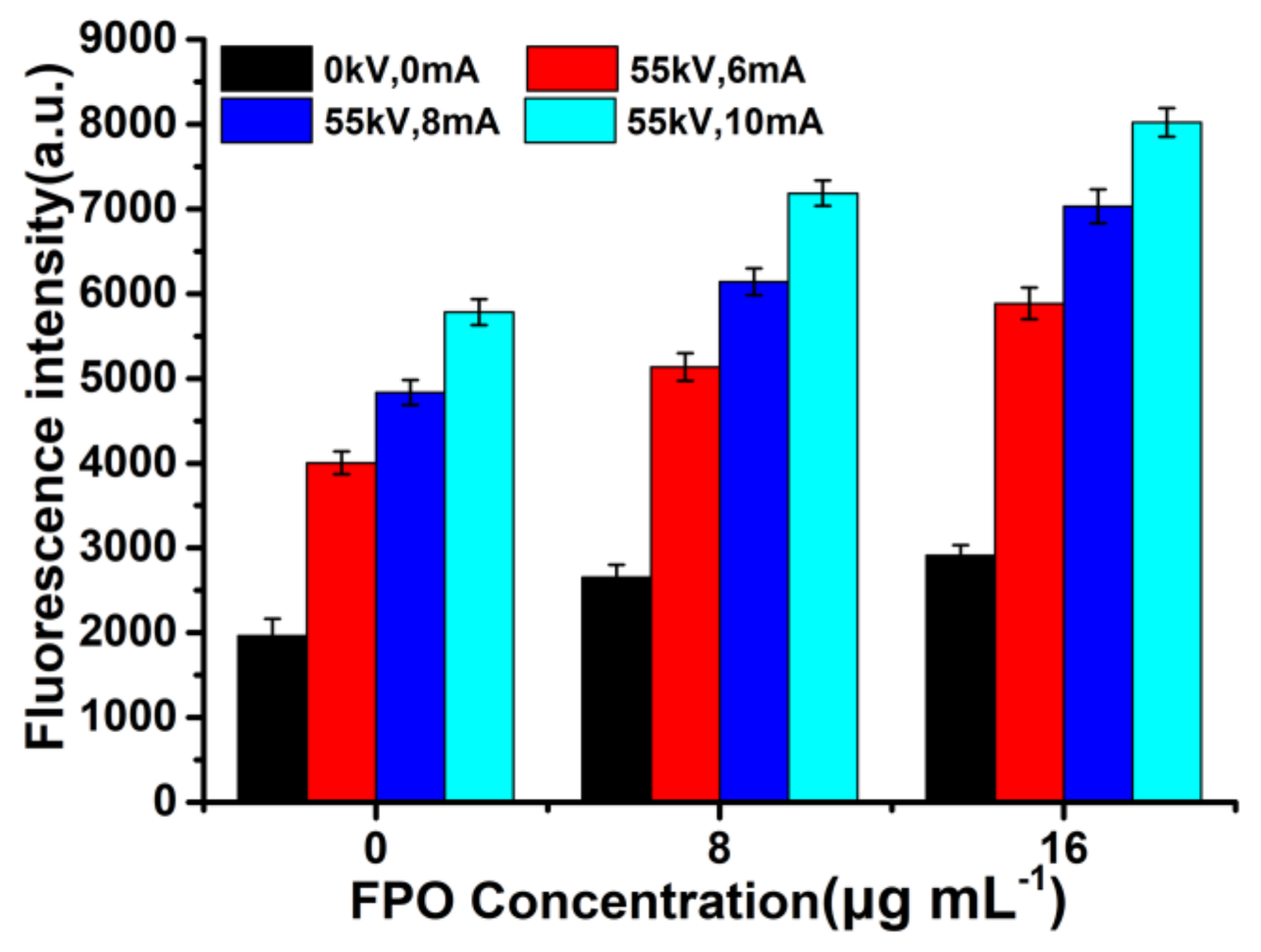
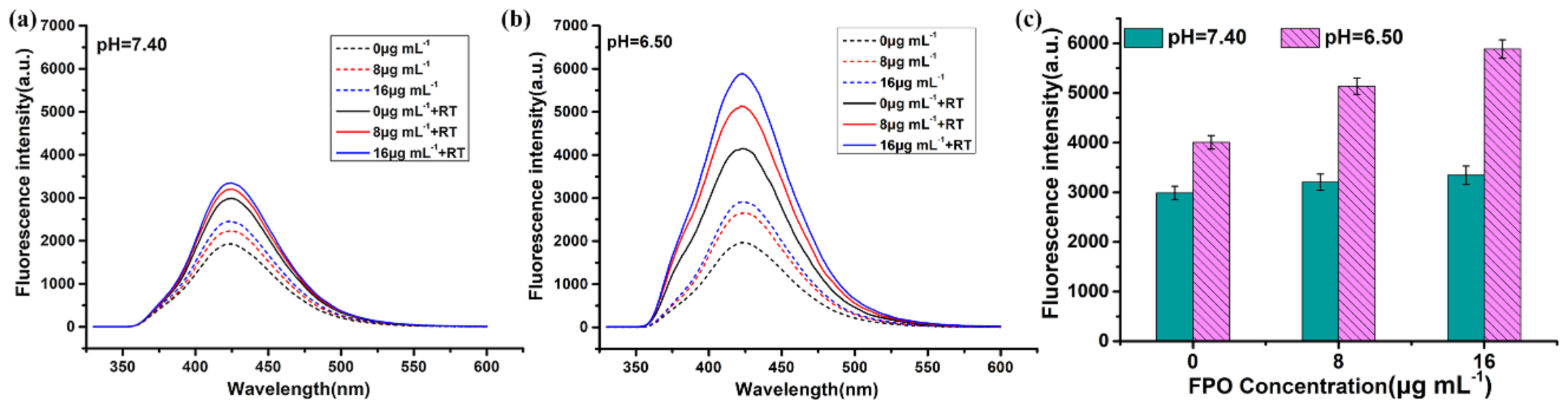
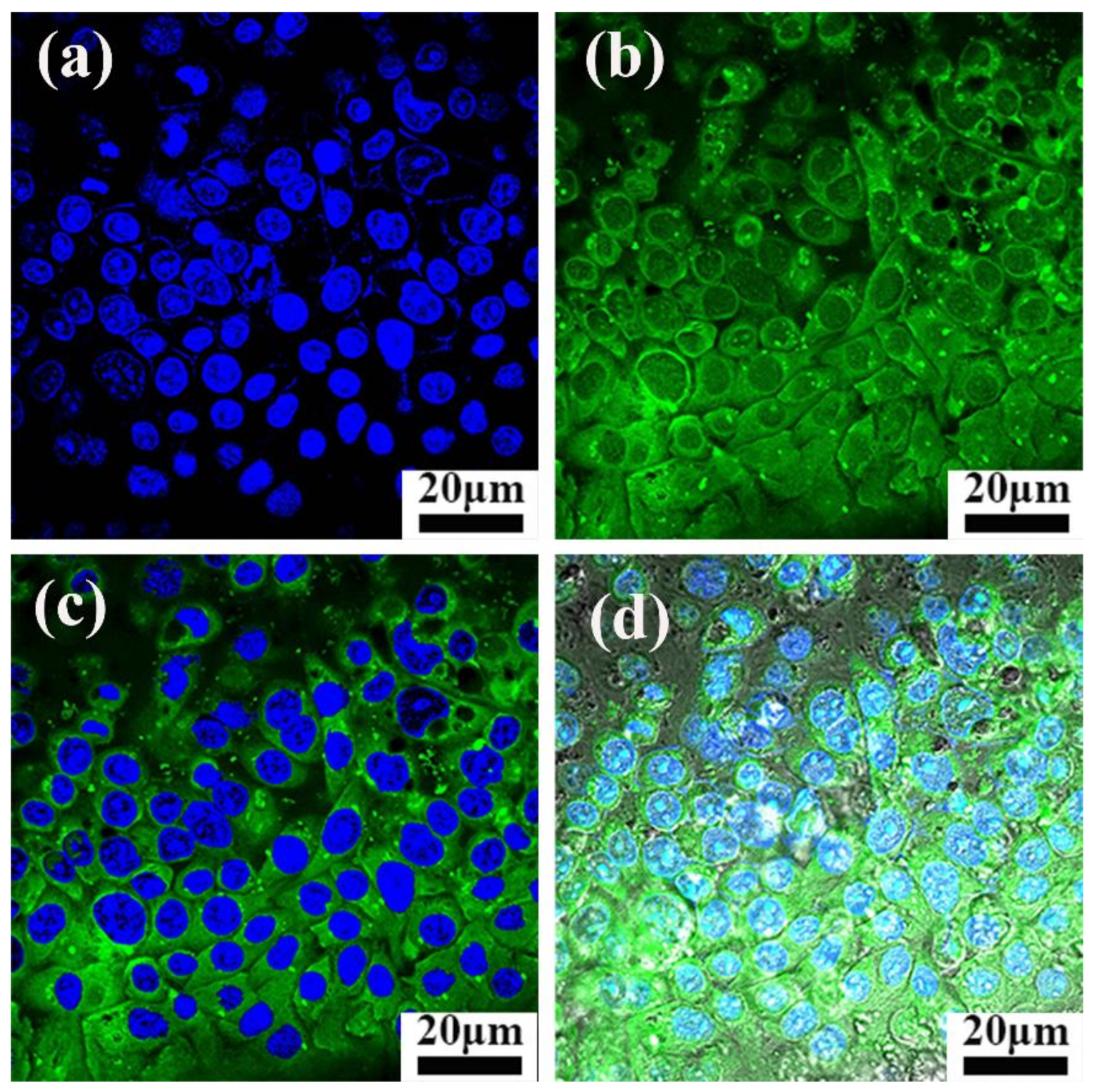

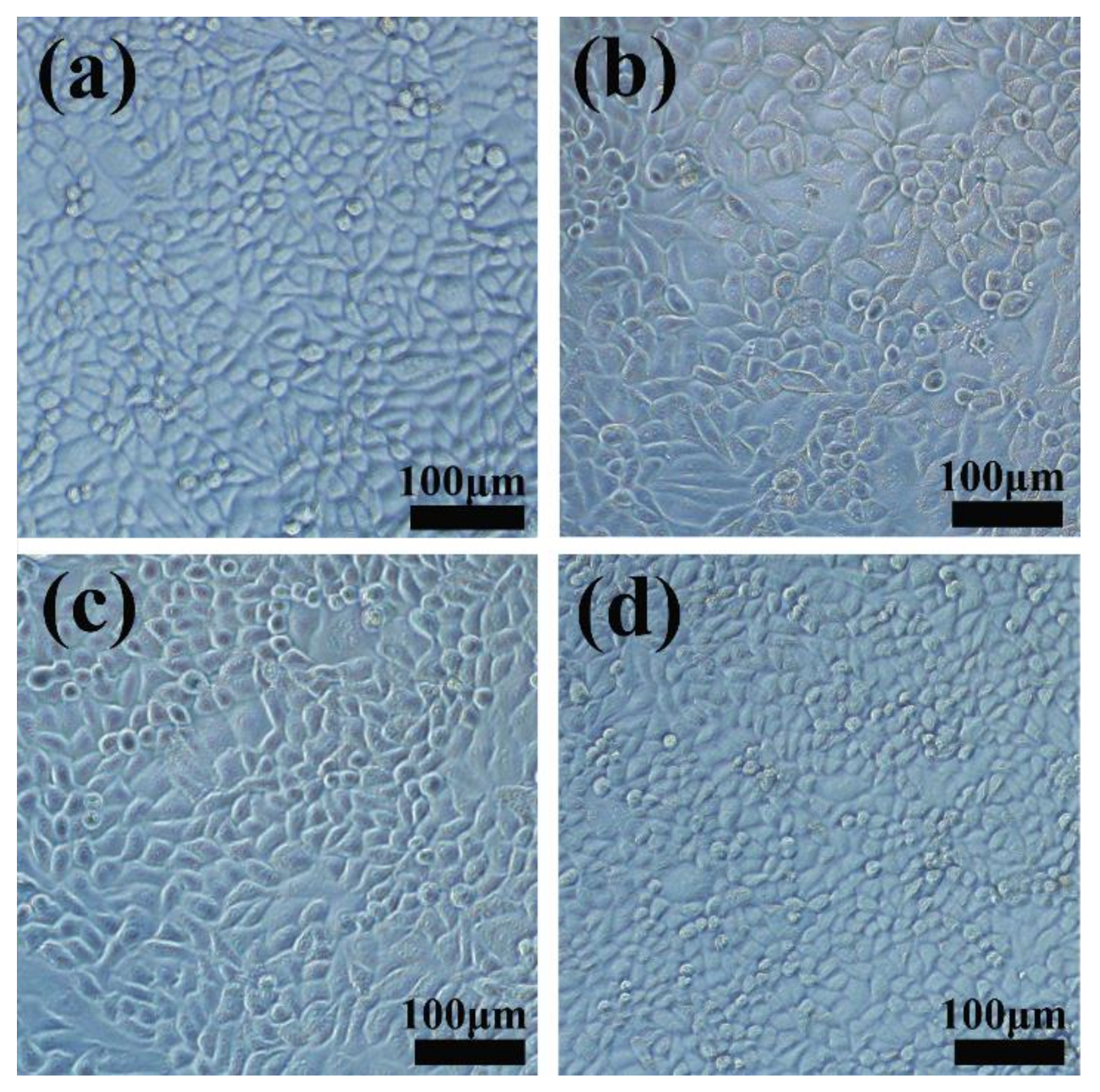
| Sample a | SBET (cm3 g−1) | Vpore (cm3 g−1) | Dpore (nm) | FPO Loading Content | Zeta Potential b (mV) |
|---|---|---|---|---|---|
| HMSNs | 1166 | 0.84 | 2.7 | / | −33.7 ± 1.3 |
| HMSNs-NH2 | 1033 | 0.71 | 2.5 | / | +36.1 ± 3.1 |
| FPO@HMSNs | 282 | 0.28 | 2 | 54.90% | −42.4 ± 1.9 |
| FPO@HMSNs-PAA | 94 | 0.13 | / | 38.32% | −56.5 ± 2.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, L.; Shao, Y.; Chang, C.; Zhu, Y. Efficient Active Oxygen Free Radical Generated in Tumor Cell by Loading-(HCONH2)·H2O2 Delivery Nanosystem with Soft-X-ray Radiotherapy. Materials 2018, 11, 596. https://doi.org/10.3390/ma11040596
Xu L, Shao Y, Chang C, Zhu Y. Efficient Active Oxygen Free Radical Generated in Tumor Cell by Loading-(HCONH2)·H2O2 Delivery Nanosystem with Soft-X-ray Radiotherapy. Materials. 2018; 11(4):596. https://doi.org/10.3390/ma11040596
Chicago/Turabian StyleXu, Lei, Yiran Shao, Chengkang Chang, and Yingchun Zhu. 2018. "Efficient Active Oxygen Free Radical Generated in Tumor Cell by Loading-(HCONH2)·H2O2 Delivery Nanosystem with Soft-X-ray Radiotherapy" Materials 11, no. 4: 596. https://doi.org/10.3390/ma11040596
APA StyleXu, L., Shao, Y., Chang, C., & Zhu, Y. (2018). Efficient Active Oxygen Free Radical Generated in Tumor Cell by Loading-(HCONH2)·H2O2 Delivery Nanosystem with Soft-X-ray Radiotherapy. Materials, 11(4), 596. https://doi.org/10.3390/ma11040596




