UiO-66-NH2/GO Composite: Synthesis, Characterization and CO2 Adsorption Performance
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of UiO-66-NH2/GO Composites
2.2. Characterizations
2.3. CO2 Adsorption Experiment
2.4. Chemical Stability Test
3. Results and Discussion
3.1. Structure Characterization
3.2. Chemical Characteristics
3.3. Stability Analysis
3.4. Low Pressure CO2 Adsorption
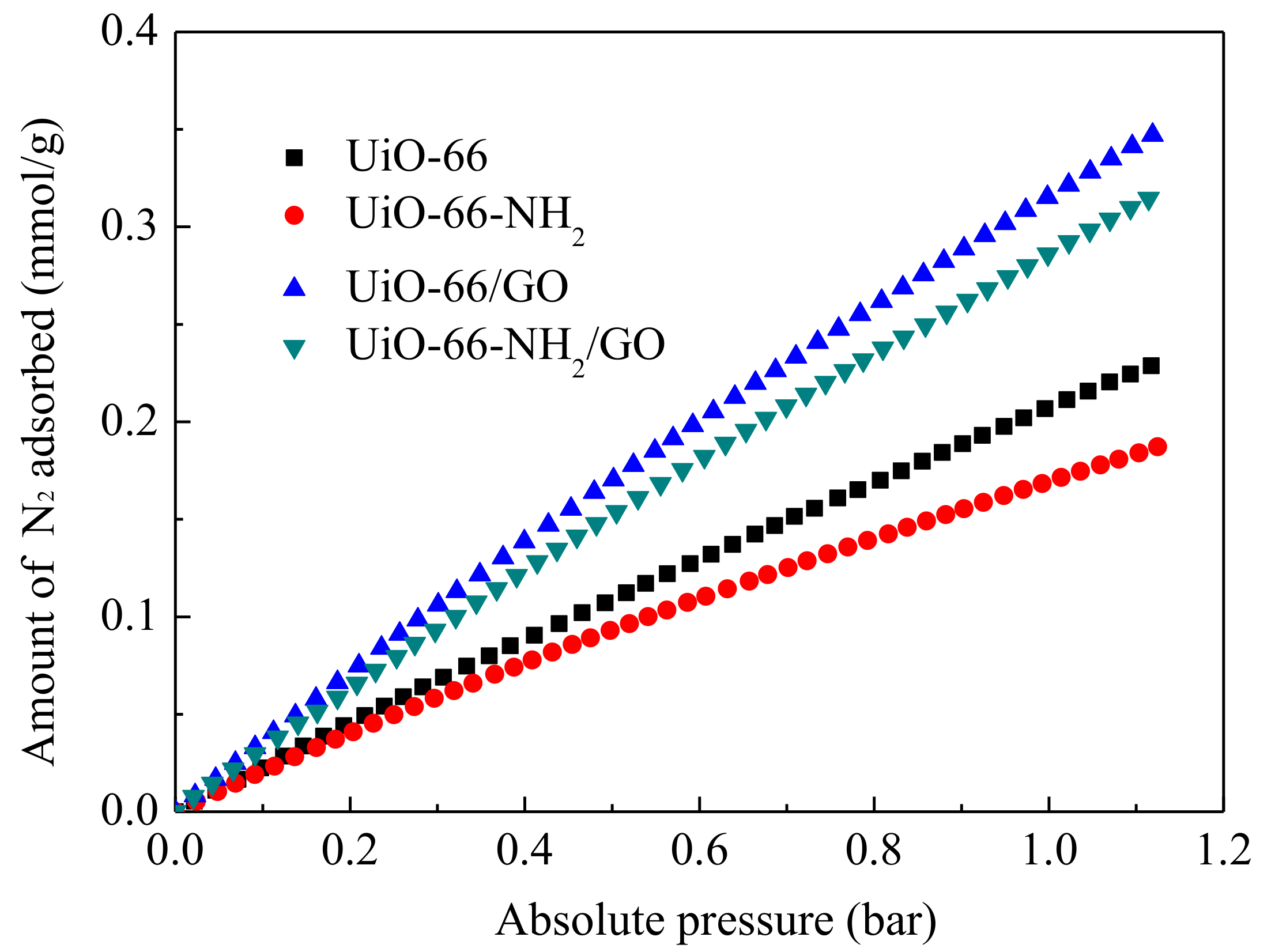
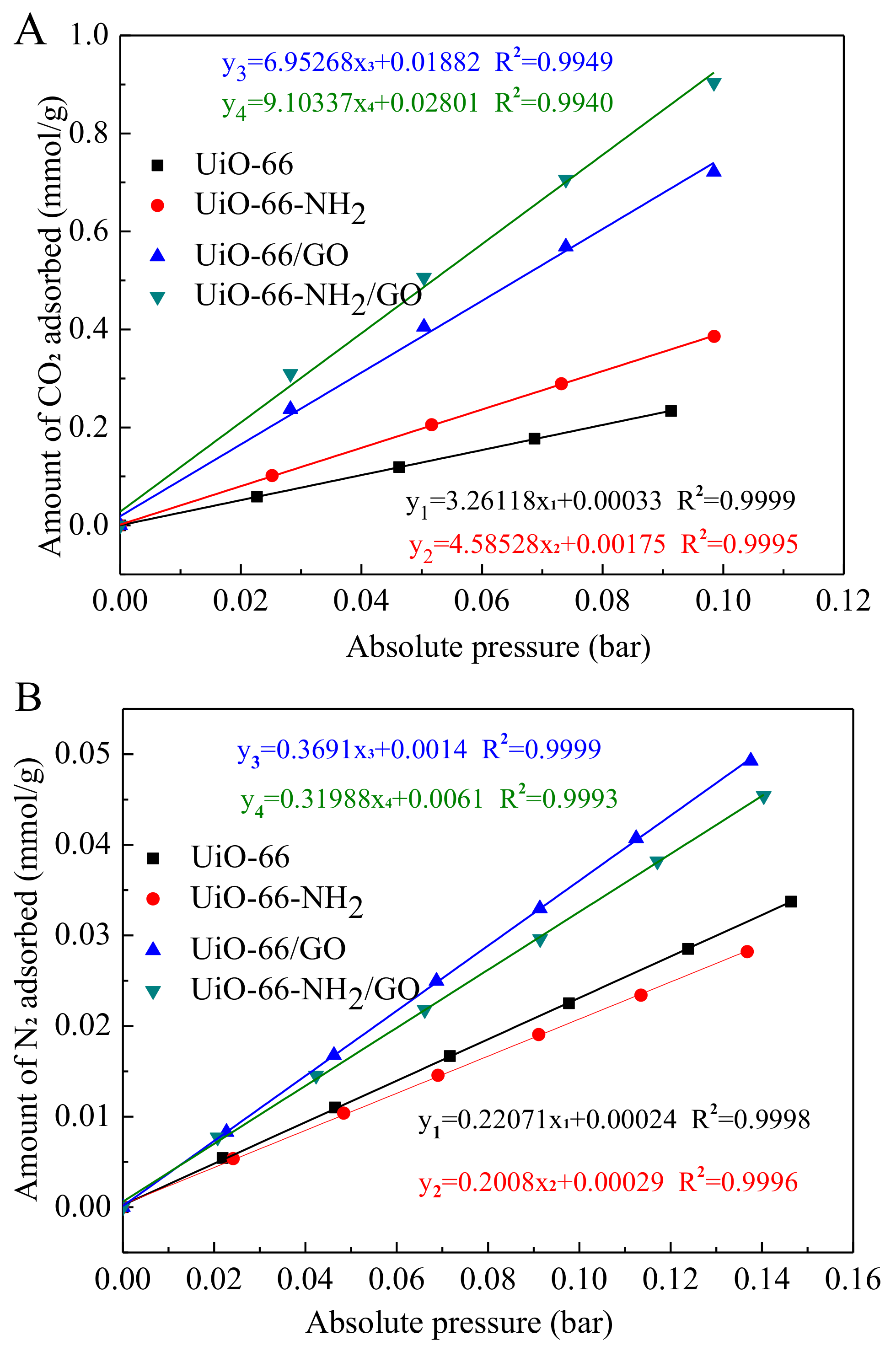
3.5. Adsorption Selectivity of CO2/N2
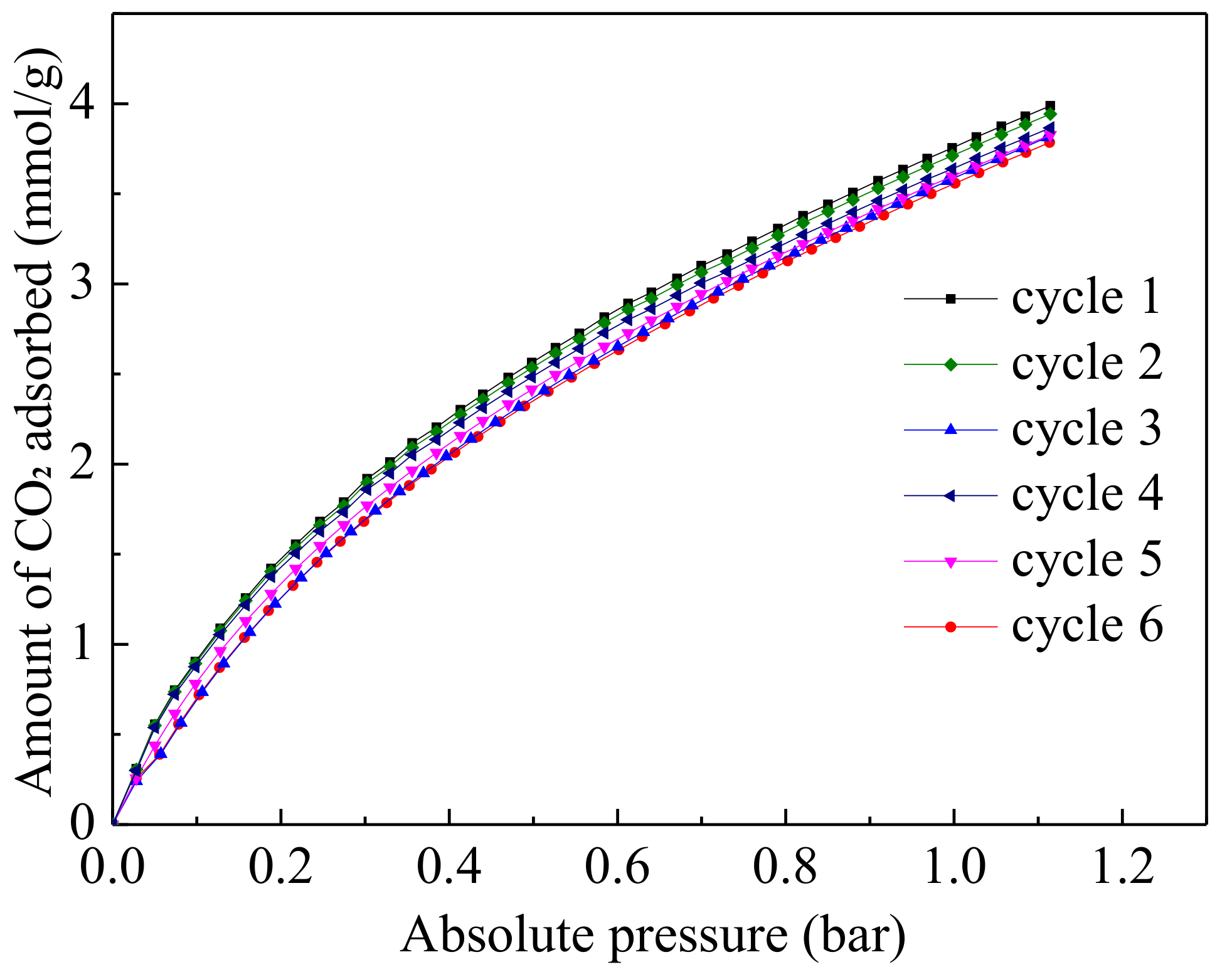
3.6. Multiple Cycles of CO2 Adsorption–Desorption
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Millward, A.R.; Yaghi, O.M. Metal-organic frameworks with exceptionally high capacity for storage of carbon dioxide at room temperature. J. Am. Chem. Soc. 2005, 127, 17998–17999. [Google Scholar] [CrossRef] [PubMed]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.H.; Ding, J.; Huang, X.; Wei, X.L.; Wang, W.L. Experimental and Computational Investigation of CO2 Capture on Mix-ligand Metal-organic Framework UiO-66. Energy Procedia 2017, 105, 4395–4401. [Google Scholar] [CrossRef]
- Chavan, S.; Vitillo, J.G.; Gianolio, D.; Zavorotynska, O.; Civalleri, B.; Jakobsen, S.; Nilsen, M.H.; Valenzano, L.; Lamberti, C.; Lillerud, K.P.; et al. H2 storage in isostructural UiO-67 and UiO-66 MOFs. Phys. Chem. Chem. Phys. 2012, 14, 1614–1626. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Jobic, H.; Salles, F.; Kolokolov, D.; Guillerm, V.; Serre, C.; Maurin, G. Probing the dynamics of CO2 and CH4 within the porous zirconium terephthalate UiO-66(Zr): A synergic combination of neutron scattering measurements and molecular simulations. Chemistry 2011, 17, 8882–8889. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qin, W.; Li, Z.; Li, Y. Enhanced stability and CO2 affinity of a UiO-66 type metal-organic framework decorated with dimethyl groups. Dalton Trans. 2012, 41, 9283–9285. [Google Scholar] [CrossRef] [PubMed]
- Garibay, S.J.; Cohen, S.M. Isoreticular synthesis and modification of frameworks with the UiO-66 topology. Chem. Commun. 2010, 46, 7700–7702. [Google Scholar] [CrossRef] [PubMed]
- Cmarik, G.E.; Kim, M.; Cohen, S.M.; Walton, K.S. Tuning the adsorption properties of UiO-66 via ligand functionalization. Langmuir 2012, 28, 15606–15613. [Google Scholar] [CrossRef] [PubMed]
- Ethiraj, J.; Albanese, E.; Civalleri, B.; Vitillo, J.G.; Bonino, F.; Chavan, S.; Shearer, G.C.; Lillerud, K.P.; Bordiga, S. Carbon dioxide adsorption in amine-functionalized mixed-ligand metal-organic frameworks of UiO-66 topology. ChemSusChem 2014, 7, 3382–3388. [Google Scholar] [CrossRef] [PubMed]
- Kandiah, M.; Nilsen, M.H.; Usseglio, S.; Jakobsen, S.; Olsbye, U.; Tilset, M.; Larabi, C.; Quadrelli, E.A.; Bonino, F.; Lillerud, K.P. Synthesis and stability of tagged UiO-66 Zr-MOFs. Chem. Mater. 2010, 2, 6632–6640. [Google Scholar] [CrossRef]
- Kandiah, M.; Usseglio, S.; Svelle, S.; Olsbye, U.; Lillerud, K.P.; Tilset, M. Post-synthetic modification of the metal-organic framework compound UiO-66. J. Mater. Chem. 2010, 20, 9848–9851. [Google Scholar] [CrossRef]
- Yang, Q.; Wiersum, A.D.; Llewellyn, P.L.; Guillerm, V.; Serre, C.; Maurin, G. Functionalizing porous zirconium terephthalate UiO-66(Zr) for natural gas upgrading: A computational exploration. Chem. Commun. 2011, 47, 9603–9605. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Wiersum, A.D.; Jobic, H.; Guillerm, V.; Serre, C.; Llewellyn, P.L.; Maurin, G. Understanding the thermodynamic and kinetic behavior of the CO2/CH4 gas mixture within the porous zirconium terephthalate UiO-66(Zr): A joint experimental and modeling approach. J. Phys. Chem. C 2011, 115, 13768–13774. [Google Scholar] [CrossRef]
- Stavitski, E.; Pidko, E.A.; Couck, S.; Remy, T.; Hensen, E.J.; Weckhuysen, B.M.; Denayer, J.; Gascon, J.; Kapteijn, F. Complexity behind CO2 capture on NH2-MIL-53(Al). Langmuir 2011, 27, 3970–3976. [Google Scholar] [CrossRef] [PubMed]
- Vaidhyanathan, R.; Iremonger, S.S.; Dawson, K.W.; Shimizu, G.K.H. An amine-functionalized metal organic framework for preferential CO2 adsorption at low pressures. Chem. Commun. 2009, 35, 5230–5232. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Zhao, Y.X.; Lv, Z.J.; Song, F.J.; Zhong, Q. Preparation and enhanced CO2 adsorption capacity of UiO-66/graphene oxide composites. J. Ind. Eng. Chem. 2015, 27, 102–107. [Google Scholar] [CrossRef]
- Zhao, Y.X.; Ding, H.L.; Zhong, Q. Synthesis and characterization of MOF-aminated graphite oxide composites for CO2 capture. Appl. Surf. Sci. 2013, 284, 138–144. [Google Scholar] [CrossRef]
- Abid, H.R.; Shang, J.; Ang, H.; Wang, S. Amino-functionalized Zr-MOF nanoparticles for adsorption of CO2 and CH4. Int. J. Smart Nano Mater. 2013, 4, 72–82. [Google Scholar] [CrossRef]
- Gascon, J.; Aktay, U.; Hernandezalonso, M.; Vanklink, G.; Kapteijn, F. Amino-based metal-organic frameworks as stable, highly active basic catalysts. J. Catal 2009, 261, 75–87. [Google Scholar] [CrossRef]
- Boutin, A.; Couck, S.; Coudert, F.; Serra-Crespo, P.; Gascon, J.; Kapteijn, F.; Fuchs, A.H.; Denayer, J.F.M. Thermodynamic analysis of the breathing of amino-functionalized MIL-53(Al) upon CO2 adsorption. Microporous Mesoporous Mater. 2011, 140, 108–113. [Google Scholar] [CrossRef]
- Nik, O.G.; Chen, X.Y.; Kaliaguine, S. Functionalized metal organic framework-polyimide mixed matrix membranes for CO2/CH4 separation. J. Membr. Sci. 2012, 413–414, 48–61. [Google Scholar] [CrossRef]
- Vermoortele, F.; Ameloot, R.; Vimont, A.; Serre, C.; Vos, D.D. An amino-modified Zr-terephthalate metal-organic framework as an acid-base catalyst for crossaldol condensation. Chem. Commun. 2011, 47, 1521–1523. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Y.; Vinh-Thang, H.; Rodrigue, D.; Kaliaguine, S. Amine-functionalized MIL-53 metal-organic framework in polyimide mixed matrix membranes for CO2/CH4 separation. Ind. Eng. Chem. Res. 2012, 51, 6895–6906. [Google Scholar] [CrossRef]
- Marx, S.; Lleist, W.; Huang, H.; Maciejewski, M.; Baiker, A. Tuning functional sites and thermal stability of mixed-linker MOFs based on MIL-53(Al). Dalton Trans. 2010, 39, 3795–3798. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, Z.; Lin, Y.S. Adsorption and diffusion of carbon dioxide on metal-organic framework (MOF-5). Ind. Eng. Chem. Res. 2009, 48, 10015–10020. [Google Scholar] [CrossRef]
- Walton, K.S.; Millward, A.R.; Dubbeldam, D.; Frost, H.; Low, J.; Yaghi, O.M.; Sunrr, R.Q. Understanding inflections and steps in carbon dioxide adsorption isotherms in metal-organic frameworks. J. Am. Chem. Soc. 2017, 130, 406–407. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Marshall, M.; Chaffee, A.L. CO2 Adsorption-based separation by metal organic framework (Cu-BTC) versus Zeolite (13X). Energy Fuels 2009, 23, 2785–2789. [Google Scholar] [CrossRef]
- Skoulidas, A.I.; Sholl, D.S. Self-diffusion and transport diffusion of light gases in metal-organic framework materials assessed using molecular dynamics simulations. J. Phys. Chem. B 2015, 109, 15760–15768. [Google Scholar] [CrossRef] [PubMed]
- Kondo, S.; Tatsuo, I. AbeI, Adsorption Science; Chemistry Industry Press: Beijing, China, 2005. [Google Scholar]
- Hui, W.; Shen, Y.C.; Vaiva, K.; Madhusudan, T.; Ping, C.; Taner, Y.; Wei, Z. Unusual and highly tunable missing-linker defects in zirconium metal-organic framework UiO-66 and their important effects on gas adsorption. J. Am. Chem. Soc. 2013, 135, 10525–10532. [Google Scholar]
- Aprea, P.; Caputo, D.; Gargiulo, N.; Iucolano, F.; Pepe, F. Modeling carbon dioxide adsorption on microporous substrates: Comparison between Cu-BTC metal-organic framework and 13X zeolitic molecular sieve. J. Chem. Eng. Data 2010, 55, 3655–3661. [Google Scholar] [CrossRef]
- An, J.; Geib, S.J.; Rosi, N.L. High and selective CO2 uptake in a cobalt adeninate metal-organic framework exhibiting pyrimidine and amino-decorated pores. J. Am. Chem. Soc. 2010, 132, 38–39. [Google Scholar] [CrossRef] [PubMed]
- Saha, D.; Bao, Z.; Jia, F.; Deng, S.G. Adsorption of CO2, CH4, N2O, and N2 on MOF-5, MOF-177, and zeolite 5A. Environ. Sci. Technol. 2010, 44, 1820–1826. [Google Scholar] [CrossRef] [PubMed]



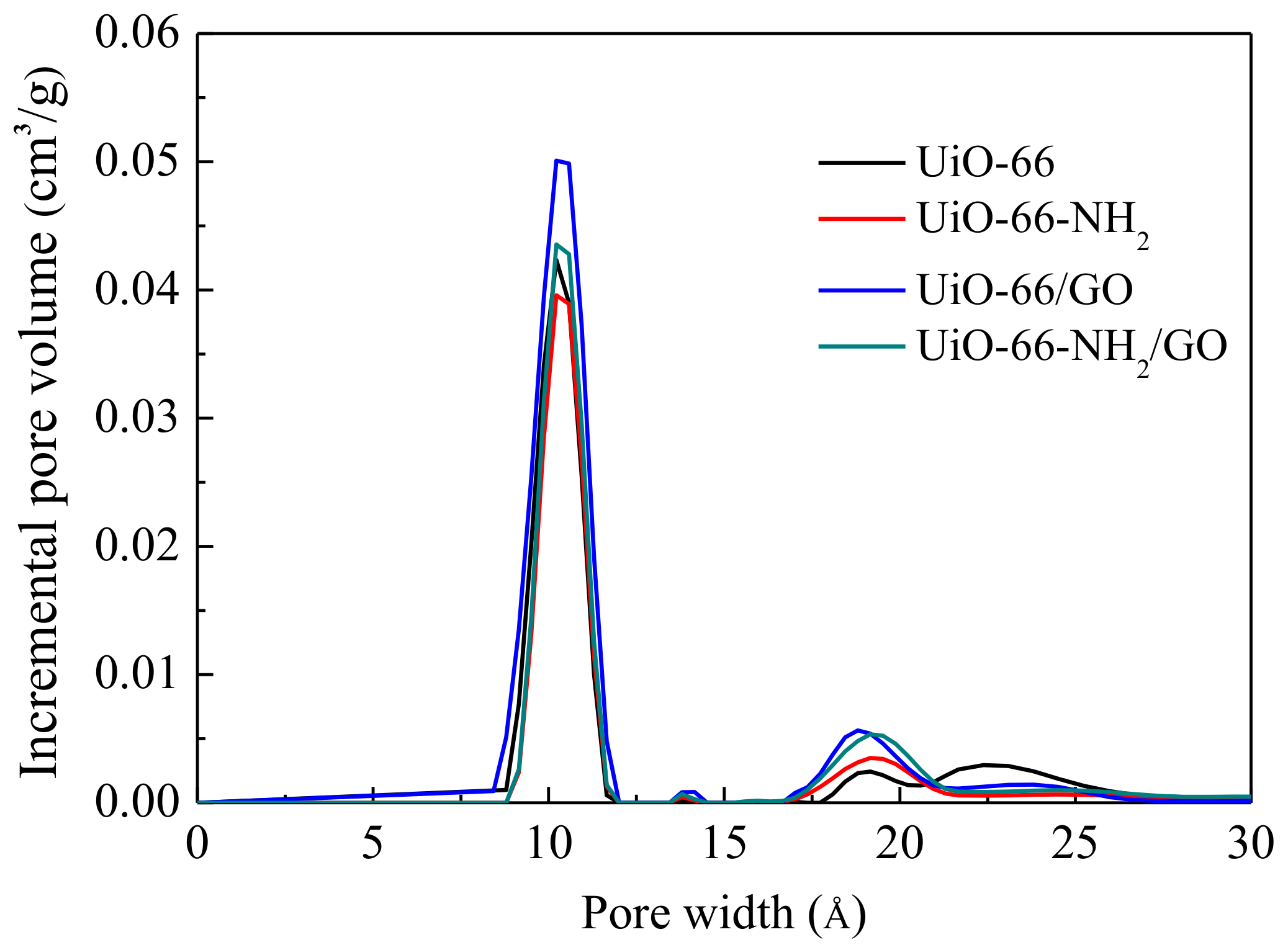
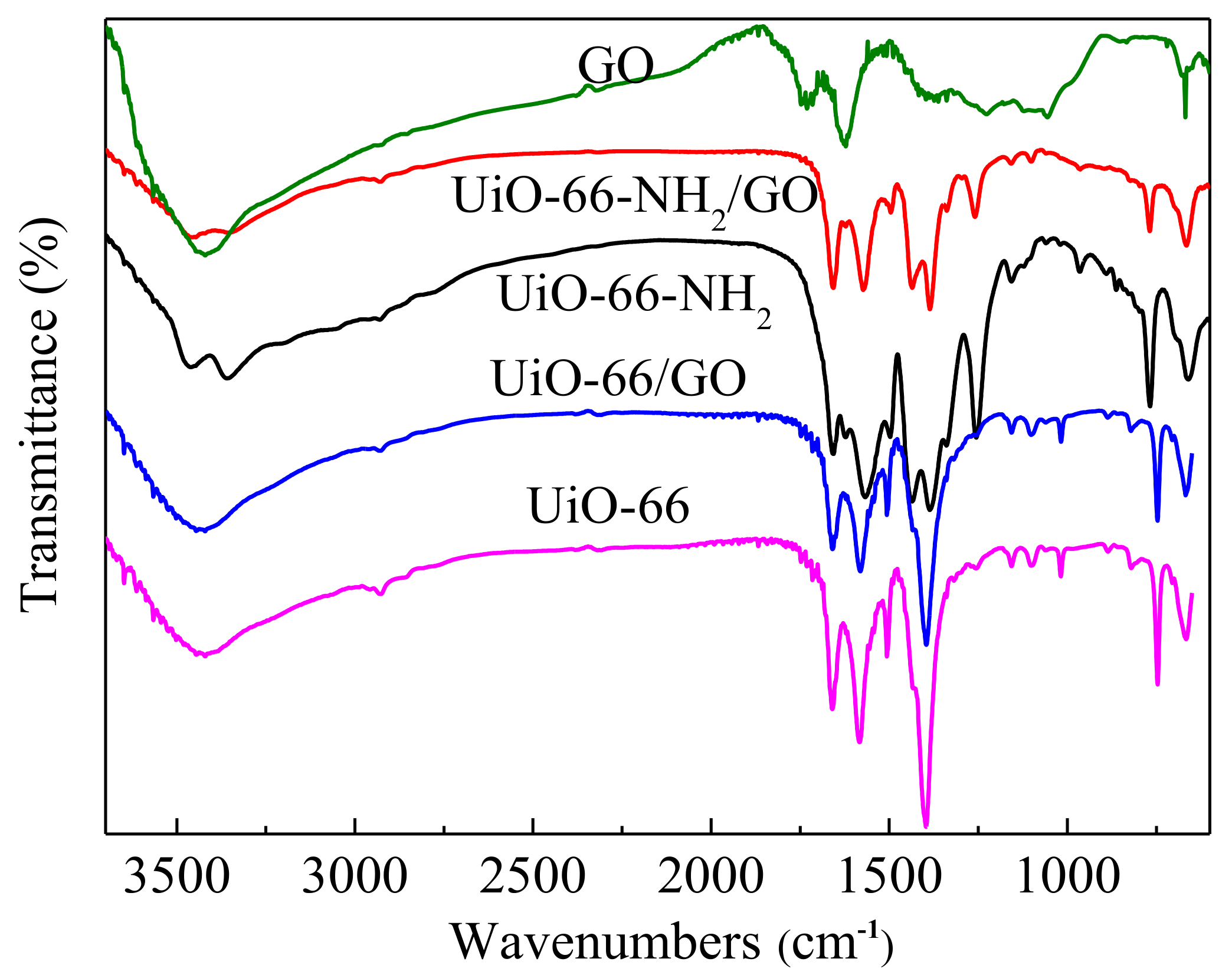

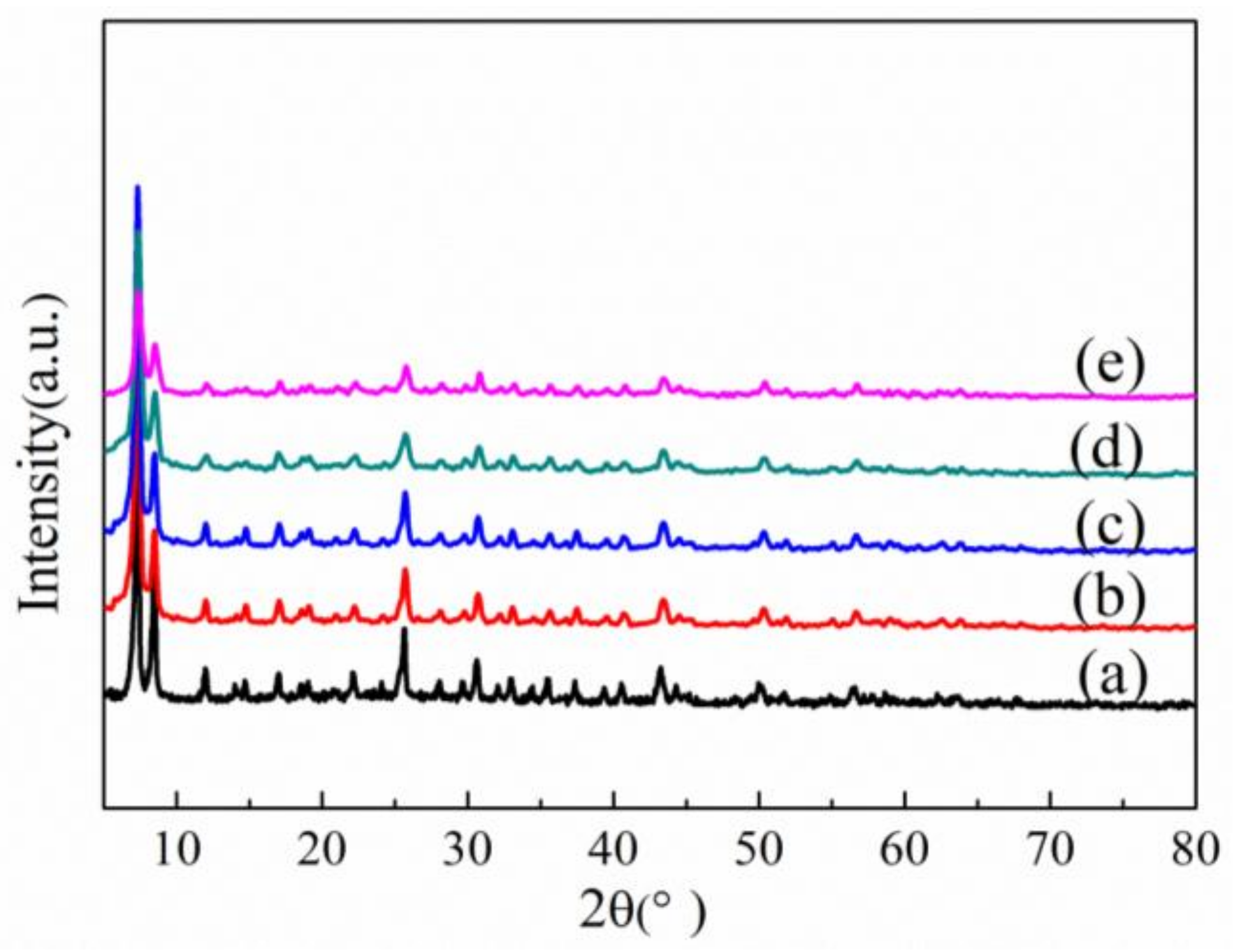

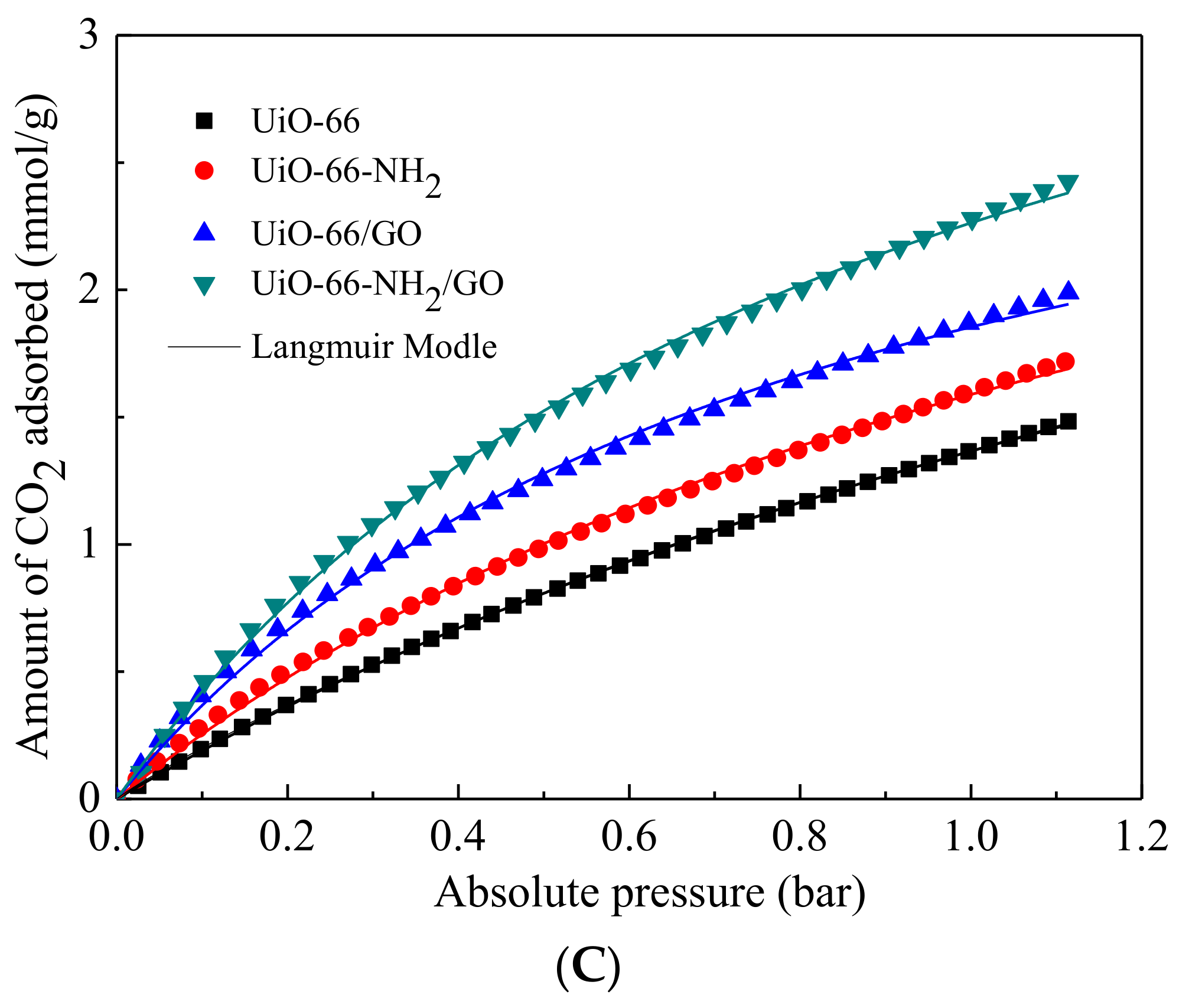
| Sample | SBET (m2/g) | Vpore (cm3/g) | Vmic (cm3/g) | Vmic/Vpore (%) |
|---|---|---|---|---|
| UiO-66 | 838 | 0.245 | 0.224 | 91 |
| UiO-66-NH2 | 822 | 0.236 | 0.214 | 90 |
| UiO-66/GO | 1184 | 0.384 | 0.304 | 79 |
| UiO-66-NH2/GO | 1052 | 0.345 | 0.286 | 83 |
| Sample | Chemical Formula | Q (mmol/g) | Temperature (K) | Ref. |
|---|---|---|---|---|
| MOF-5 | Zn4O(BDC)3 | 2.10 | 296 | [26] |
| IRMOF-1 | Zn4O(BDC)3 | 1.92 | 208 | [2] |
| MOF-177 | Zn4O(BTB)2 | 0.8 | 298 | [27] |
| zeolite 13X | - | 1.77 | 293 | [28] |
| Activated carbon | - | 1.5 | 298 | [29] |
| UiO-66 | Zr6O4(OH)(BDC)6 | 1.77 | 298 | [4] |
| UiO-66-NH2 | Zr6O4(OH)(BDC-NH2)6 | 3.05 | 298 | [4] |
| UiO-66 | Zr6O4(OH)(BDC)6 | 2.27 | 298 | present work |
| UiO-66/GO | - | 3.37 | 298 | present work |
| UiO-66-NH2 | Zr6O4(OH)(BDC-NH2)6 | 2.59 | 298 | present work |
| UiO-66-NH2/GO | - | 3.80 | 298 | present work |
| Sample | 273 K | 298 K | 318 K | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Qm | b | R2 | Qm | b | R2 | Qm | b | R2 | |
| UiO-66 | 11.78 | 0.45 | 0.999 | 15.49 | 0.17 | 0.999 | 4.42 | 0.45 | 0.999 |
| UiO-66-NH2 | 12.63 | 0.45 | 0.999 | 8.28 | 0.45 | 0.999 | 3.82 | 0.71 | 0.998 |
| UiO-66/GO | 10.83 | 1.26 | 0.997 | 6.03 | 1.20 | 0.997 | 3.37 | 1.22 | 0.997 |
| UiO-66-NH2/GO | 10.92 | 1.39 | 0.996 | 6.38 | 1.41 | 0.996 | 4.39 | 1.06 | 0.998 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, Y.; Zhang, H.; Song, F.; Huang, T.; Ji, J.; Zhong, Q.; Chu, W.; Xu, Q. UiO-66-NH2/GO Composite: Synthesis, Characterization and CO2 Adsorption Performance. Materials 2018, 11, 589. https://doi.org/10.3390/ma11040589
Cao Y, Zhang H, Song F, Huang T, Ji J, Zhong Q, Chu W, Xu Q. UiO-66-NH2/GO Composite: Synthesis, Characterization and CO2 Adsorption Performance. Materials. 2018; 11(4):589. https://doi.org/10.3390/ma11040589
Chicago/Turabian StyleCao, Yan, Hongmei Zhang, Fujiao Song, Tao Huang, Jiayu Ji, Qin Zhong, Wei Chu, and Qi Xu. 2018. "UiO-66-NH2/GO Composite: Synthesis, Characterization and CO2 Adsorption Performance" Materials 11, no. 4: 589. https://doi.org/10.3390/ma11040589
APA StyleCao, Y., Zhang, H., Song, F., Huang, T., Ji, J., Zhong, Q., Chu, W., & Xu, Q. (2018). UiO-66-NH2/GO Composite: Synthesis, Characterization and CO2 Adsorption Performance. Materials, 11(4), 589. https://doi.org/10.3390/ma11040589




