Synthesis and Characterization of Sulfonated Graphene Oxide Reinforced Sulfonated Poly (Ether Ether Ketone) (SPEEK) Composites for Proton Exchange Membrane Materials
Abstract
1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of SG and SPEEK
2.3. Preparation of Composite PEMs
2.4. Characterization
2.4.1. Chemical Structure and Micro-Morphologies Characterization
2.4.2. Water Uptake, IEC, DS, and Dissolvability
2.4.3. Proton Conductivity and Methanol Permeability
3. Results and Discussion
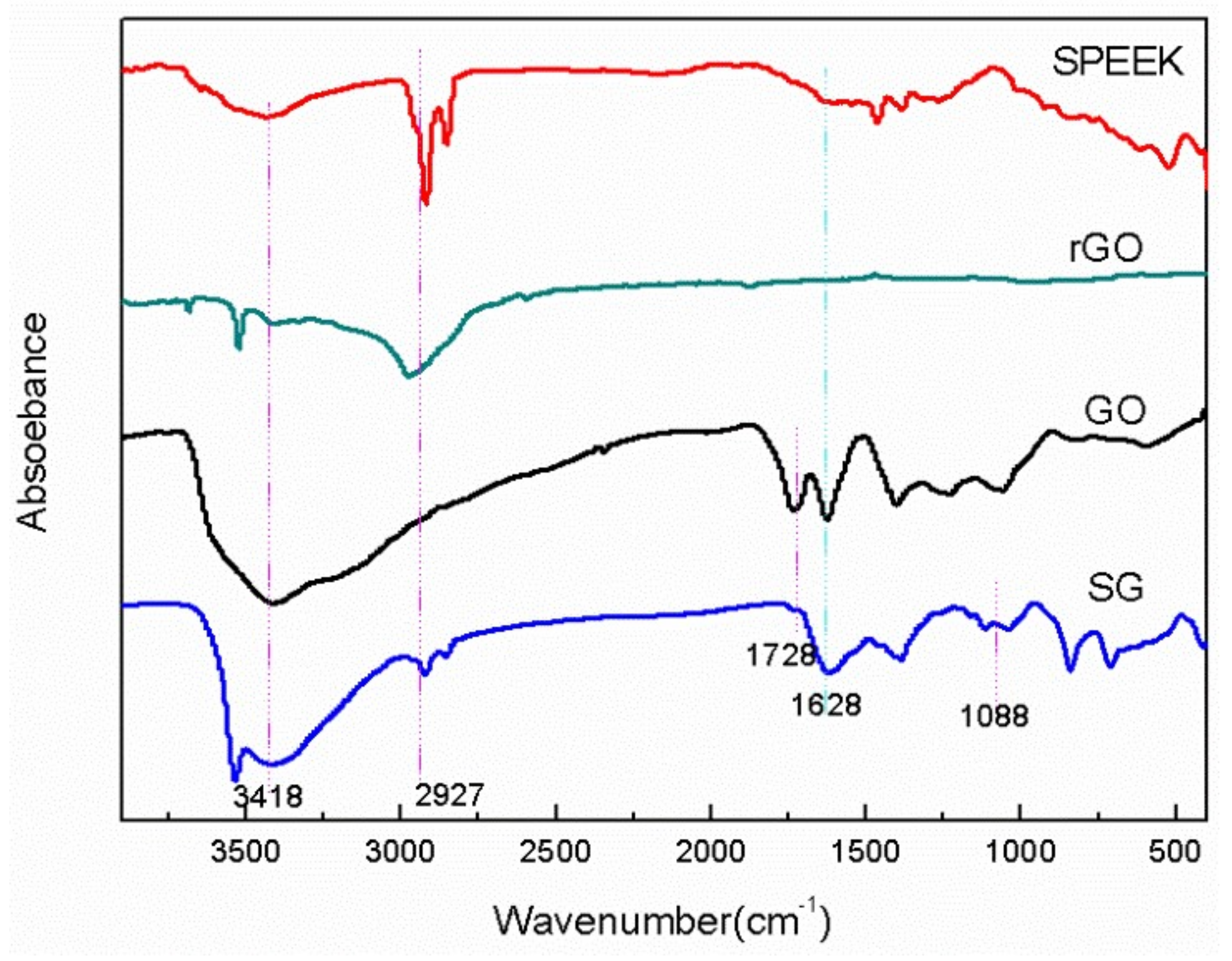
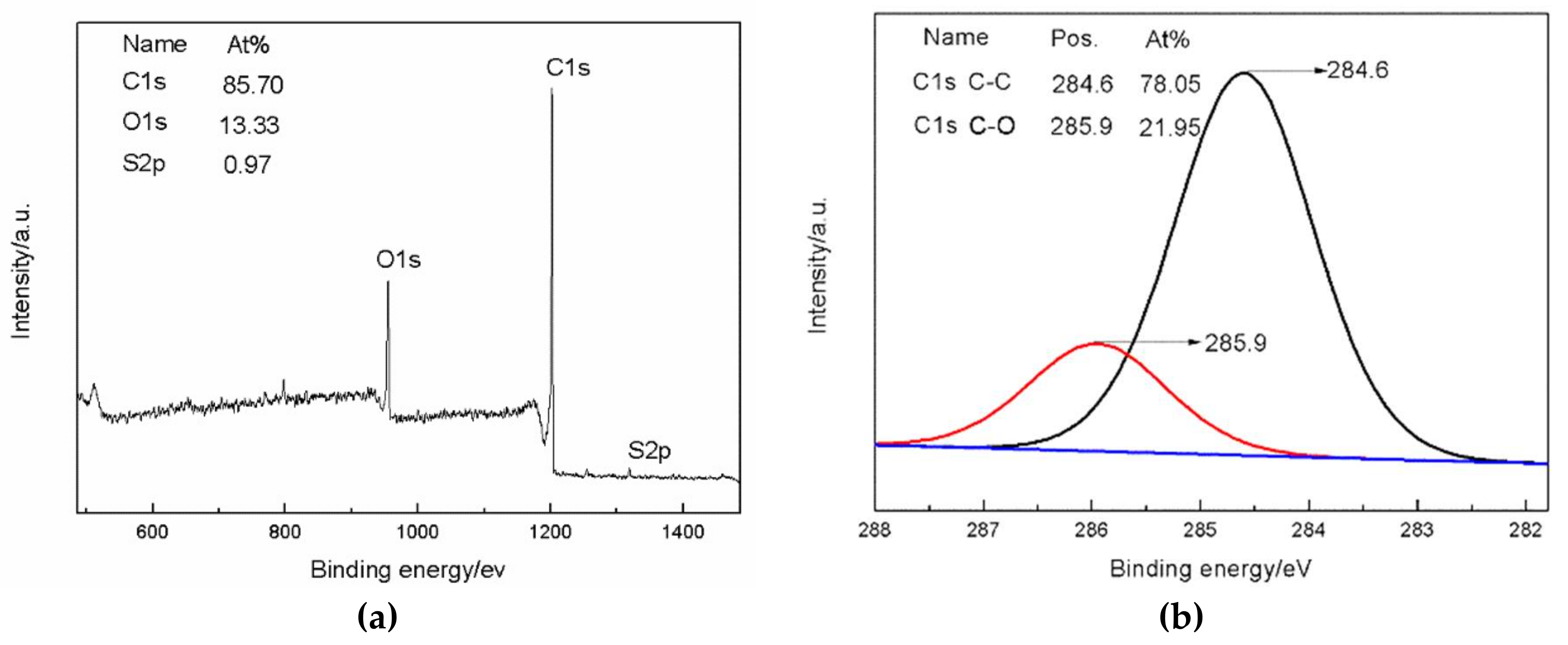
3.1. Structure Characterization
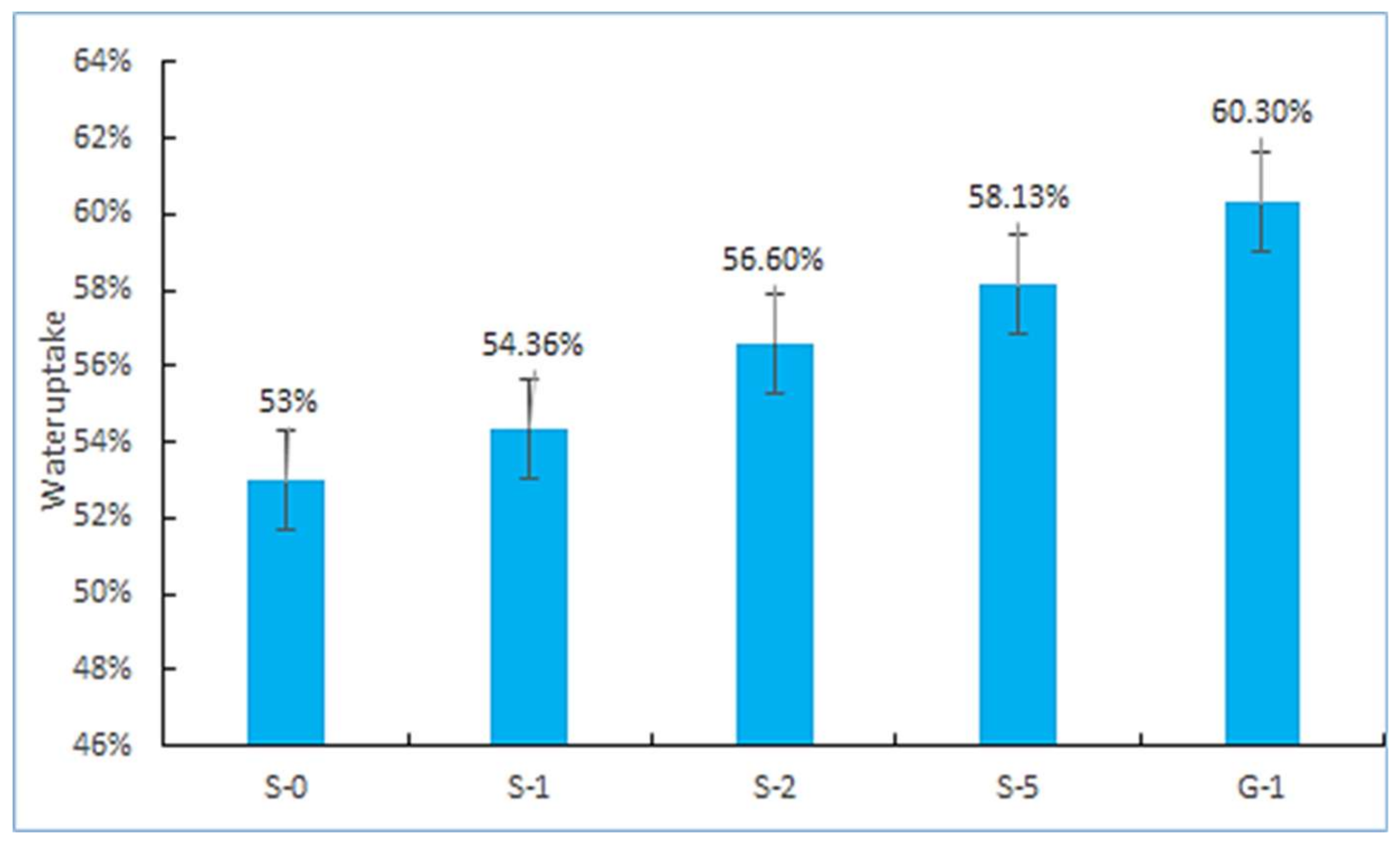
3.2. IEC, Water Uptake, and Dissolvability
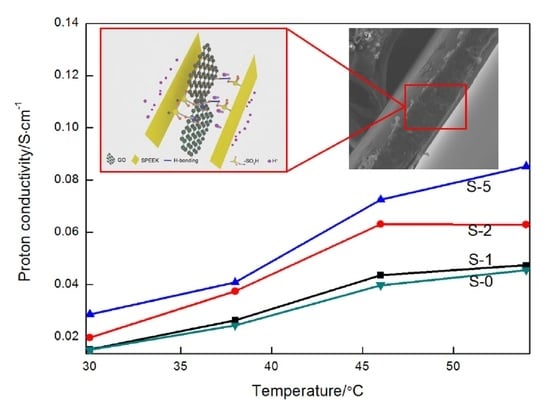
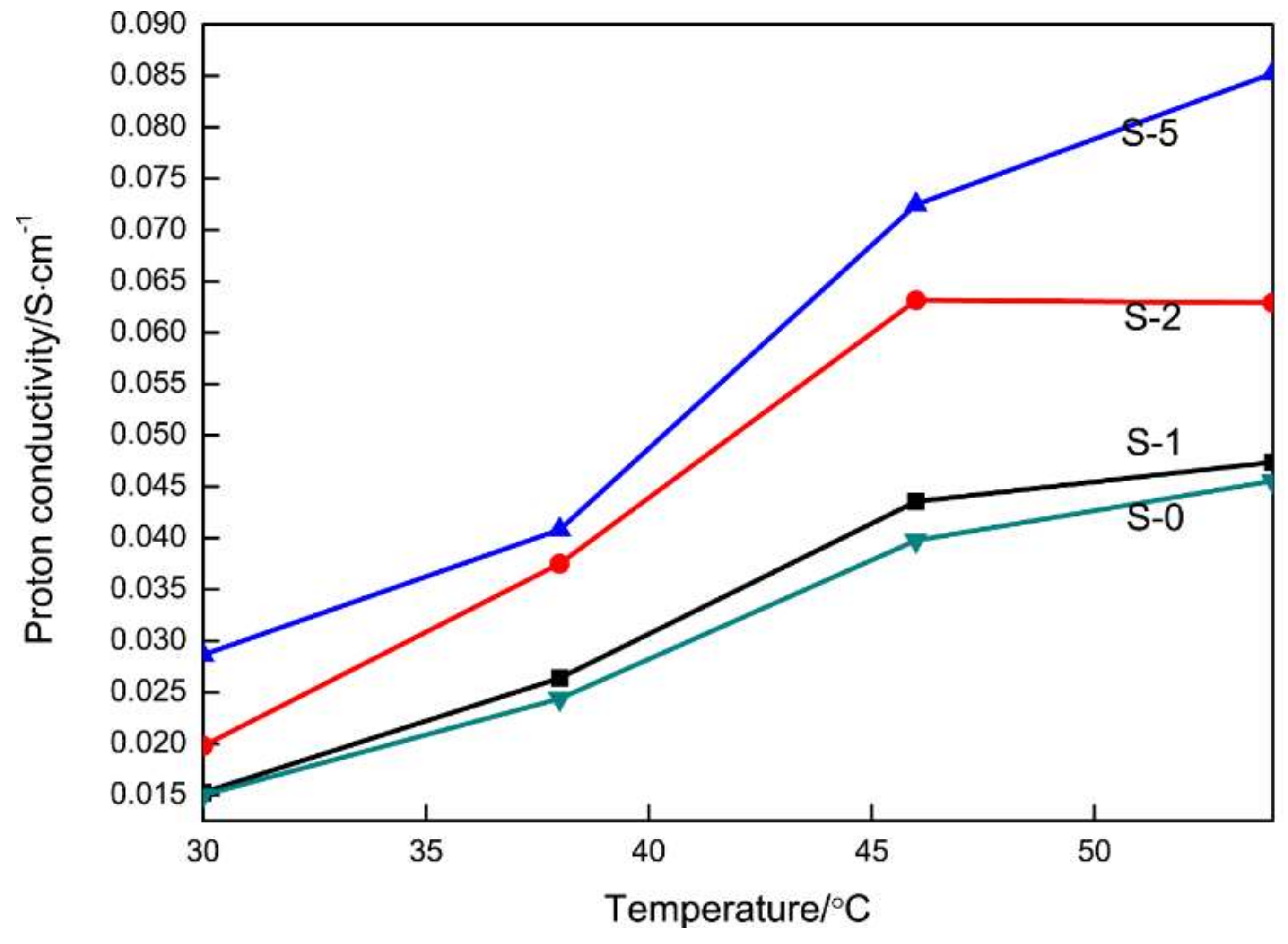
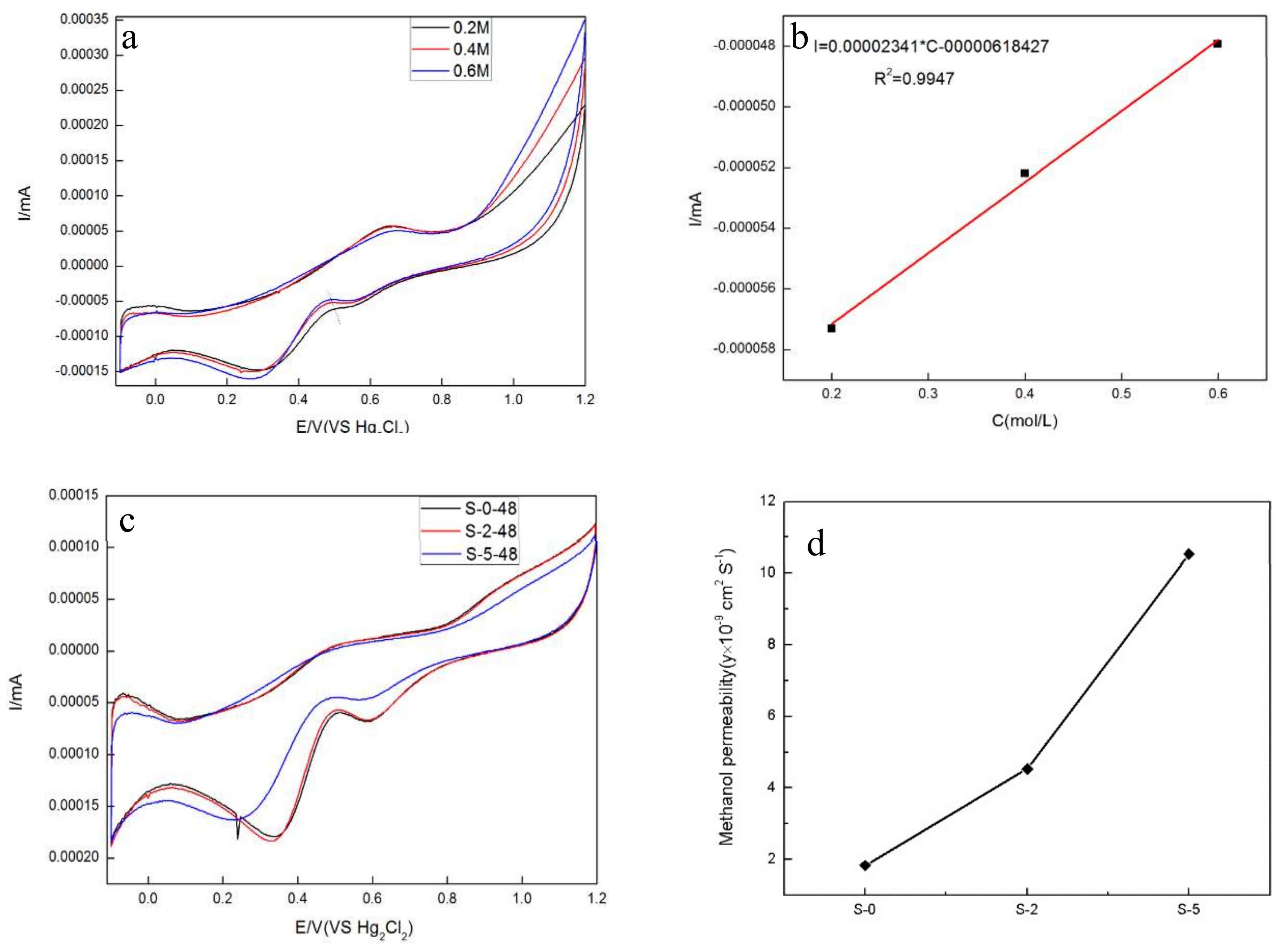
3.3. Proton Conductivity and Methanol Permeability
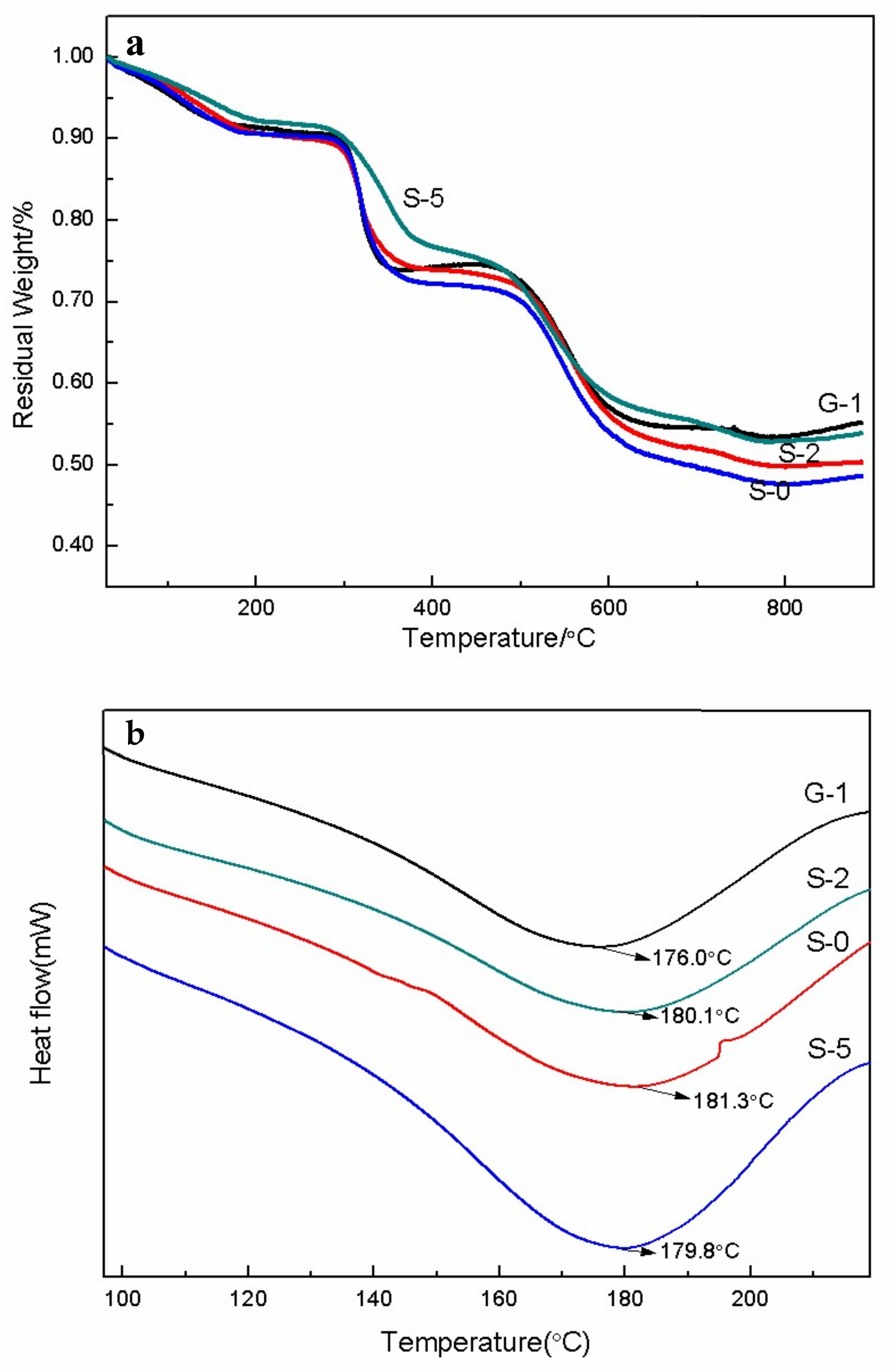
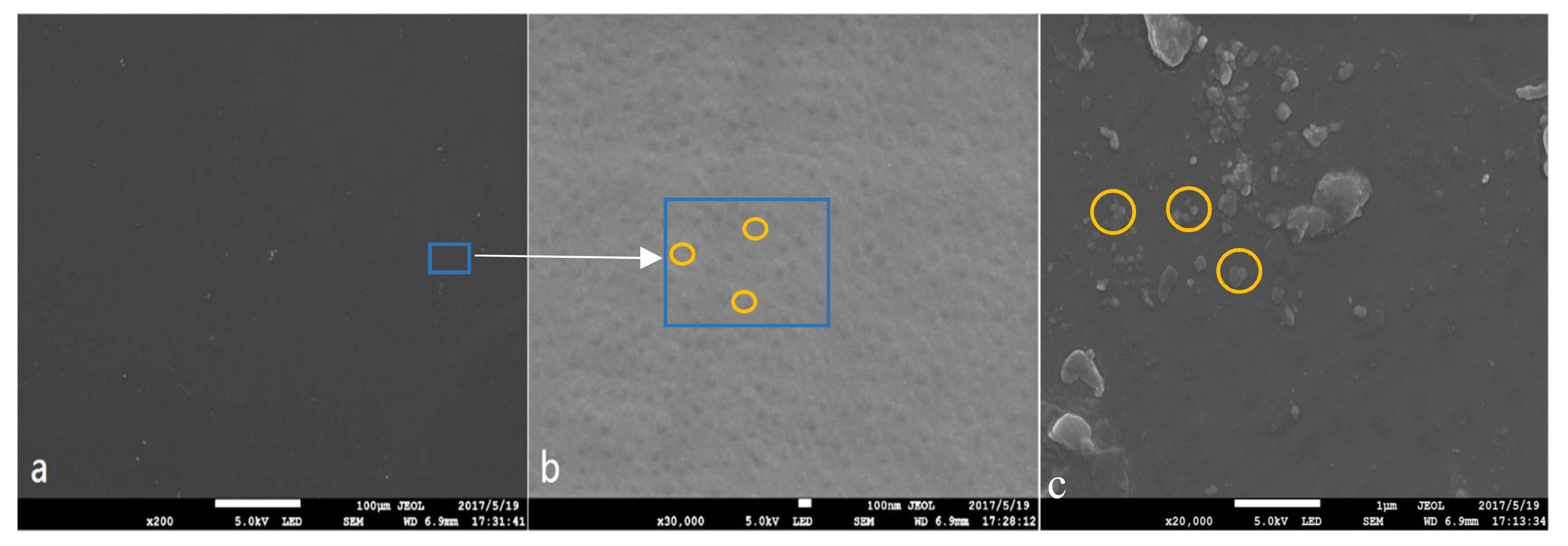
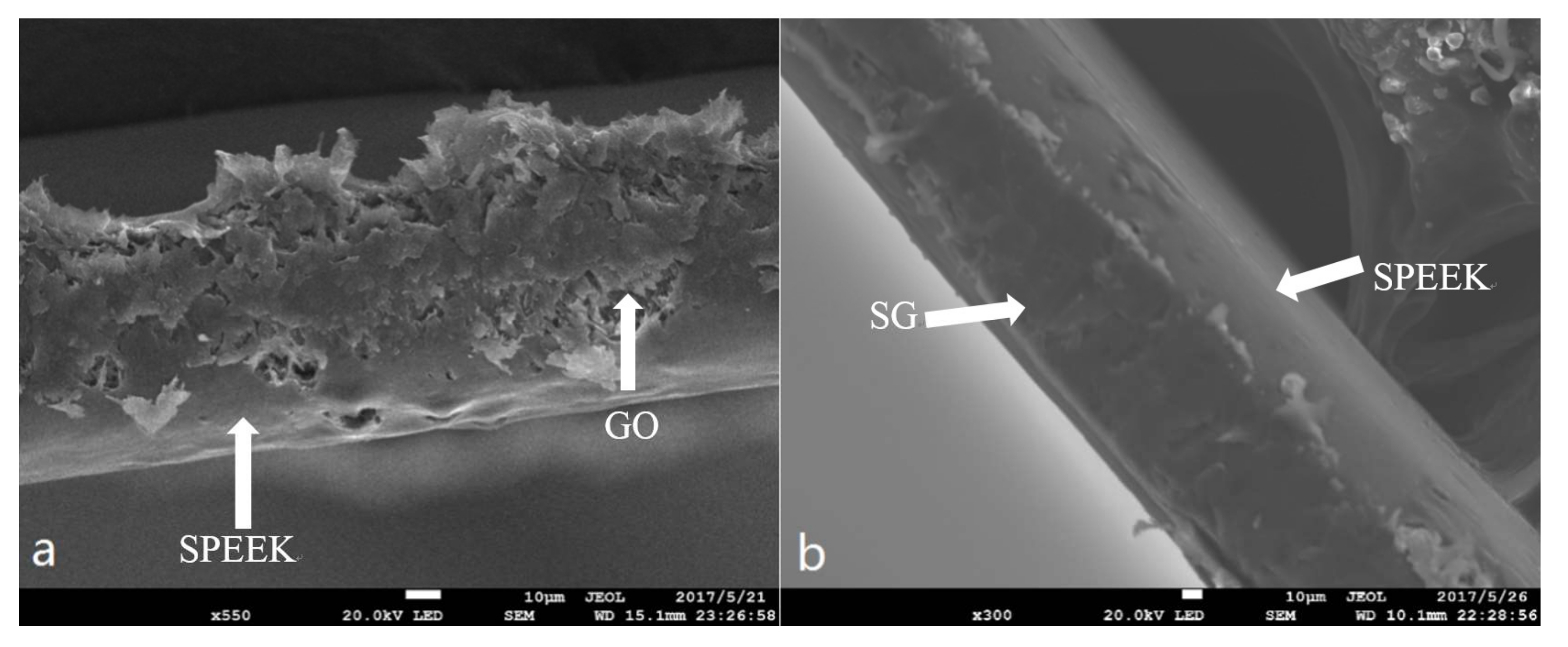
3.4. Thermal Stability and Morphology Study
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mauritz, K.A.; Moore, R.B. State of understanding of nafion. Chem. Rev. 2004, 104, 4535–4586. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, J.; Zheng, P.; Feng, M.; Chen, J.; Liu, X. Phthalonitrile end-capped sulfonated polyarylene ether nitriles for low-swelling proton exchange membranes. J. Polym. Res. 2016, 23, 256. [Google Scholar] [CrossRef]
- Kraytsberg, A.; Ein-Eli, Y. Review of advanced materials for proton exchange membrane fuel cells. Energy Fuels 2014, 28, 7303–7330. [Google Scholar] [CrossRef]
- Neburchilov, V.; Martin, J.; Wang, H.; Zhang, J. A review of polymer electrolyte membranes for direct methanol fuel cells. J. Power Sources 2007, 169, 221–238. [Google Scholar] [CrossRef]
- Kumar, R.; Mamlouk, M.; Scott, K. Sulfonated polyether ether ketone—Sulfonated graphene oxide composite membranes for polymer electrolyte fuel cells. RSC Adv. 2013, 4, 617–623. [Google Scholar] [CrossRef]
- Munjewar, S.S.; Thombre, S.B.; Mallick, R.K. A comprehensive review on recent material development of passive direct methanol fuel cell. Ionics 2017, 23, 1–18. [Google Scholar] [CrossRef]
- Hazarika, M.; Jana, T. Novel proton exchange membrane for fuel cell developed from blends of polybenzimidazole with fluorinated polymer. Eur. Polym. J. 2013, 49, 1564–1576. [Google Scholar] [CrossRef]
- Schmidt-Rohr, K.; Chen, Q. Parallel cylindrical water nanochannels in nafion fuel-cell membranes. Nat. Mater. 2008, 7, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Vinothkannan, M.; Kannan, R.; Kim, A.R.; Kumar, G.G.; Nahm, K.S.; Dong, J.Y. Facile enhancement in proton conductivity of sulfonated poly (ether ether ketone) using functionalized graphene oxide—Synthesis, characterization, and application towards proton exchange membrane fuel cells. Colloid Polym. Sci. 2016, 294, 1197–1207. [Google Scholar] [CrossRef]
- Kim, T.W.; Sahimi, M.; Tsotsis, T.T. Preparation and characterization of hybrid hydrotalcite-sulfonated polyetheretherketone (speek) cation-exchange membranes. Ind. Eng. Chem. Res. 2009, 48, 9504–9513. [Google Scholar] [CrossRef]
- Devrim, Y.; Devrim, H.; Eroglu, I. Polybenzimidazole/SiO2 hybrid membranes for high temperature proton exchange membrane fuel cells. Int. J. Hydrogen Energy 2016, 41, 10044–10052. [Google Scholar] [CrossRef]
- Castricum, H.L.; Sah, A.; Geenevasen, J.A.J.; Kreiter, R.; Blank, D.H.A.; Vente, J.F.; Elshof, J.E.T. Structure of hybrid organic–Inorganic sols for the preparation of hydrothermally stable membranes. J. Sol-Gel Sci. Technol. 2008, 48, 11–17. [Google Scholar] [CrossRef][Green Version]
- Shang, Y.; Xie, X.; Jin, H.; Guo, J.; Wang, Y.; Feng, S.; Wang, S.; Xu, J. Synthesis and characterization of novel sulfonated naphthalenic polyimides as proton conductive membrane for dmfc applications. Eur. Polym. J. 2006, 42, 2987–2993. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M.; Kowsari, E. Synthesis and characterization of poly(vinyl alcohol)/sulfonated graphene oxide nanocomposite membranes for use in proton exchange membrane fuel cells (pemfcs). Ind. Eng. Chem. Res. 2014, 53, 16621–16632. [Google Scholar] [CrossRef]
- Zhao, L.; Hong, C.; Lin, L.; Wu, H.; Su, Y.; Zhang, X.; Liu, A. Controllable nanoscale engineering of vertically aligned MoS2 ultrathin nanosheets by nitrogen doping of 3d graphene hydrogel for improved electrocatalytic hydrogen evolution. Carbon 2017, 116, 223–231. [Google Scholar] [CrossRef]
- Liu, S.; Wang, L.; Zhang, B.; Liu, B.; Wang, J.; Song, Y. Novel sulfonated polyimide/polyvinyl alcohol blend membranes for vanadium redox flow battery applications. J. Mater. Chem. A 2015, 3, 2072–2081. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Zhao, C.; Fu, T.; Shi, Y.; Hui, N. Composite membranes based on highly sulfonated peek and PBI: Morphology characteristics and performance. J. Membr. Sci. 2008, 308, 66–74. [Google Scholar] [CrossRef]
- Amir-Al-Ahmed; Sultan, A.S.; Zaidi, S.M.J. Sulfonated Poly(Ether Ether Ketone) (Speek): A Promising Membrane Material for Polymer Electrolyte Fuel Cell; Springer: Dordrecht, The Netherlands, 2012; pp. 437–451. [Google Scholar]
- Tang, Z.; Zhang, Z.; Han, Z.; Shen, S.; Li, J.; Yang, J. One-step synthesis of hydrophobic-reduced graphene oxide and its oil/water separation performance. J. Mater. Sci. 2016, 51, 1–8. [Google Scholar] [CrossRef]
- Beydaghi, H.; Javanbakht, M. Aligned nanocomposite membranes containing sulfonated graphene oxide with superior ionic conductivity for direct methanol fuel cell application. Ind. Eng. Chem. Res. 2015, 54, 7028–7037. [Google Scholar] [CrossRef]
- Wang, L.; Lai, A.; Zhuo, Y.; Zhang, Q.; Zhu, A.; Liu, Q. Properties of hybrid spek-c/go composite proton exchange membranes. CIESC J. 2015, 66, 3605–3610. [Google Scholar]
- Gahlot, S.; Sharma, P.P.; Kulshrestha, V.; Jha, P.K. Sgo/spes-based highly conducting polymer electrolyte membranes for fuel cell application. ACS Appl. Mater. Interfaces 2014, 6, 5595. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Cao, Y.; Kumar, R.; Wu, X.; Wang, X.; Scott, K. A polybenzimidazole/sulfonated graphite oxide composite membrane for high temperature polymer electrolyte membrane fuel cells. J. Mater. Chem. 2011, 21, 11359–11364. [Google Scholar] [CrossRef]
- Ning, C.; Qian, L.; Jin, L.; Yong, W.; Bai, Y.; Szunerits, S.; Boukherroub, R. Facile synthesis of fluorinated polydopamine/chitosan/reduced graphene oxide composite aerogel for efficient oil/water separation. Chem. Eng. J. 2017, 326, 17–28. [Google Scholar]
- Heo, Y.; Im, H.; Kim, J. The effect of sulfonated graphene oxide on sulfonated poly (ether ether ketone) membrane for direct methanol fuel cells. J. Membr. Sci. 2013, 425–426, 11–22. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, X.; Manthiram, A. Sulfonated poly(ether ether ketone) membranes with sulfonated graphene oxide fillers for direct methanol fuel cells. Int. J. Hydrogen Energy 2013, 38, 5875–5884. [Google Scholar] [CrossRef]
- Yao, H.; Tong, C.; Lei, G.; Liu, L.; Lü, C. Enhanced performance of the sulfonated polyimide proton exchange membranes by graphene oxide: Size effect of graphene oxide. J. Membr. Sci. 2014, 458, 36–46. [Google Scholar]
- Li, D.; Wang, L.H.; Liu, S.; Ren, X.L.; Han, X.T. Preparation of sulfonated poly(ether ether ketone)/graphene oxide blend membranes and their application in vanadium redox flow battery. Acta Polym. Sin. 2015, 1280–1287. [Google Scholar]
- Yue, D.; Wang, L.H.; Han, X.T. The preparation and properties of sulfonated poly (eter ether ketone) membranes for vanadium redox flow battery application. Acta Polym. Sin. 2013, 13, 1476–1482. [Google Scholar] [CrossRef]
- Zaidi, S.M.J. Comparative study of electrochemical methods for determination of methanol permeation through proton-exchange membranes. Arab. J. Sci. Eng. 2011, 36, 689–701. [Google Scholar] [CrossRef]
- Xie, F.; Meng, H.; Shen, P.K. Diffusion study in a novel three-dimensional electrode for direct methanol fuel cells. Electrochim. Acta 2008, 53, 5039–5044. [Google Scholar] [CrossRef]
- Zhang, L.; Shi, T.; Wu, S.; Zhou, H. Sulfonated graphene oxide: The new and effective material for synthesis of polystyrene-based nanocomposites. Colloid Polym. Sci. 2013, 291, 2061–2068. [Google Scholar] [CrossRef]
- Paik, Y.; Chae, S.A.; Han, O.H.; Sang, Y.H.; Ha, H.Y. Influence of water and degree of sulfonation on the structure and dynamics of speek studied by solid-state c and h nmr. Polymer 2009, 50, 2664–2673. [Google Scholar] [CrossRef]
- Wang, L.; Yi, B.L.; Zhang, H.M.; Xing, D.M. Characteristics of polyethersulfone/sulfonated polyimide blend membrane for proton exchange membrane fuel cell. J. Phys. Chem. B 2008, 112, 4270–4275. [Google Scholar] [CrossRef] [PubMed]
- Yoonoo, C.; Dawson, C.P.; Roberts, E.P.L.; Holmes, S.M. Nafion®/mordenite composite membranes for improved direct methanol fuel cell performance. J. Membr. Sci. 2011, 369, 367–374. [Google Scholar] [CrossRef]
- Zakaria, Z.; Kamarudin, S.K.; Timmiati, S.N. Membranes for direct ethanol fuel cells: An overview. Appl. Energy 2016, 163, 334–342. [Google Scholar] [CrossRef]
- Corti, H.R. Membranes for Direct Alcohol Fuel Cells; Springer: Dordrecht, The Netherlands, 2014; pp. 121–230. [Google Scholar]
- Kim, D.S.; Liu, B.; Guiver, M.D. Influence of silica content in sulfonated poly(arylene ether ether ketone ketone) (SPAEEKK) hybrid membranes on properties for fuel cell application. Polymer 2006, 47, 7871–7880. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Majedi, F.S.; Kabiri, K.; Solati-Hashjin, M.; Moaddel, H. Novel nanocomposite proton exchange membranes based on Nafion® and AMPS-modified montmorillonite for fuel cell applications. J. Membr. Sci. 2010, 365, 286–293. [Google Scholar] [CrossRef]
- Jung, H.Y.; Kim, M.W.; Lim, J.H.; Jin, H.C.; Roh, S.H. The effect of sGO content in speek/sGO composite membrane for unitized regenerative fuel cell. KEPCO J. Electr. Power Energy 2016, 2, 127–131. [Google Scholar] [CrossRef]
- Liu, A.; Li, Z.; Zhang, J.; Lin, L.; Wu, H. Solvent-assisted oxygen incorporation of vertically aligned MoS2 ultrathin nanosheets decorated on reduced graphene oxide for improved electrocatalytic hydrogen evolution. ACS Appl. Mater. Interfaces 2016, 8, 25210–25218. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhao, L.; Liu, A.; Li, X.; Wu, H.; Lu, C. Three-dimensional MoS2/rGO hydrogel with extremely high double-layer capacitance as active catalyst for hydrogen evolution reaction. Electrochim. Acta 2015, 182, 652–658. [Google Scholar] [CrossRef]



| Sample | GO/g | SG/g | SPEEK/g |
|---|---|---|---|
| G-1 | 0.012 | 0 | 1.188 |
| S-0 | 0 | 0 | 1.200 |
| S-1 | 0 | 0.012 | 1.188 |
| S-2 | 0 | 0.024 | 1.176 |
| S-5 | 0 | 0.060 | 1.140 |
| Samples | C1s (at %) | O1s (at %) | S2p (at %) | C/O |
|---|---|---|---|---|
| GO | 67.49 | 32.51 | 0 | 2.08 |
| SG | 85.70 | 13.33 | 0.97 | 6.43 |
| G-1 | S-0 | S-1 | S-2 | S-5 | |
|---|---|---|---|---|---|
| chloroform | + | + | + | + | + |
| DMF | + | - | - | - | + |
| methanol | - | - | / | / | - |
| NMP | + | + | + | + | + |
| acetone | / | / | / | / | - |
| DMSO | + | - | - | + | + |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, N.; Zhou, C.; Wang, Y.; Ju, H.; Tan, D.; Li, J. Synthesis and Characterization of Sulfonated Graphene Oxide Reinforced Sulfonated Poly (Ether Ether Ketone) (SPEEK) Composites for Proton Exchange Membrane Materials. Materials 2018, 11, 516. https://doi.org/10.3390/ma11040516
Cao N, Zhou C, Wang Y, Ju H, Tan D, Li J. Synthesis and Characterization of Sulfonated Graphene Oxide Reinforced Sulfonated Poly (Ether Ether Ketone) (SPEEK) Composites for Proton Exchange Membrane Materials. Materials. 2018; 11(4):516. https://doi.org/10.3390/ma11040516
Chicago/Turabian StyleCao, Ning, Chaofan Zhou, Yong Wang, Hong Ju, Dongyang Tan, and Jin Li. 2018. "Synthesis and Characterization of Sulfonated Graphene Oxide Reinforced Sulfonated Poly (Ether Ether Ketone) (SPEEK) Composites for Proton Exchange Membrane Materials" Materials 11, no. 4: 516. https://doi.org/10.3390/ma11040516
APA StyleCao, N., Zhou, C., Wang, Y., Ju, H., Tan, D., & Li, J. (2018). Synthesis and Characterization of Sulfonated Graphene Oxide Reinforced Sulfonated Poly (Ether Ether Ketone) (SPEEK) Composites for Proton Exchange Membrane Materials. Materials, 11(4), 516. https://doi.org/10.3390/ma11040516