Study on the Visible-Light Photocatalytic Performance and Degradation Mechanism of Diclofenac Sodium under the System of Hetero-Structural CuBi2O4/Ag3PO4 with H2O2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Hetero-Structural CuBi2O4/Ag3PO4
2.3. Characterization
2.4. Photocatalytic Performance Measurement
2.5. Analysis of Reactive Species
3. Results
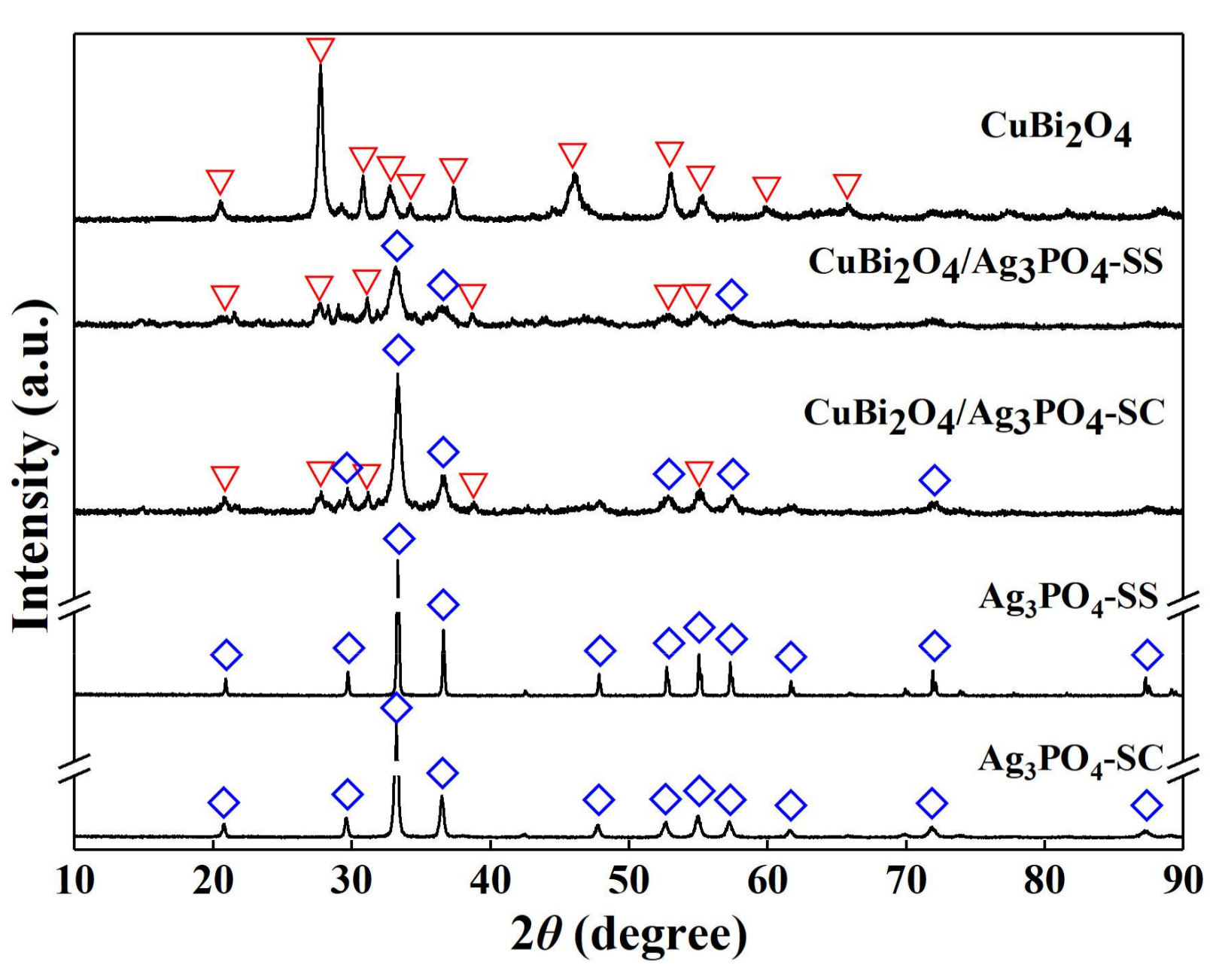
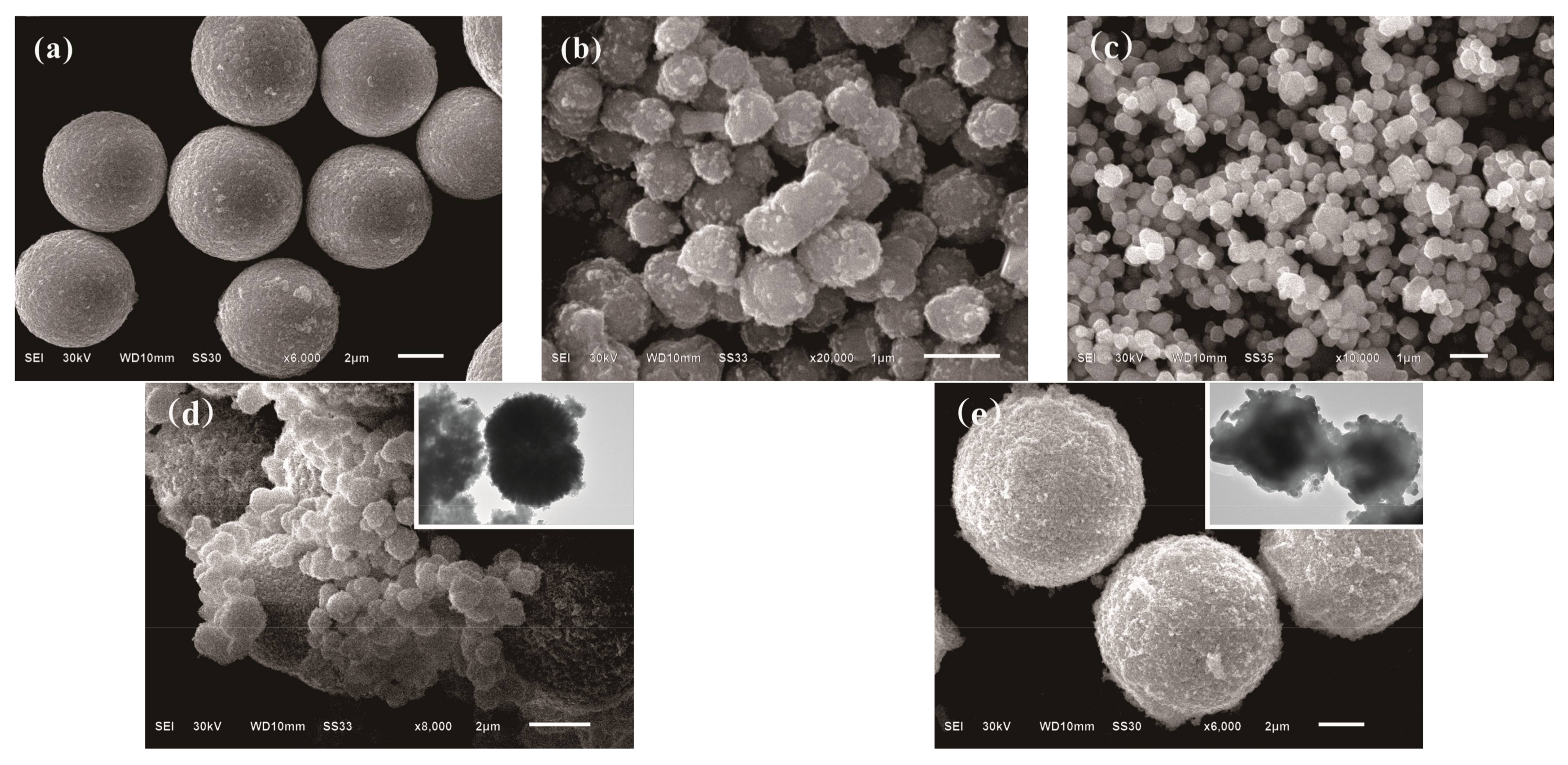
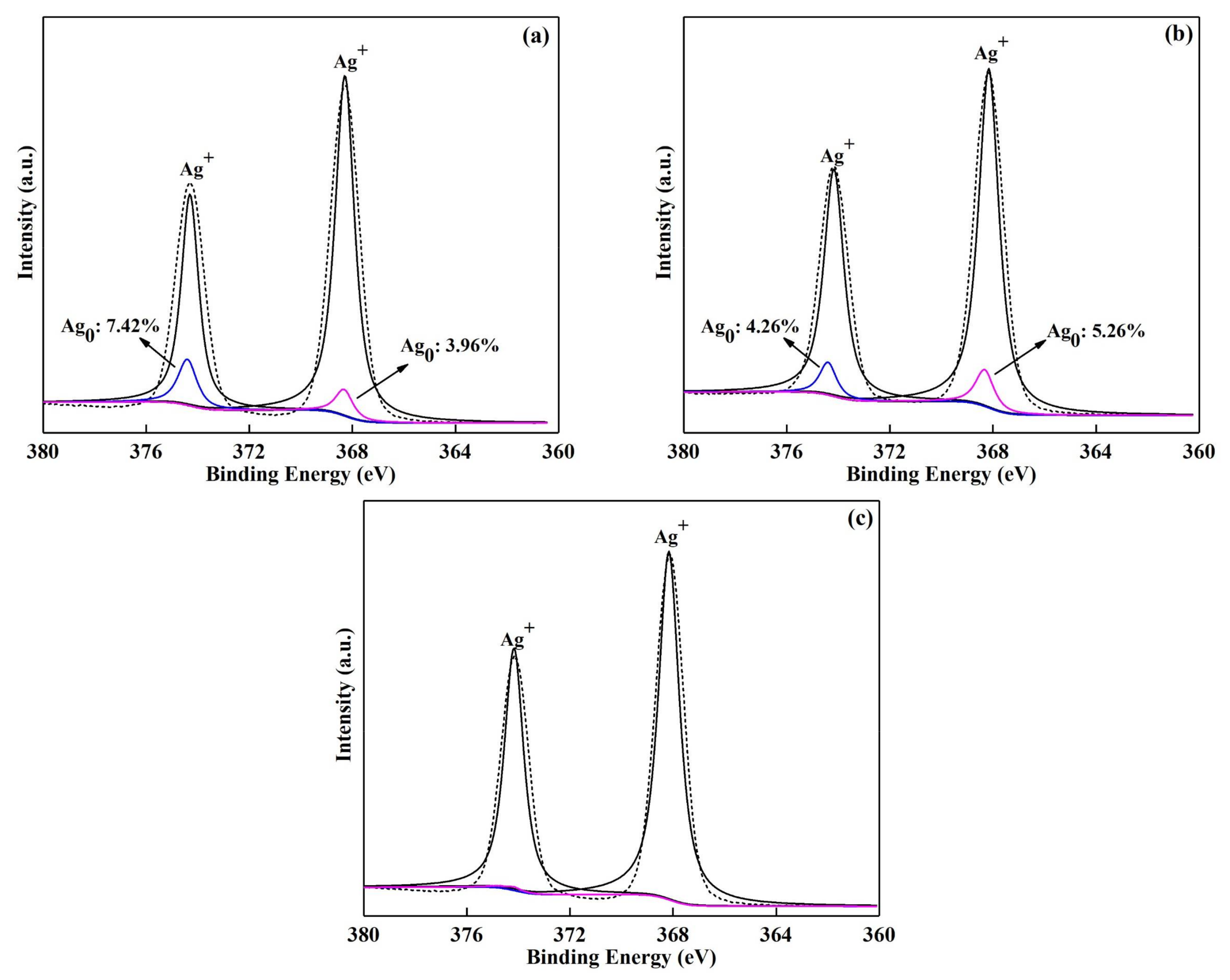
3.1. Characterizations
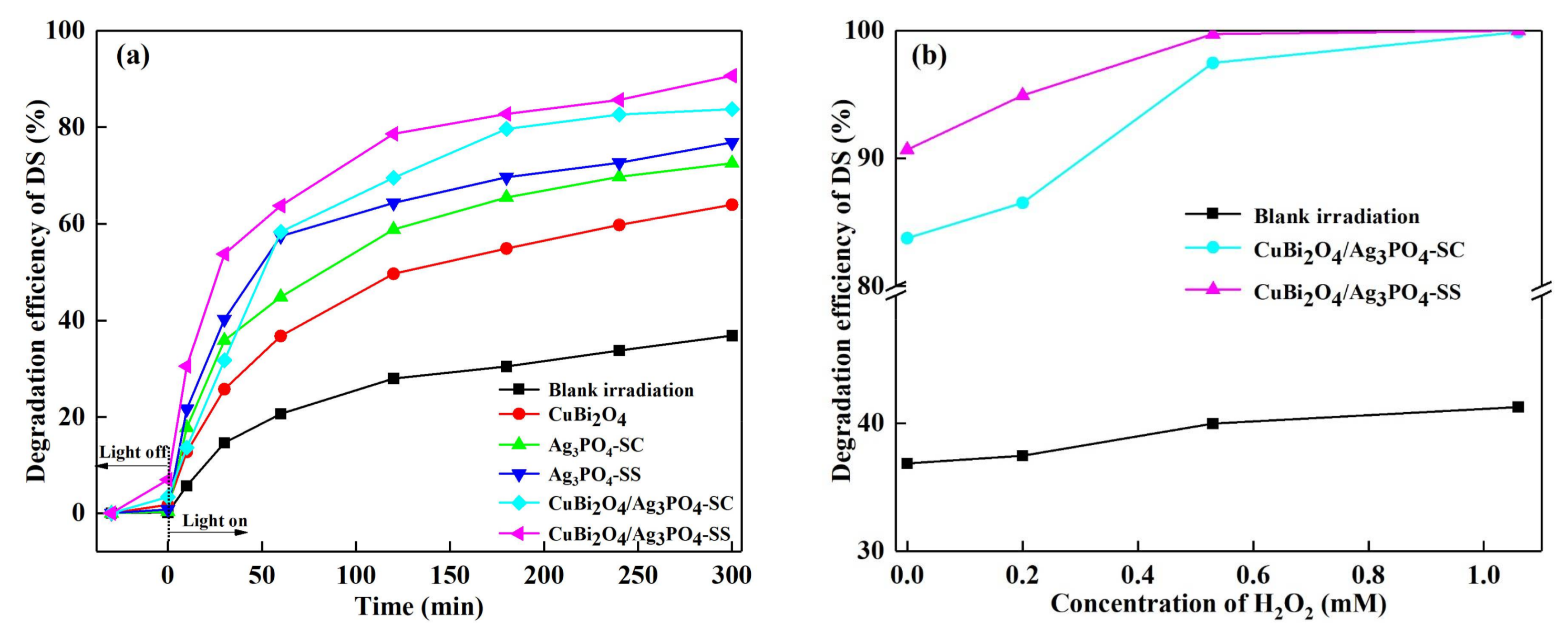
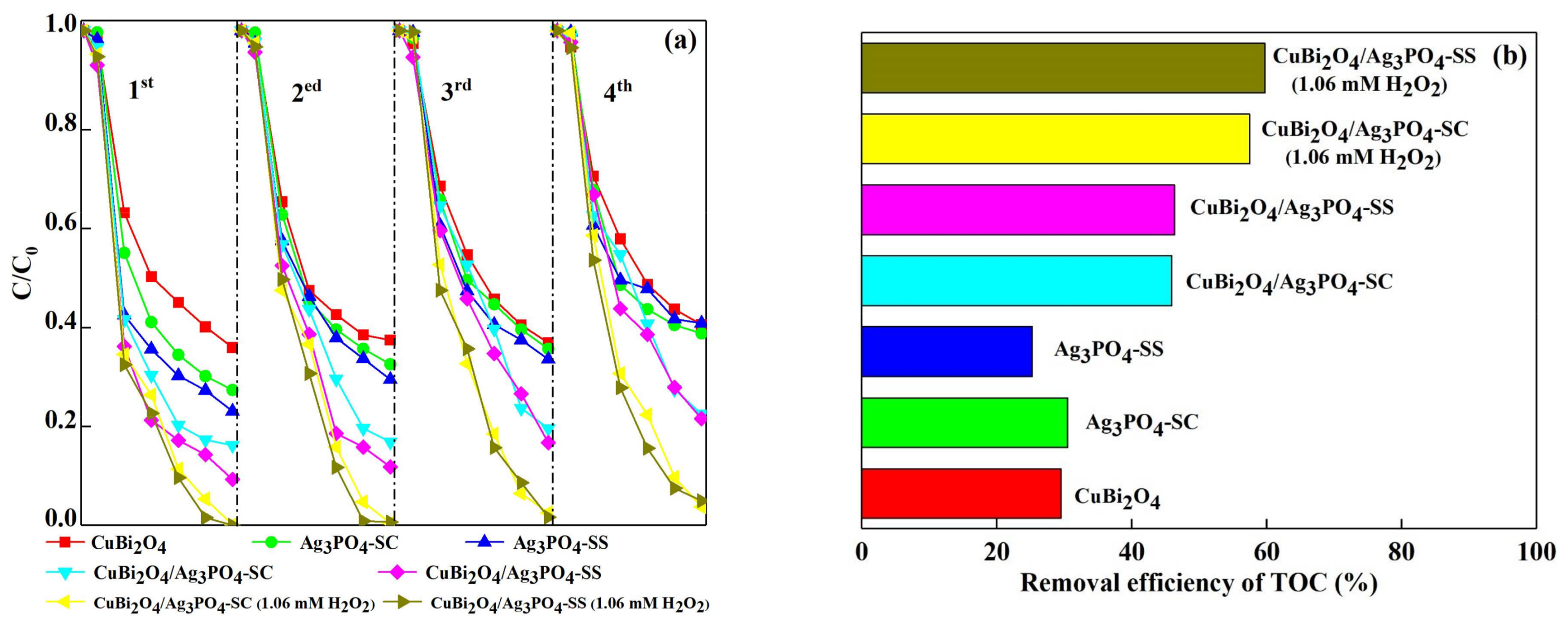
3.2. Photocatalytic Activity for DS Degradation
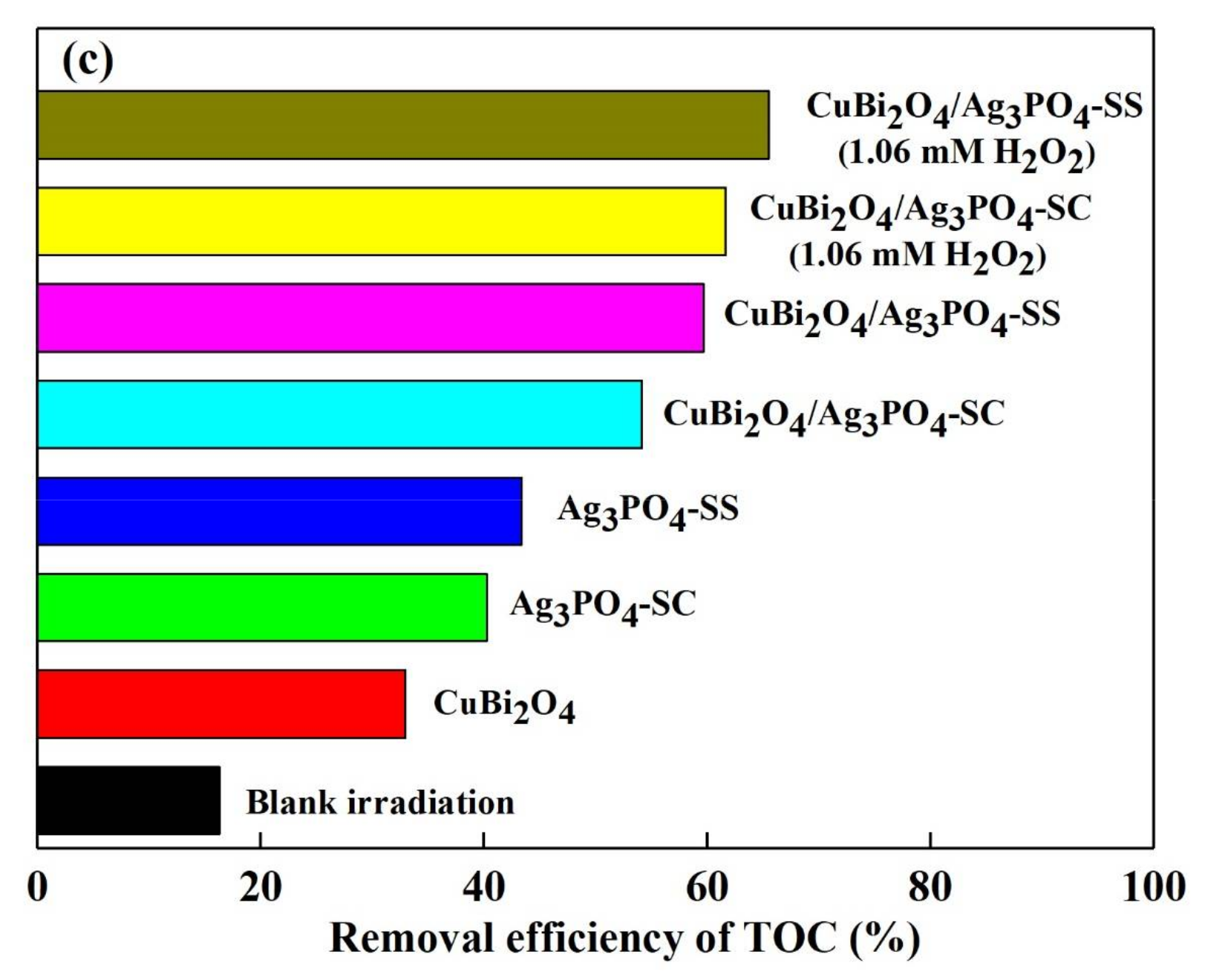
3.3. Photocatalytic Stability of the Catalysts
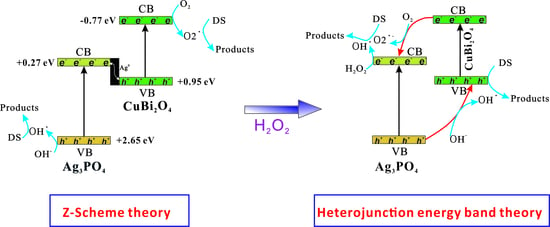
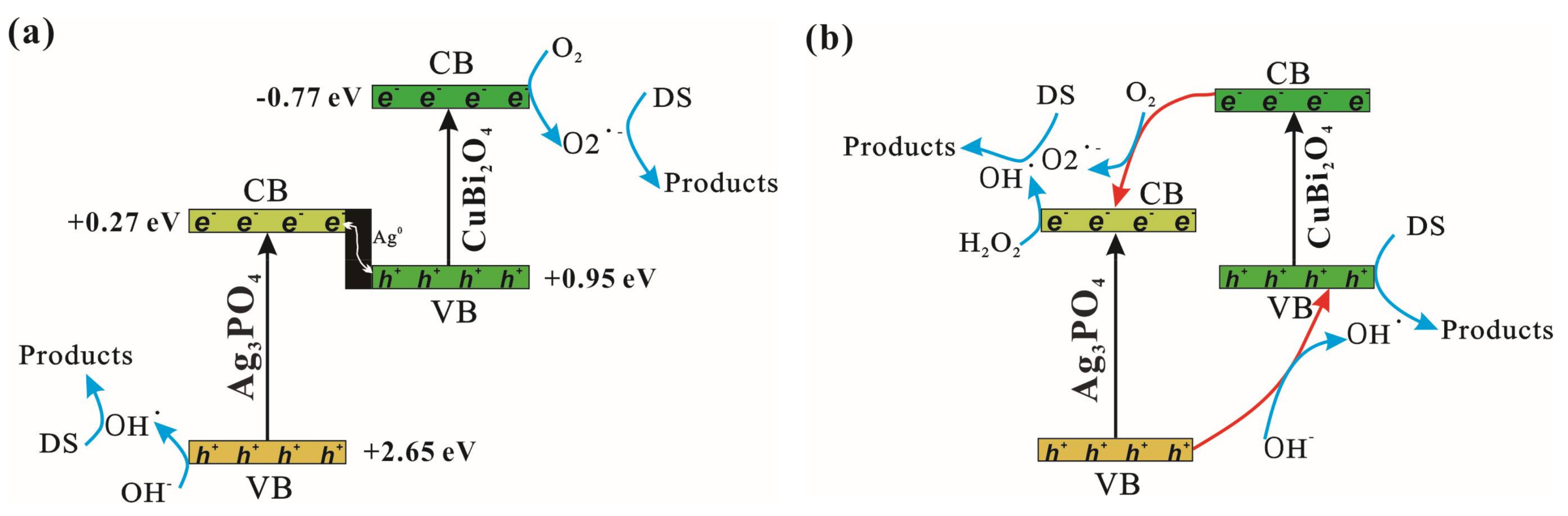
3.4. Photocatalytic Mechanism Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Oliveira, T.D.; Guégan, R.; Thiebault, T.; Milbeau, C.L.; Muller, F.; Teixeira, V.; Giovanela, M.; Boussafir, M. Adsorption of diclofenac onto organoclays: Effects of surfactant and environmental (pH and temperature) conditions. J. Hazard. Mater. 2016, 323, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Su, Y.; Adeleye, A.S.; Zhang, Y.; Zhou, X. Optimal design and characterization of sulfide-modified nanoscale zerovalent iron for diclofenac removal. Appl. Catal. B Environ. 2017, 201, 211–220. [Google Scholar] [CrossRef]
- Mezzelani, M.; Gorbi, S.; Fattorini, D.; d’Errico, G.; Consolandi, G.; Milan, M.; Bargelloni, L.; Regoli, F. Long-term exposure of Mytilus galloprovincialis to diclofenac, Ibuprofen and Ketoprofen: Insights into bioavailability, biomarkers and transcriptomic changes. Chemosphere 2018, 198, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Vieno, N.; Sillanpää, M. Fate of diclofenac in municipal wastewater treatment plant—A review. Environ. Int. 2014, 69, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Sari, S.; Ozdemir, G.; Yangin-Gomec, C.; Zengin, G.E.; Topuz, E.; Aydin, E.; Pehlivanoglu-Mantas, E.; Okutman, T.D. Seasonal variation of diclofenac concentration and its relation with wastewater characteristics at two municipal wastewater treatment plants in Turkey. J. Hazard. Mater. 2014, 272, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.X.; Xu, P.Q.; Cong, H.Y.; Yin, G.T. Synthesis and Characterization of WO3/Graphene Nanocomposites for Enhanced Photocatalytic Activities by One-Step In-Situ Hydrothermal Reaction. Materials 2018, 11, 147–162. [Google Scholar]
- Tran, H.T.T.; Kosslick, H.; Ibad, M.F.; Fischer, C.; Bentrup, U.; Vuong, T.H.; Nguyen, L.Q.; Schulz, A. Photocatalytic Performance of Highly Active Brookite in the Degradation of Hazardous Organic Compounds Compared to Anatase and Rutile. Appl. Catal. B Environ. 2017, 200, 647–658. [Google Scholar] [CrossRef]
- Shi, L.; Wang, F.; Liang, L.; Chen, K.; Liu, M.; Zhu, R.; Sun, J. In site acid template induced facile synthesis of porous graphitic carbon nitride with enhanced visible-light photocatalytic activity. Catal. Commun. 2017, 89, 129–132. [Google Scholar] [CrossRef]
- Chang, F.; Zheng, J.; Wang, X.; Xu, Q.; Deng, B.; Hu, X.; Liu, X. Heterojuncted non-metal binary composites silicon carbide/g-C3N4 with enhanced photocatalytic performance. Mater. Sci. Semicond. Process. 2018, 75, 183–192. [Google Scholar] [CrossRef]
- Liang, Y.C.; Lo, Y.R.; Wang, C.C.; Xu, N.C. Shell Layer Thickness-Dependent Photocatalytic Activity of Sputtering Synthesized Hexagonally Structured ZnO-ZnS Composite Nanorods. Materials 2018, 11, 87. [Google Scholar] [CrossRef] [PubMed]
- Martin, D.J.; Liu, G.; Moniz, S.J.; Bi, Y.; Beale, A.M.; Ye, J.; Tang, J. Efficient visible driven photocatalyst, silver phosphate: Performance, understanding and perspective. Chem. Soc. Rev. 2015, 44, 7808–7828. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Dai, Y.Z.; Guo, J.; Bu, F.Z.; Wang, X.Y. Synthesis of micro-nano Ag3PO4/ZnFe2O4 with different organic additives and its enhanced photocatalytic activity under visible light irradiation. Mater. Sci. Semicond. Process. 2016, 41, 335–342. [Google Scholar] [CrossRef]
- Yi, Z.; Ye, J.; Kikugawa, N.; Kako, T.; Ouyang, S.; Stuartwilliams, H.; Yang, H.; Cao, J.; Luo, W.; Li, Z. An orthophosphate semiconductor with photooxidation properties under visible-light irradiation. Nat. Mater. 2010, 9, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.J.; Dai, Y.Z.; Wang, X.Y. Methods and mechanism for improvement of photocatalytic activity and stability of Ag3PO4: A review. J. Alloys Compd. 2015, 649, 910–932. [Google Scholar] [CrossRef]
- Zhao, G.Y.; Liu, L.J.; Li, J.R.; Liu, Q. Efficient removal of dye MB: Through the combined action of adsorption and photodegradation from NiFe2O4/Ag3PO4. J. Alloys Compd. 2016, 664, 169–174. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, W.; Zhang, Z.; Fang, X. High-Efficiency Visible-Light-Driven Ag3PO4/AgI Photocatalysts: Z-Scheme Photocatalytic Mechanism for Their Enhanced Photocatalytic Activity. J. Phys. Chem. C 2017, 117, 19346–19352. [Google Scholar] [CrossRef]
- Shi, W.; Guo, F.; Yuan, S. In situ synthesis of Z-scheme Ag3PO4/CuBi2O4 photocatalysts and enhanced photocatalytic performance for the degradation of tetracycline under visible light irradiation. Appl. Catal. B Environ. 2017, 209, 720–728. [Google Scholar] [CrossRef]
- Abroushan, E.; Farhadi, S.; Zabardasti, A. Ag3PO4/CoFe2O4 magnetic nanocomposite: Synthesis, characterization and applications in catalytic reduction of nitrophenols and sunlight-assisted photocatalytic degradation of organic dye pollutants. RSC Adv. 2017, 7, 18293–18304. [Google Scholar] [CrossRef]
- Ren, J.; Chai, Y.; Liu, Q.; Zhang, L.; Dai, W.L. Intercorrelated Ag3PO4 nanoparticles decorated with graphic carbon nitride: Enhanced stability and photocatalytic activities for water treatment. Appl. Surf. Sci. 2017, 403, 177–186. [Google Scholar] [CrossRef]
- Chava, R.K.; Do, J.Y.; Kang, M. Fabrication of CdS-Ag3PO4 heteronanostructures for improved visible photocatalytic hydrogen evolution. J. Alloys Compd. 2017, 727, 86–93. [Google Scholar] [CrossRef]
- Wang, F.; Yang, H.; Zhang, Y.; Wang, F.; Yang, H.; Zhang, Y.; Wang, F.; Yang, H.; Zhang, Y. Enhanced photocatalytic performance of CuBi2O4 particles decorated with Ag nanowires. Mater. Sci. Semicond. Process. 2018, 73, 58–66. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.; Lim, T.T. Hierarchically-structured Co-CuBi2O4 and Cu-CuBi2O4 for sulfanilamide removal via peroxymonosulfate activation. Catal. Today 2017, 280, 2–7. [Google Scholar] [CrossRef]
- Nishikawa, M.; Yuto, S.; Hasegawa, T.; Shiroishi, W.; Hou, H.; Nakabayashi, Y.; Nosaka, Y.; Saito, N. Compositing effects of CuBi2O4 on visible-light responsive photocatalysts. Mater. Sci. Semicond. Process. 2017, 57, 12–17. [Google Scholar] [CrossRef]
- Yin, H.; Cao, M.L.; Yu, X.X.; Li, C.; Shen, Y.; Zhu, M.Q. Hierarchical CuBi2O4 microspheres as lithium-ion battery anodes with superior high-temperature electrochemical performance. RSC Adv. 2017, 7, 13250–13256. [Google Scholar] [CrossRef]
- Wang, F.; Chemseddine, A.; Abdi, F.F.; Krol, R.V.D.; Berglund, S.P. Spray pyrolysis of CuBi2O4 photocathodes: Improved solution chemistry for highly homogeneous thin films. J. Mater. Chem. A 2017, 25, 12838–12847. [Google Scholar] [CrossRef]
- Zhang, Y.; Xie, Y.; Li, J.; Bai, T.; Wang, J. Photocatalytic activity and adsorption performance of p-CuBi2O4/n-TiO2 p-n heterojunction composites prepared by in situ sol-gel coating method. J. Sol Gel Sci. Technol. 2014, 71, 38–42. [Google Scholar] [CrossRef]
- Ming, G.; Na, Z.; Zhao, Y.; Jing, L.; Lu, L. Sunlight-Assisted Degradation of Dye Pollutants in Ag3PO4 Suspension. Ind. Eng. Chem. Res. 2012, 51, 5167–5173. [Google Scholar]
- Eswar, K.R.; Katkar, V.V.; Ramamurthy, P.C.; Madras, G. Novel AgBr/Ag3PO4 Decorated Ceria Nanoflake Composites for Enhanced Photocatalytic Activity toward Dyes and Bacteria under Visible Light. Ind. Eng. Chem. Res. 2015, 54, 8031–8042. [Google Scholar] [CrossRef]
- Patil, R.; Kelkar, S.; Naphade, R.; Ogale, S. Low temperature grown CuBi2O4 with flower morphology and its composite with CuO nanosheets for photoelectrochemical water splitting. J. Mater. Chem. A 2014, 2, 92–95. [Google Scholar] [CrossRef]
- Martha, S.; Nashim, A.; Parida, K.M. Facile synthesis of highly active g-C3N4 for efficient hydrogen production under visible light. J. Mater. Chem. A 2013, 1, 7816–7824. [Google Scholar] [CrossRef]
- Teng, W.; Li, X.; Zhao, Q.; Chen, G. Fabrication of Ag/Ag3PO4/TiO2 heterostructure photoelectrodes for efficient decomposition of 2-chlorophenol under visible light irradiation. J. Mater. Chem. A 2013, 1, 9060–9068. [Google Scholar] [CrossRef]
- Guo, J.; Ouyang, S.; Zhou, H.; Kako, T.; Ye, J. Ag3PO4/In(OH)3 Composite Photocatalysts with Adjustable Surface-Electric Property for Efficient Photodegradation of Organic Dyes under Simulated Solar-Light Irradiation. J. Phys. Chem. C 2013, 117, 17716–17724. [Google Scholar] [CrossRef]
- Zhou, P.; Yu, J.; Jaroniec, M. All-Solid-State Z-Scheme Photocatalytic Systems. Adv. Mater. 2014, 26, 4920–4935. [Google Scholar] [CrossRef] [PubMed]
- Kanagaraj, T.; Thiripuranthagan, S. Photocatalytic activities of novel SrTiO3-BiOBr heterojunction catalysts towards the degradation of reactive dyes. Appl. Catal. B Environ. 2017, 207, 218–232. [Google Scholar] [CrossRef]
- Liu, W.; Wang, M.; Xu, C.; Chen, S.; Fu, X. Ag3PO4/ZnO: An efficient visible-light-sensitized composite with its application in photocatalytic degradation of Rhodamine B. Mater. Res. Bull. 2013, 48, 106–113. [Google Scholar] [CrossRef]
- Su, M.; He, C.; Sharma, V.K.; Asi, M.A.; Xia, D.; Li, X.Z.; Deng, H.; Xiong, Y. Mesoporous zinc ferrite: Synthesis, characterization, and photocatalytic activity with HO/visible light. J. Hazard. Mater. 2012, 211–212, 95–103. [Google Scholar] [CrossRef] [PubMed]


| Samples | BET Surface Area (m2 g−1) |
|---|---|
| CuBi2O4 | 2.307 |
| Ag3PO4-SC | 0.406 |
| Ag3PO4-SS | 0.984 |
| CuBi2O4/Ag3PO4-SC | 12.218 |
| CuBi2O4/Ag3PO4-SS | 14.596 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Li, N.; Xu, S.; Wang, H.; Cai, Y. Study on the Visible-Light Photocatalytic Performance and Degradation Mechanism of Diclofenac Sodium under the System of Hetero-Structural CuBi2O4/Ag3PO4 with H2O2. Materials 2018, 11, 511. https://doi.org/10.3390/ma11040511
Chen X, Li N, Xu S, Wang H, Cai Y. Study on the Visible-Light Photocatalytic Performance and Degradation Mechanism of Diclofenac Sodium under the System of Hetero-Structural CuBi2O4/Ag3PO4 with H2O2. Materials. 2018; 11(4):511. https://doi.org/10.3390/ma11040511
Chicago/Turabian StyleChen, Xiaojuan, Ning Li, Song Xu, Hailong Wang, and Yumin Cai. 2018. "Study on the Visible-Light Photocatalytic Performance and Degradation Mechanism of Diclofenac Sodium under the System of Hetero-Structural CuBi2O4/Ag3PO4 with H2O2" Materials 11, no. 4: 511. https://doi.org/10.3390/ma11040511
APA StyleChen, X., Li, N., Xu, S., Wang, H., & Cai, Y. (2018). Study on the Visible-Light Photocatalytic Performance and Degradation Mechanism of Diclofenac Sodium under the System of Hetero-Structural CuBi2O4/Ag3PO4 with H2O2. Materials, 11(4), 511. https://doi.org/10.3390/ma11040511