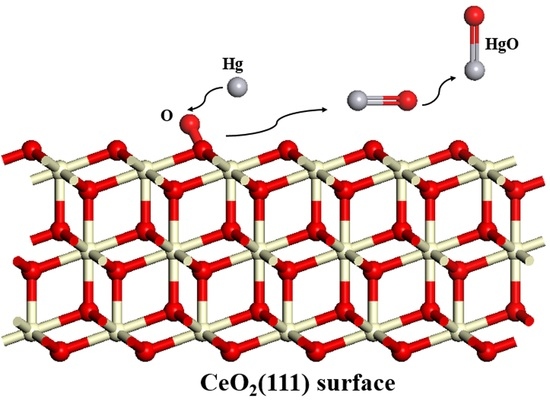
Mechanism of Mercury Adsorption and Oxidation by Oxygen over the CeO2 (111) Surface: A DFT Study
Abstract
:1. Introduction
2. Computational Details
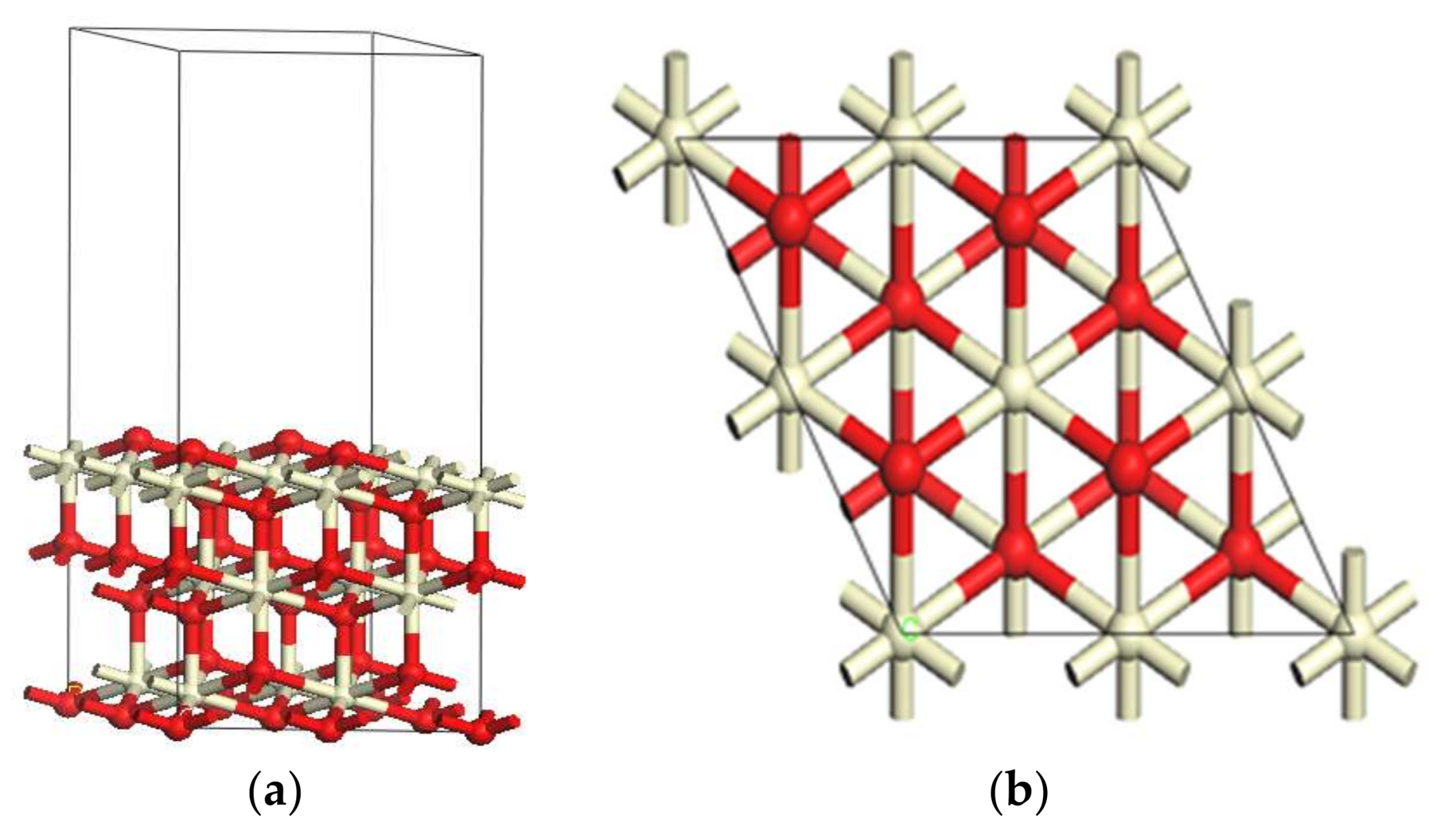
2.1. Catalyst Models
2.2. Computational Method
3. Results and Discussion
3.1. Adsorption of Hg0 on the CeO2 (111) Surface
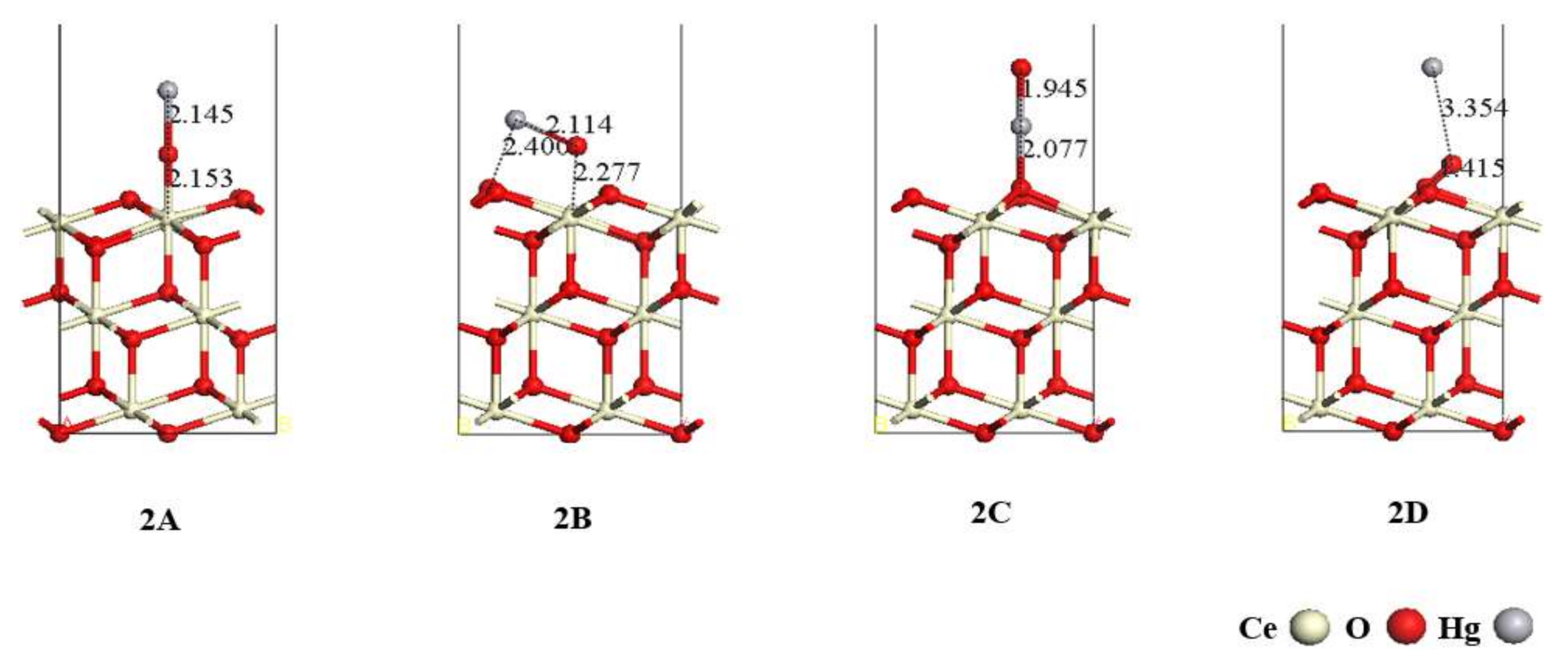
3.2. Adsorption of HgO on the CeO2 (111) Surface
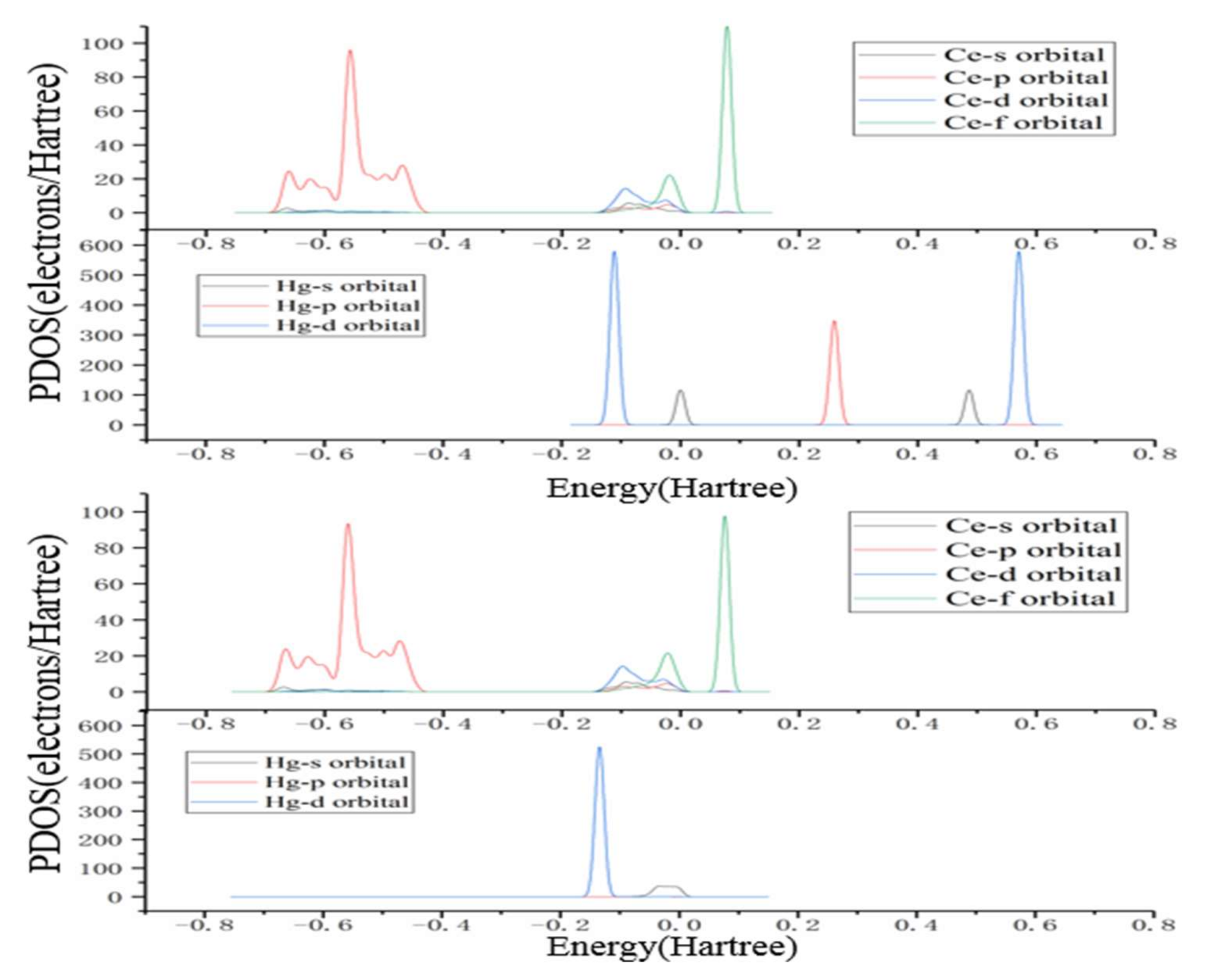
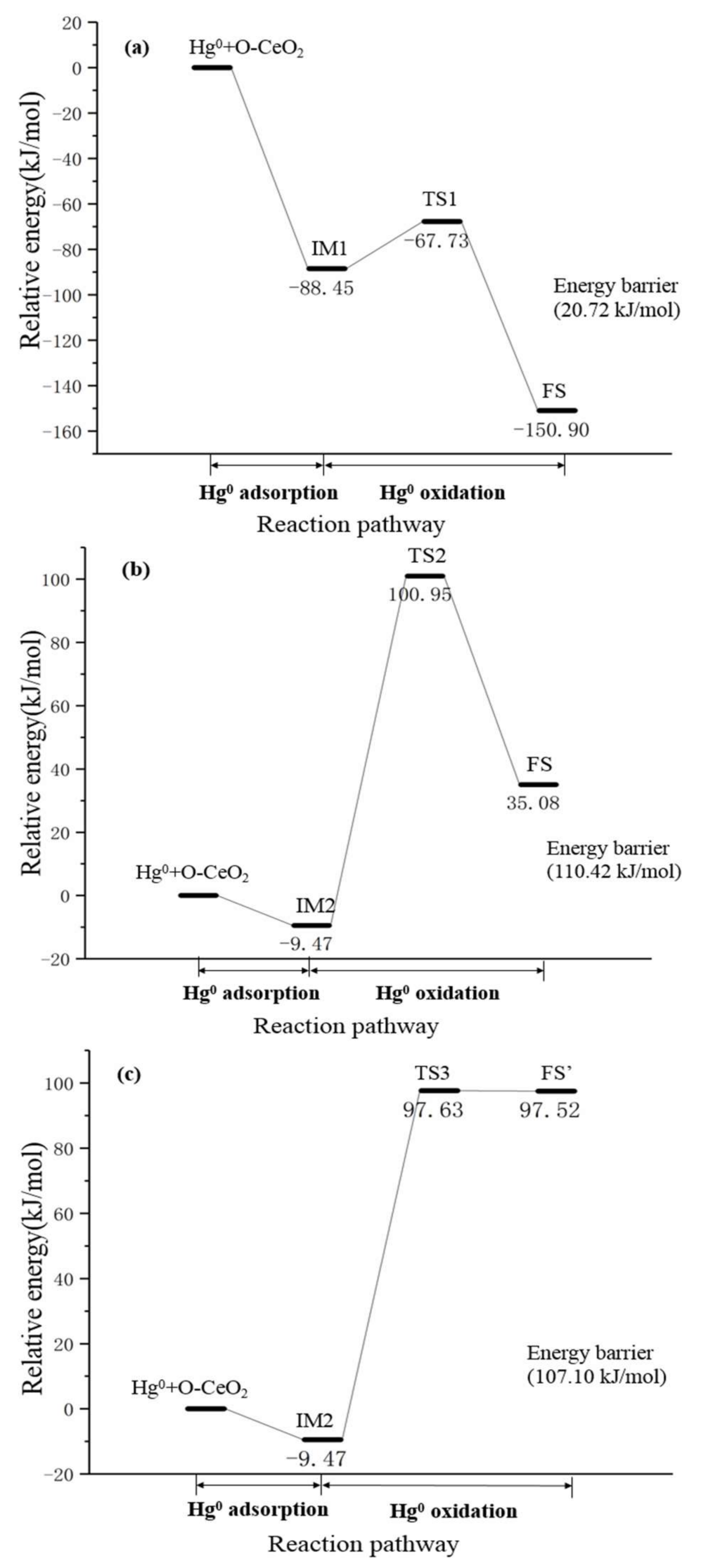
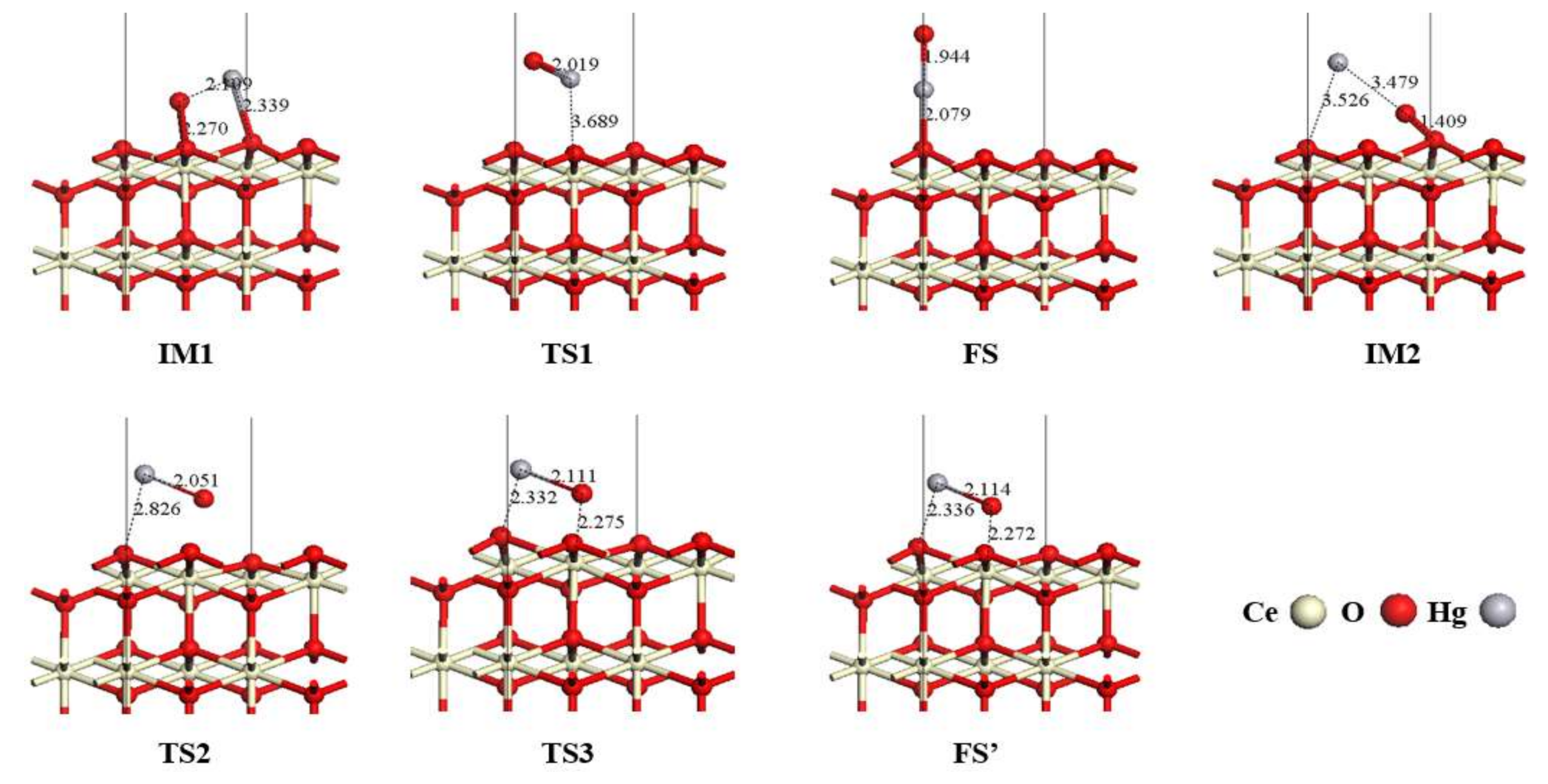
3.3. Hg0 Oxidation Mechanism on the CeO2 (111) Surface
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, S.X.; Zhang, L.; Wang, L.; Wu, Q.R.; Wang, F.Y.; Hao, J.M. A review of atmospheric mercury emissions, pollution and control in China. Front. Environ. Sci. Eng. 2014, 8, 631–649. [Google Scholar] [CrossRef]
- Liu, R.H.; Xu, W.Q.; Tong, L.; Zhu, T.Y. Mechanism of Hg0 oxidation in the presence of HCl over a commercial V2O5-WO3/TiO2 SCR catalyst. J. Environ. Sci.-China 2015, 36, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.H.; Lai, C.W.; Sharifah, B.A.H. In Situ Anodization of WO3-Decorated TiO2 Nanotube Arrays for Efficient Mercury Removal. Materials 2015, 8, 5702–5714. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.S.; Fan, M.Y.; Hu, J.W.; Ruan, W.Q.; Xiong, K.N.; Wei, X.H. Optimizing Low-Concentration Mercury Removal from Aqueous Solutions by Reduced Graphene Oxide-Supported Fe3O4 Composites with the Aid of an Artificial Neural Network and Genetic Algorithm. Materials 2017, 10, 1279. [Google Scholar] [CrossRef] [PubMed]
- Streets, D.G.; Lu, Z.F.; Levin, L.; Ter Schure, A.; Sunderland, E.M. Historical releases of mercury to air, land, and water from coal combustion. Sci. Total Environ. 2017, 615, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.C.; Shen, Z.M.; Tang, Q.L.; Yang, B.W.; Fan, M.H. A DFT study of Hg0, adsorption on Co3O4 (110) surface. Chem. Eng. J. 2016, 289, 349–355. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.X.; Streets, D.G.; Hao, J.M.; Chan, M.; Jiang, J.K. Trends in Anthropogenic Mercury Emissions in China from 1995 to 2003. Environ. Sci. Technol. 2006, 40, 5312–5318. [Google Scholar] [CrossRef] [PubMed]
- Li, H.L.; Zhu, L.; Wu, S.K.; Liu, Y.; Shih, K.M. Synergy of CuO and CeO2 combination for mercury oxidation under low-temperature selective catalytic reduction atmosphere. Int. J. Coal Geol. 2017, 170, 69–76. [Google Scholar] [CrossRef]
- Zhao, L.K.; Li, C.T.; Zhang, X.N.; Zeng, G.M.; Zhang, J.; Xie, Y.E. A review on oxidation of element mercury from coal-fired flue gas with selective catalytic reduction catalysts. Catal. Sci. Technol. 2015, 5, 3459–3472. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Liu, X.W.; Zhao, B.; Chen, Z.G.; Shao, H.Z.; Wang, L.L.; Xu, M.H. Effects of existing energy saving and air pollution control devices on mercury removal in coal-fired power plants. Fuel Process. Technol. 2015, 131, 99–108. [Google Scholar] [CrossRef]
- Huang, W.J.; Xu, H.M.; Qu, Z.; Zhao, S.J.; Chen, W.M.; Yan, N.Q. Significance of Fe2O3, modified SCR catalyst for gas-phase elemental mercury oxidation in coal-fired flue gas. Fuel Process. Technol. 2016, 149, 23–28. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, X.W.; Zhou, Z.J.; Shao, H.Z.; Xu, M.H. Catalytic oxidation of elemental mercury by Mn–Mo/CNT at low temperature. Chem. Eng. J. 2016, 284, 1233–1241. [Google Scholar] [CrossRef]
- Jung, J.E.; Geatches, D.; Lee, K.; Aboud, S.; Brown, G.E.; Wilcox, J. First-Principles Investigation of Mercury Adsorption on the α-Fe2O3 (1102) Surface. J. Phys. Chem. C 2015, 119, 26512–26518. [Google Scholar] [CrossRef]
- Zhao, L.K.; Li, C.T.; Zhang, X.N.; Zeng, G.M.; Zhang, J.; Xie, Y.E. Oxidation of elemental mercury by modified spent TiO2-based SCR-DeNOx catalysts in simulated coal-fired flue gas. Environ. Sci. Pollut. Res. 2016, 23, 1471–1481. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.N.; Li, C.T.; Zhao, L.K.; Zhang, J.; Zeng, G.M.; Xie, Y.E.; Yu, M.E. Simultaneous removal of elemental mercury and NO from flue gas by V2O5–CeO2/TiO2 catalysts. Appl. Surf. Sci. 2015, 347, 392–400. [Google Scholar] [CrossRef]
- Li, H.L.; Wu, C.Y.; Li, Y.; Zhang, J.Y. CeO2-TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue gas. Environ. Sci. Technol. 2011, 45, 7394–7400. [Google Scholar] [CrossRef] [PubMed]
- Phuong, T.M.P.; Thang, L.M.; Tien, T.N.; Isabel, V.D. CeO2 Based Catalysts for the Treatment of Propylene in Motorcycle’s Exhaust Gases. Materials 2014, 7, 7379–7397. [Google Scholar] [CrossRef]
- Fan, X.P.; Li, C.T.; Zeng, G.M.; Zhang, X.; Tao, S.S.; Lu, P.; Tan, Y.; Luo, D.Q. Hg0 Removal from Simulated Flue Gas over CeO2/HZSM-5. Energy Fuels 2012, 26, 2082–2089. [Google Scholar] [CrossRef]
- Zhao, L.; He, Q.S.; Li, L.; Lu, Q.; Dong, C.Q.; Yang, Y.P. Research on the catalytic oxidation of Hg0 by modified SCR catalysts. J. Fuel Chem. Technol. 2015, 43, 628–634. [Google Scholar] [CrossRef]
- Zhang, B.K.; Liu, J.; Shen, F.H. Heterogeneous Mercury Oxidation by HCl over CeO2 Catalyst: Density Functional Theory Study. J. Phys. Chem. C 2015, 119, 15047–15055. [Google Scholar] [CrossRef]
- Wang, S.X.; Zhang, L.; Li, G.H.; Wu, Y.; Hao, J.M.; Pirrone, N.; Sproviery, F.; Ancora, M.P. Mercury emission and speciation of coal-fired power plants in China. Atmos. Chem. Phys. 2010, 10, 1183–1192. [Google Scholar] [CrossRef]
- He, C.; Shen, B.X.; Chi, G.L.; Li, F.K. Elemental mercury removal by CeO2/TiO2-PILCs under simulated coal-fired flue gas. Chem. Eng. J. 2016, 300, 1–8. [Google Scholar] [CrossRef]
- Zhao, L.L.; Li, C.T.; Zhang, J.; Zhang, X.N.; Zhan, F.M.; Ma, J.F.; Xie, Y.E.; Zeng, G.M. Promotional effect of CeO2 modified support on V2O5–WO3/TiO2 catalyst for elemental mercury oxidation in simulated coal-fired flue gas. Fuel 2015, 153, 361–369. [Google Scholar] [CrossRef]
- Presto, A.A.; Granite, E.J. Survey of catalysts for oxidation of mercury in flue gas. Environ. Sci. Technol. 2006, 40, 5601–5609. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.K.; Liu, J.; Zhang, J.Y.; Zheng, C.G.; Chang, M. Mercury oxidation mechanism on Pd (100) surface from first-principles calculations. Chem. Eng. J. 2014, 237, 344–351. [Google Scholar] [CrossRef]
- Zhang, B.K.; Liu, J.; Yang, Y.J.; Chang, M. Oxidation mechanism of elemental mercury by HCl over MnO2 catalyst: Insights from first principles. Chem. Eng. J. 2015, 280, 354–362. [Google Scholar] [CrossRef]
- Xu, Z.M.; Lv, X.J.; Chen, J.A.; Jiang, L.X.; Lai, Y.Q.; Li, J. First principles study of adsorption and oxidation mechanism of elemental mercury by HCl over MoS2 (100) surface. Chem. Eng. J. 2016, 308, 1225–1232. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, J.; Zhang, B.K.; Liu, F. Density functional theory study on the heterogeneous reaction between Hg0, and HCl over spinel-type MnFe2O4. Chem. Eng. J. 2017, 308, 897–903. [Google Scholar] [CrossRef]
- Delley, B. From molecules to solids with the DMol3 approach. J. Chem. Phys. 2000, 113, 7756–7764. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rec. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Wang, Y. Generalized gradient approximation for the exchange-correlation hole of a many-electron system. Phys. Rev. B 1996, 54, 16533–16539. [Google Scholar] [CrossRef]
- Delley, B. Hardness conserving semilocal pseudopotentials. Phys. Rev. B 2002, 66, 155125. [Google Scholar] [CrossRef]
- Song, Y.L.; Yin, L.L.; Zhang, J.; Hu, X.P.; Gong, X.Q. A DFT+U study of CO oxidation at CeO2 (110) and (111) surfaces with oxygen vacancies. Surf. Sci. 2013, 618, 140–147. [Google Scholar] [CrossRef]
- Huang, M.; Fabris, S. CO adsorption and oxidation on ceria surfaces from DFT+ U calculations. J. Phys. Chem. C 2008, 112, 8643–8648. [Google Scholar] [CrossRef]
- Ganduglia-Pirovano, M.V.; Da, S.J.; Sauer, J. Density-functional calculations of the structure of near-surface oxygen vacancies and electron localization on CeO2 (111). Phys. Rev. Lett. 2009, 102, 026101. [Google Scholar] [CrossRef] [PubMed]
- Fronzi, M.; Soon, A.; Delley, B.; Traversa, E.; Stampfl, C. Stability and morphology of cerium oxide surfaces in an oxidizing environment: A first-principles investigation. J. Chem. Phys. 2009, 131, 104701. [Google Scholar] [CrossRef]
- Fronzi, M.; Piccinin, S.; Delley, B.; Traversa, E.; Stampl, C. Water adsorption on the stoichiometric and reduced CeO2 (111) surface: A first-principles investigation. Phys. Chem. Chem. Phys. 2009, 11, 9188–9199. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Adams, J.B.; Schilfgaarde, M. Density-functional calculation of CeO2 surfaces and prediction of effects of oxygen partial pressure and temperature on stabilities. J. Chem. Phys. 2005, 123, 064701. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Sherman, B.J.; Lo, C.S. Carbon dioxide activation and dissociation on ceria (110): A density functional theory study. J. Chem. Phys. 2013, 138, 014702. [Google Scholar] [CrossRef] [PubMed]
- Kumari, N.; Haider, M.A.; Agarwal, M.; Shina, N.; Basu, S. Role of Reduced CeO2 (110) Surface for CO2 Reduction to CO and Methanol. J. Phys. Chem. C 2016, 120, 16626–16635. [Google Scholar] [CrossRef]
- Gerward, L.; Olsen, J.S.; Vaitheeswaran, G.; Kanchana, V.; Svane, A. Bulk modulus of CeO2 and PrO2—An experimental and theoretical study. J. Alloys Compd. 2005, 400, 56–61. [Google Scholar] [CrossRef]
- Halgren, T.A.; Lipscomb, W.N. The synchronous-transit method for determining reaction pathways and locating molecular transition states. Chem. Phys. Lett. 1977, 49, 225–232. [Google Scholar] [CrossRef]
- Yang, Y.J.; Liu, J.; Zhang, B.K.; Liu, F. Mechanistic studies of mercury adsorption and oxidation by oxygen over spinel-type MnFe2O4. J. Hazard. Mater. 2017, 321, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.Y.; Li, Z.X. A DFT study of the Hg0 oxidation mechanism on the V2O5-TiO2 (001) surface. Mol. Catal. 2017, 433, 372–382. [Google Scholar] [CrossRef]

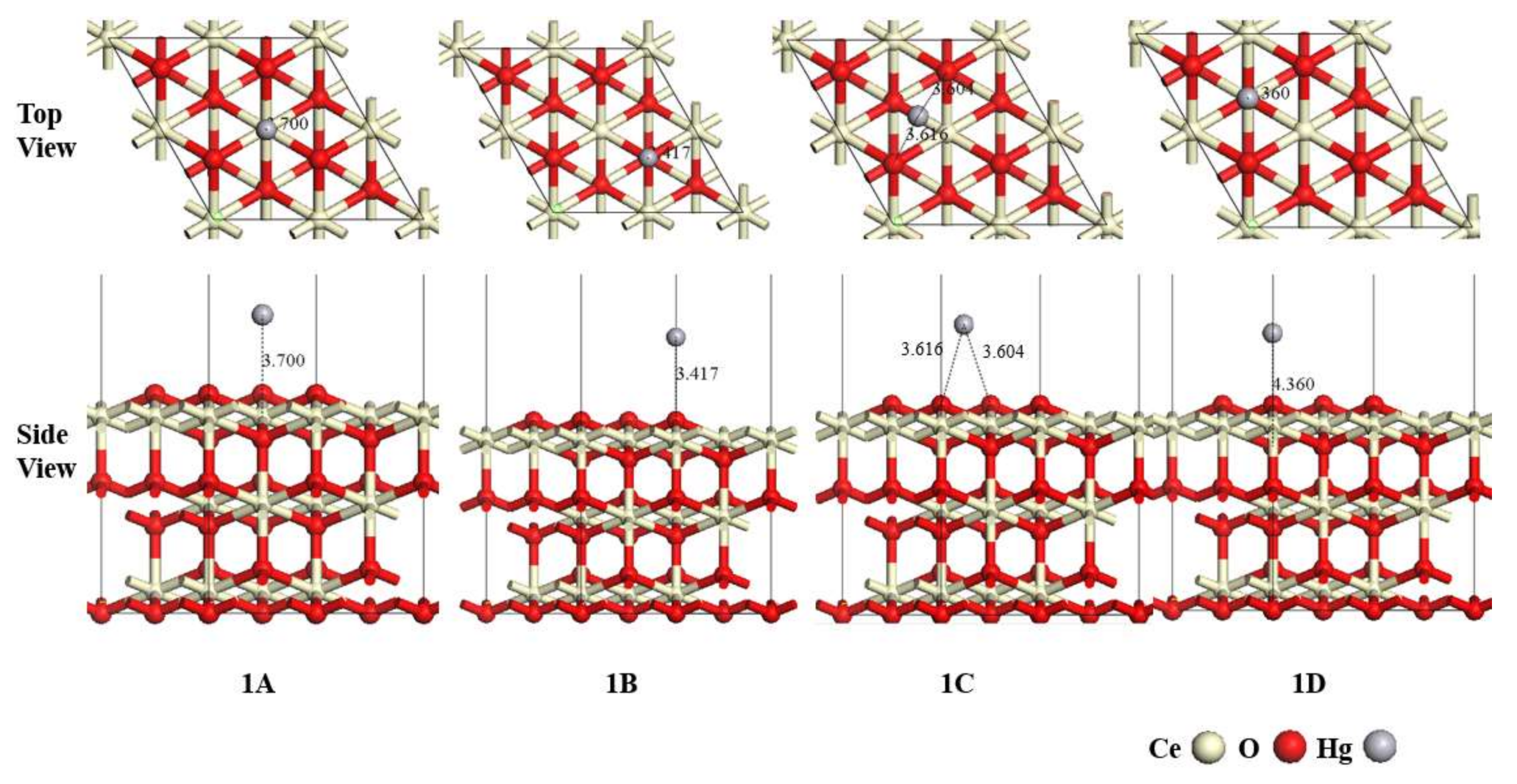
| Configurations | Eads (kJ/mol) | RX-Hg (Å) * | QHg (e) |
|---|---|---|---|
| 1A | −5.71 | 3.700 | 0.012 |
| 1B | −1.54 | 3.417 | 0.008 |
| 1C | −5.30 | 3.616/3.604 | 0.009 |
| 1D | −5.25 | 4.360 | 0.011 |
| Configurations | Eads (kJ/mol) | RX-Hg (Å) * | RO-Hg (Å) | RX-O (Å) |
|---|---|---|---|---|
| 2A | −69.90 | - | 2.145 | 2.153 |
| 2B | −101.99 | 2.114 | 2.277 | 2.400 |
| 2C | −163.81 | 2.077 | 1.945 | - |
| 2D | −198.37 | - | 3.354 | 1.415 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, L.; Wu, Y.; Han, J.; Lu, Q.; Yang, Y.; Zhang, L. Mechanism of Mercury Adsorption and Oxidation by Oxygen over the CeO2 (111) Surface: A DFT Study. Materials 2018, 11, 485. https://doi.org/10.3390/ma11040485
Zhao L, Wu Y, Han J, Lu Q, Yang Y, Zhang L. Mechanism of Mercury Adsorption and Oxidation by Oxygen over the CeO2 (111) Surface: A DFT Study. Materials. 2018; 11(4):485. https://doi.org/10.3390/ma11040485
Chicago/Turabian StyleZhao, Li, Yangwen Wu, Jian Han, Qiang Lu, Yongping Yang, and Laibao Zhang. 2018. "Mechanism of Mercury Adsorption and Oxidation by Oxygen over the CeO2 (111) Surface: A DFT Study" Materials 11, no. 4: 485. https://doi.org/10.3390/ma11040485
APA StyleZhao, L., Wu, Y., Han, J., Lu, Q., Yang, Y., & Zhang, L. (2018). Mechanism of Mercury Adsorption and Oxidation by Oxygen over the CeO2 (111) Surface: A DFT Study. Materials, 11(4), 485. https://doi.org/10.3390/ma11040485